Phytohormone Profiles of Lettuce and Pepper Grown Aeroponically with Elevated Root-Zone Carbon Dioxide Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Measurements
2.2. Multi-Hormone Analysis
2.3. Leaf Area Expansion in Response to ACC, GA3 and BA Application
- (1)
- 0.1, 10 and 100 µM GA3;
- (2)
- 5, 50 and 500 µM BA;
- (3)
- 0.1, 10 and 100 µM ACC.
2.4. Statistics
3. Results
3.1. Root-Zone Carbon Dioxide Enrichment of Aeroponically Grown Lettuce and Pepper Plants
3.2. Leaf Area Expansion in Pepper Plants Grown Aeroponically
3.3. Leaf Area Expansion in Response to ACC, GA3 and BA Application
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Enoch, H.Z.; Olesen, J.M. Plant response to irrigation with water enriched carbon dioxide. New Phytol. 1993, 125, 249–258. [Google Scholar] [CrossRef]
- Cramer, M.D.; Richards, M.D. The effect of rhizosphere dissolved inorganic carbon on gas exchange characteristics and growth rates of tomato seedlings. J. Exp. Bot. 1999, 50, 79–87. [Google Scholar] [CrossRef]
- He, J.; Austin, P.T.; Lee, S.K. Effects of elevated root zone CO2 and air temperature on photosynthetic gas exchange, nitrate uptake, and total reduced nitrogen content in aeroponically grown lettuce plants. J. Exp. Bot. 2010, 61, 3959–3969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Austin, P.T.; Nichols, M.A.; Lee, S.K. Elevated root-zone CO2 protects lettuce plants from midday depression of photosynthesis. Environ. Exp. Bot. 2007, 61, 94–110. [Google Scholar] [CrossRef]
- Taylor, G.; Ranasinghe, S.; Bosac, C.; Gardner, S.D.L.; Ferris, R. Elevated CO2 and plant growth: Cellular mechanisms and responses of whole plant. J. Exp. Bot. 1994, 45, 1761–1774. [Google Scholar] [CrossRef]
- Ferris, R.; Sabatti, M.; Miglietta, F.; Mills, R.F.; Taylor, G. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant Cell Environ. 2001, 24, 305–315. [Google Scholar] [CrossRef]
- Hasegawa, T.; Sakai, H.; Tokida, T.; Nakamura, H.; Zhu, C.; Usui, Y.; Yoshimoto, M.; Fukoaka, M.; Wakatsuki, H.; Katayanagi, N.; et al. Rice cultivar response to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct. Plant Biol. 2013, 40, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Hui, D.; Zhang, D. Elevated CO2 stimulated net accumulation of carbon and nitrogen in land ecosystems: A metaanalysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Teng, N.; Wang, J.; Chen, T.; Wu, X.; Wang, Y.; Lin, J. Elevated CO2 induces physiological, biochemical and structural changes in leaves of Arabidopsis thaliana. New Phytol. 2006, 172, 92–103. [Google Scholar] [CrossRef]
- Wang, Y.; Shao-Ting, D.; Ling-Ling, L.; Li-Dong, H.; Ping, F.; Xian-Yong, L.; Yong-Song, Z.; Hai-Long, W. Effect of CO2 elevation on root growth and its relationship with indole acetic acid and ethylene in tomato seedlings. Pedosphere 2009, 19, 570–576. [Google Scholar] [CrossRef]
- Seneweera, S.; Aben, S.K.; Basra, A.S.; Jones, B.; Conroy, J.P. Involvement of ethylene in the morphological and developmental response of rice to elevated atmospheric CO2 concentrations. Plant Growth Regul. 2003, 39, 143–153. [Google Scholar] [CrossRef]
- Li, P.H.; Sioson, A.; Mane, S.P.; Ulanov, A.; Grothaus, G.; Heath, L.S.; Murali, T.M.; Bohnert, H.J.; Grene, R. Response diversity of Arabidopsis thaliana ecotypes in elevated CO2 in the field. Plant Mol. Biol. 2006, 62, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, L.; Li, Y.; Wang, Z. Systematic comparison of C3 and C4 plants based on metabolic network analysis. BMC Syst. Biol. 2012, 6 (Suppl 2). [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.L.; Chen, Y.D.; Liu, Y.L.; Qi, X. Effect of rhizosphere CO2 concentration on root growth and activity of netted muskmelon. Trans. CSAE 2009, 25, 210–215, (In Chinese with English abstract). [Google Scholar]
- Davies, P.J. The plant hormones: Their nature, occurrence, and functions. In Plant Hormones: Biosynthesis, Signal Transduction Action; Davies, P.J., Ed.; Springer: Ithaca, NY, USA, 2010. [Google Scholar]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Sigurdsson, B.D.; Thorgeirsson, H.; Linder, S. Growth and dry-matter partitioning of young Populus trichocarpa in response to carbon dioxide concentration and mineral nutrient availability. Tree Physiol. 2001, 21, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Pritchard, S.G.; Rogers, H.H.; Prior, S.A.; Peterson, C.M. Elevated CO2 and plant structure: A review. Glob. Chang. Biol. 1999, 5, 807–837. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, D.; Mandel, T.; Kuhlemeier, C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Sieburth, L.E. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol. 1999, 121, 1179–1190. [Google Scholar] [CrossRef] [Green Version]
- Ljung, K.; Bhalerao, R.P.; Sandberg, G. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J. 2001, 28, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; Wyrzykowska, J.; Muller, P.; David, K.; Couch, D.; Perrot-Rechenmann, C.; Fleming, A.J. Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 2008, 20, 2746–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sairanen, I.; Novák, O.; Pencik, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar] [CrossRef] [Green Version]
- Lilley, J.L.; Gee, G.W.; Sairanen, I.; Ljung, K.; Nemhauser, J.L. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012, 160, 2261–2270. [Google Scholar] [CrossRef] [Green Version]
- Hachiya, T.; Sugiura, D.; Kojima, M.; Sato, S.; Yanagisawa, S.; Sakakibara, H.; Terashima, I.; Noguchi, K. High CO2 triggers preferential root growth of Arabidopsis thaliana via two distinct systems under low pH and low N stresses. Plant Cell Physiol. 2014, 55, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Jin, C.; Jin, G.; Zhou, Q.; Lin, X.; Tang, C.; Zhang, Y. Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant Cell Environ. 2011, 34, 1304–1317. [Google Scholar] [CrossRef]
- Piñero, M.C.; Houdusse, F.; Garcia-Mina, J.M.; Garnica, M.; del Amor, F.M. Regulation of hormonal responses of sweet pepper as affected by salinity and elevated CO2 concentration. Physiol. Plant. 2013, 151, 375–389. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinins. Arab. Book 2014, 12, e0168. [Google Scholar] [CrossRef] [Green Version]
- Shani, E.; Yanai, O.; Ori, N. The role of hormones in shoot apical meristem function. Curr. Opin. Plant Biol. 2006, 9, 484–489. [Google Scholar] [CrossRef]
- Taiz, l.; Zeiger, E. Plant Physiology, 5th ed.; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Schaz, U.; Dull, B.; Reinbothe, C.; Beck, E. Influence of root-bed size on the response of tobacco to elevated CO2 as mediated by cytokinins. Aob. Plants 2014, 6, plu010. [Google Scholar] [CrossRef] [Green Version]
- Yong, J.W.; Wong, S.C.; Letham, D.S.; Hocart, C.H.; Farquhar, G.D. Effects of elevated [CO2] and nitrogen nutrition on cytokinins in the xylem sap and leaves of cotton. Plant Physiol. 2000, 124, 767–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moubayidin, L.; Di Mambro, R.; Sabatini, S. Cytokinin-auxin crosstalk. Trends Plant Sci. 2009, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Bar, M.; Ori, N. Leaf development and morphogenesis. Development 2014, 141, 4219–4230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fofanova, T.A.; Khokhlova, V.A. Tissue Specific Response of Detached Pumpkin Cotyledon Cells to the Treatment with Phytohormones. Fiziol. Rast. 1983, 30, 421–430. [Google Scholar]
- Kulaeva, O.N.; Khokhlova, V.A.; Fofanova, T.A. Cytokinins and Abscisic Acid in the Regulation of Growth and Intracellular Differentiation, Gormonal’naya Regulyatsiya Ontogeneza Rastenii (Hormonal Regulation of Plant Development); Chailakhyan, M.K., Ed.; Nauka: Moscow, Russia, 1984; pp. 71–86. [Google Scholar]
- Nielsen, T.H.; Ulvskov, P. Cytokinins and leaf development in sweet pepper (Capsicum annum L.). Planta 1992, 188, 78–84. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Davies, P.J.; Reid, J.B. Genetic dissection of the relative roles of auxin and gibberellin in the regulation of stem elongation in intact light-grown peas. Plant Physiol. 1996, 110, 1029–1034. [Google Scholar] [CrossRef] [Green Version]
- Cosgrove, D.J.; Sovonick-Dunford, S.A. Mechanism of gibberellin-dependent stem elongation in peas. Plant Physiol. 1989, 89, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Li, C.R.; Gan, L.J.; Xia, K.; Zhou, X.; Hew, C.S. Responses of carboxylating enzymes, sucrose metabolizing enzymes and plant hormones in a tropical epiphytic CAM orchid to CO2 enrichment. Plant Cell Environ. 2002, 25, 369–377. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; He, C.; Duan, A. Genes responsive to elevated CO2 concentrations in triploid white poplar and integrated gene network analysis. PLoS ONE 2014, 9, e98300. [Google Scholar] [CrossRef]
- Wang, X.; Han, F.; Yang, M.; Yang, P.; Shen, S. Exploring the response of rice (Oryza sativa) leaf to giberellins: A proteomic strategy. Rice 2013, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riemann, M.; Dhakarey, R.; Hazman, M.; Miro, B.; Kohli, A.; Nick, P. Exploring jasmonates in the hormonal network of drought and salinity responses. Front. Plant Sci. 2015, 6, 1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [Green Version]
- Zavala, J.A.; Nabity, P.D.; DeLucia, E.H. An emerging understanding of mechanisms governing insect herbivory under elevated CO2. Annu. Rev. Entomol. 2013, 58, 79–97. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.C.; Guo, H.J.; Ge, F. Plant–aphid interactions under elevated CO2: Some cues from aphid feeding behavior. Front. Plant Sci. 2016, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Misra, B.B.; Armas, E.; Huhman, D.; Alborn, H.T.; Sumner, L.W.; Chen, S. Jasmonate-mediated stomatal closure under elevated CO2 revealed by time-resolved metabolomics. Plant J. 2016, 88, 947–962. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.A.; Hetherington, A.M. Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol. 1997, 114, 1557–1560. [Google Scholar] [CrossRef] [Green Version]
- Merilo, E.; Jalakas, P.; Kollist, H.; Brosché, M. The role of ABA recycling and transporter proteins in rapid stomatal responses to reduced air humidity, elevated CO2 and exogenous ABA. Mol. Plant 2015, 8, 657–659. [Google Scholar] [CrossRef] [Green Version]
- Xue, S.; Hu, H.; Ries, A.; Merilo, E.; Kollist, H.; Schroeder, J.I. Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J. 2011, 30, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.C.; Hsu, J.H.; Wang, L.C. Identification of novel inhibitors of 1-aminocyclopropane-1-carboxylic acid synthase by chemical screening in Arabidopsis thaliana. J. Biol. Chem. 2010, 285, 33445–33456. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.G.; Fernandez-Maculet, J.C.; Yang, S.F. Purification and characterization of 1-aminocyclopropane-1-carboxylate oxidase from apple fruit. Proc. Nat. Acad. Sci. USA 1992, 89, 9789–9793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleecker, A.B.; Estelle, M.A.; Somerville, C.; Kende, H. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 1988, 241, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Park, J.H.; Lee, G.I.; Paek, K.H.; Park, S.K.; Nam, H.G. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 1997, 12, 527–535. [Google Scholar] [CrossRef]
- He, C.; Davies, F.T.; Lacey, R.E. Ethylene reduces gas exchange and growth of lettuce plants under hypobaric and normal atmospheric conditions. Physiol. Plant. 2009, 135, 258–271. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347. [Google Scholar]
- Liu, F.; Jensen, C.R.; Andersen, M.N. Hydraulic and chemical signals in the control of leaf expansion and stomatal conductance in soybean exposed to drought stress. Funct. Plant Biol. 2003, 30, 65–73. [Google Scholar] [CrossRef]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef]
- Leibar-Porcel, E.; McAinsh, M.R.; Dodd, I.C. Elevated Root-Zone Dissolved Inorganic Carbon Alters Plant Nutrition of Lettuce and Pepper Grown Hydroponically and Aeroponically. Agronomy 2020, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- De Ollas, C.; Arbona, V.; Gomez-Cadenas, A.; Dodd, I.C. Attenuated accumulation of jasmonates modifies stomatal responses to water deficit. J. Exp. Bot. 2018, 69, 2103–2116. [Google Scholar] [CrossRef] [Green Version]
- Castro, P.; Puertolas, J.; Dodd, I.C. Stem girdling uncouples soybean stomatal conductance from leaf water potential by enhancing leaf xylem ABA concentration. Environ. Exp. Bot. 2019, 159, 146–156. [Google Scholar] [CrossRef] [Green Version]
- Vlot, A.C.; Dempsey, D.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovácik, J.; Grúz, J.; Backor, M.; Strnad, M.; Repcák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143. [Google Scholar] [CrossRef]
- Hayat, S.; Fariduddin, Q.; Ali, B.; Ahmad, A. Effect of salicylic acid on growth and enzyme activities of wheat seedlings. Acta Agron. Hung. 2005, 53, 433–437. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Mashayekhi, K.; Alizadeh, M.; Ebrahimi, P. Effect of salicylic acid on somatic embryogenesis and chlorogenic acid levels of carrot (Daucus carota cv. Nantes) explants. J. Ornam. Hortic. 2011, 1, 105–113. [Google Scholar]
- Armengot, L.; Marquès-Bueno, M.M.; Soria-Garcia, A.; Müller, M.; Munné-Bosch, S.; Martínez, M.C. Functional interplay between protein kinase CK2 and salicylic acid sustains PIN transcriptional expression and root development. Plant J. 2014, 78, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Agtuca, B.; Rieger, E.; Hilger, K.; Song, L.; Robert, C.A.M.; Erb, M.; Karve, A.; Ferrieri, R.A. Carbon-11 Reveals Opposing Roles of Auxin and Salicylic Acid in Regulating Leaf Physiology, Leaf Metabolism, and Resource Allocation Patterns that Impact Root Growth in Zea mays. J. Plant Growth Regul. 2013, 33, 328–339. [Google Scholar] [CrossRef]
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565. [Google Scholar] [CrossRef]
- McManus, M.T. The Plant Hormone Ethylene—Annual Plant Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2012; p. 10. [Google Scholar]
- Shiu, O.Y.; Oetiker, J.H.; Yip, W.K.; Yang, S.F. The promoter of LEACS7, an early flooding induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proc. Nat. Acad. Sci. USA 1998, 95, 10334–10339. [Google Scholar] [CrossRef] [Green Version]
- Sobeih, W.; Dodd, I.C.; Bacon, M.A.; Grierson, D.; Davies, W.J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial rootzone drying. J. Exp. Bot. 2004, 55, 2353–2363. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.; Brown, K.M. Ethylene and plant responses to nutritional stress. Physiol. Plant. 1997, 100, 613–619. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Albacete, A.; Martinez-Andujar, C.; Acosta, M.; Romero-Aranda, R.; Dodd, I.C.; Lutts, S.; Pérez-Alfocea, F. Hormonal changes during salinity-induced leaf senescence in tomato (Solanum lycopersicum L.). J. Exp. Bot. 2008, 59, 3039–3050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abeles, F.B.; Morgan, P.W.; Saltveit, M.E. Ethylene in Plant Biology; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Pierik, R.; Tholen, D.; Poorter, H.; Visser, E.J.; Voesenek, L.A.C.J. The Janus face of ethylene: Growth inhibition and stimulation. Trends Plant Sci. 2006, 11, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Konings, H.; Jackson, M.B. A relationship between rates of ethylene production by roots and the promoting or inhibiting effects of exogenous ethylene and water on root elongation. J. Plant Physiol. 1979, 92, 385–397. [Google Scholar] [CrossRef]
- Fiorani, F.; Bögemann, G.M.; Visser, E.J.W.; Lambers, H.; Voesenek, L.A.C.J. Ethylene emission and responsiveness to applied ethylene vary among Poa species that inherently differ in leaf elongation rates. Plant Physiol. 2002, 129, 1382–1390. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, Y.S.; Walch-Liu, P.; Neumann, G.; Romheld, V.; von Wiren, N.; Bangerth, F. Root-derived cytokinins as long-distance signals for NO3- induced stimulation of leaf growth. J. Exp. Bot. 2005, 56, 1143–1152. [Google Scholar] [CrossRef]
- Achard, P.; Vriezen, W.H.; Van Der Straeten, D.; Harberd, N.P. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 2003, 15, 2816. [Google Scholar] [CrossRef] [Green Version]


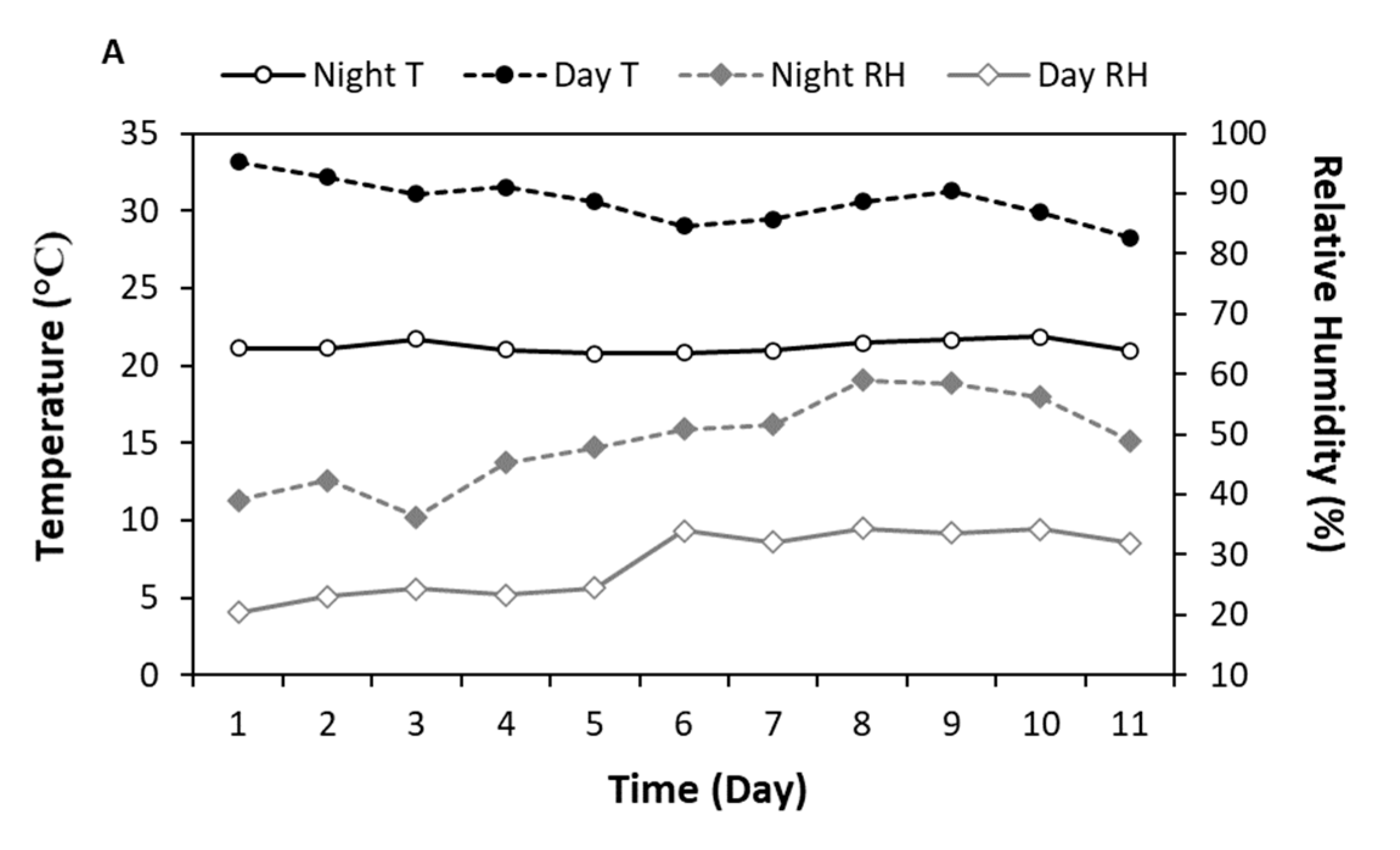
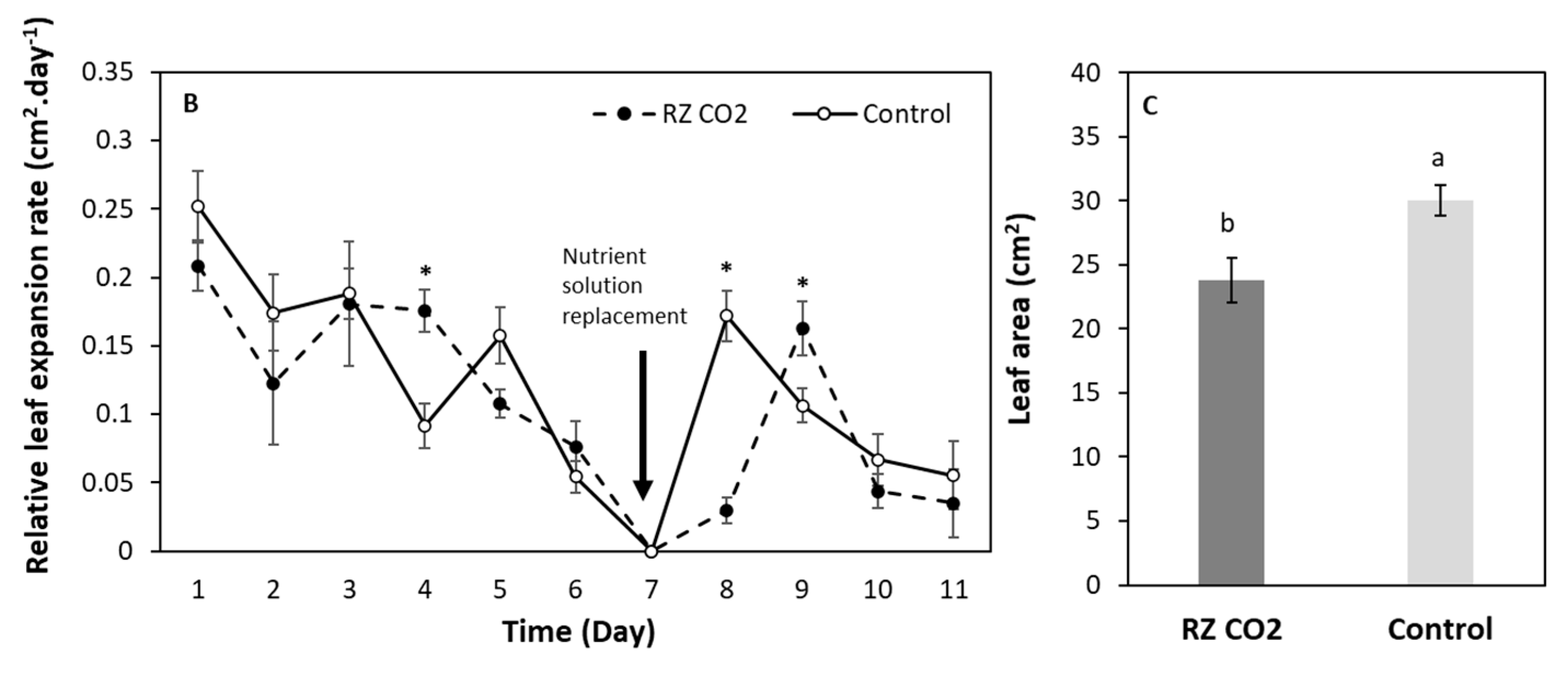
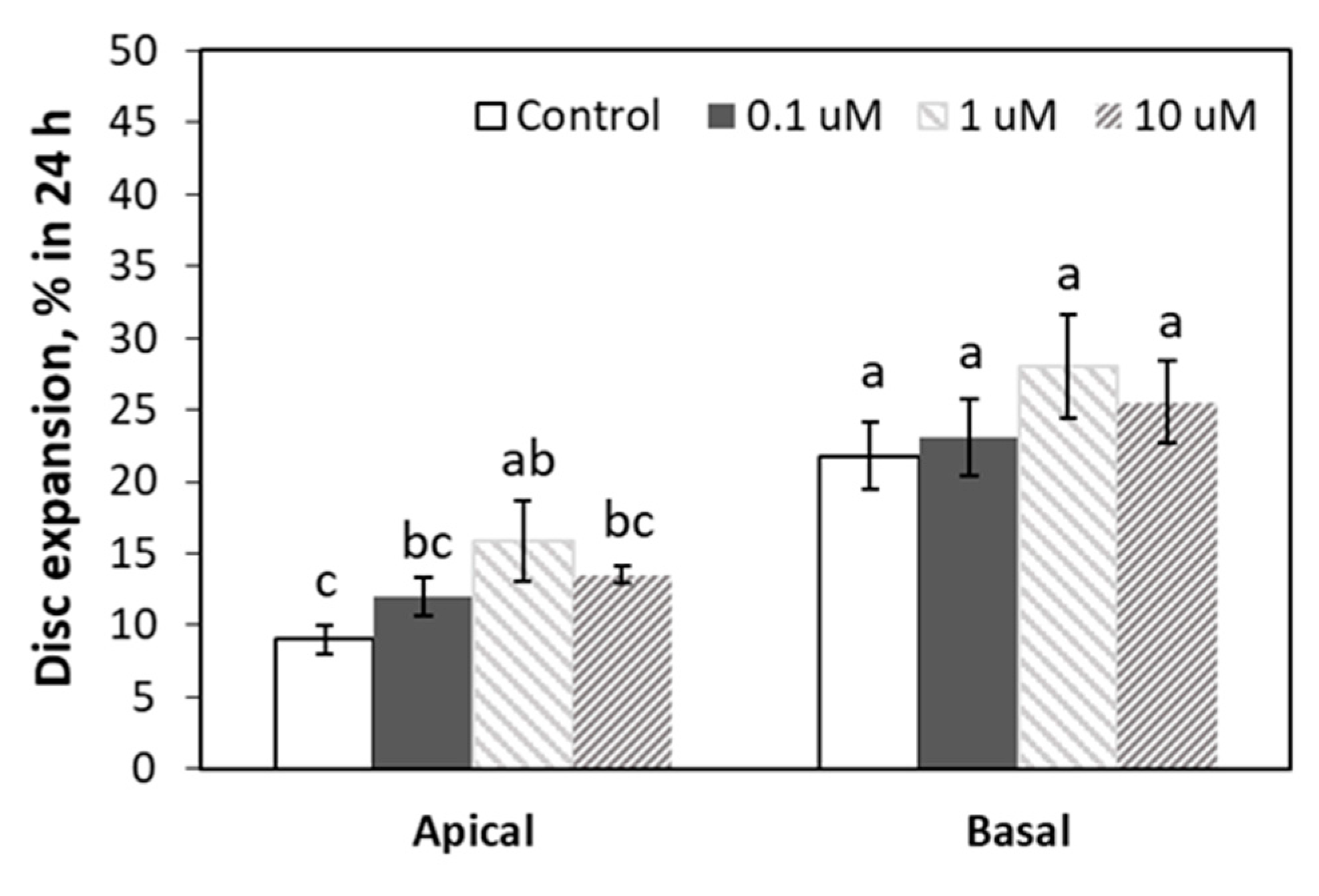
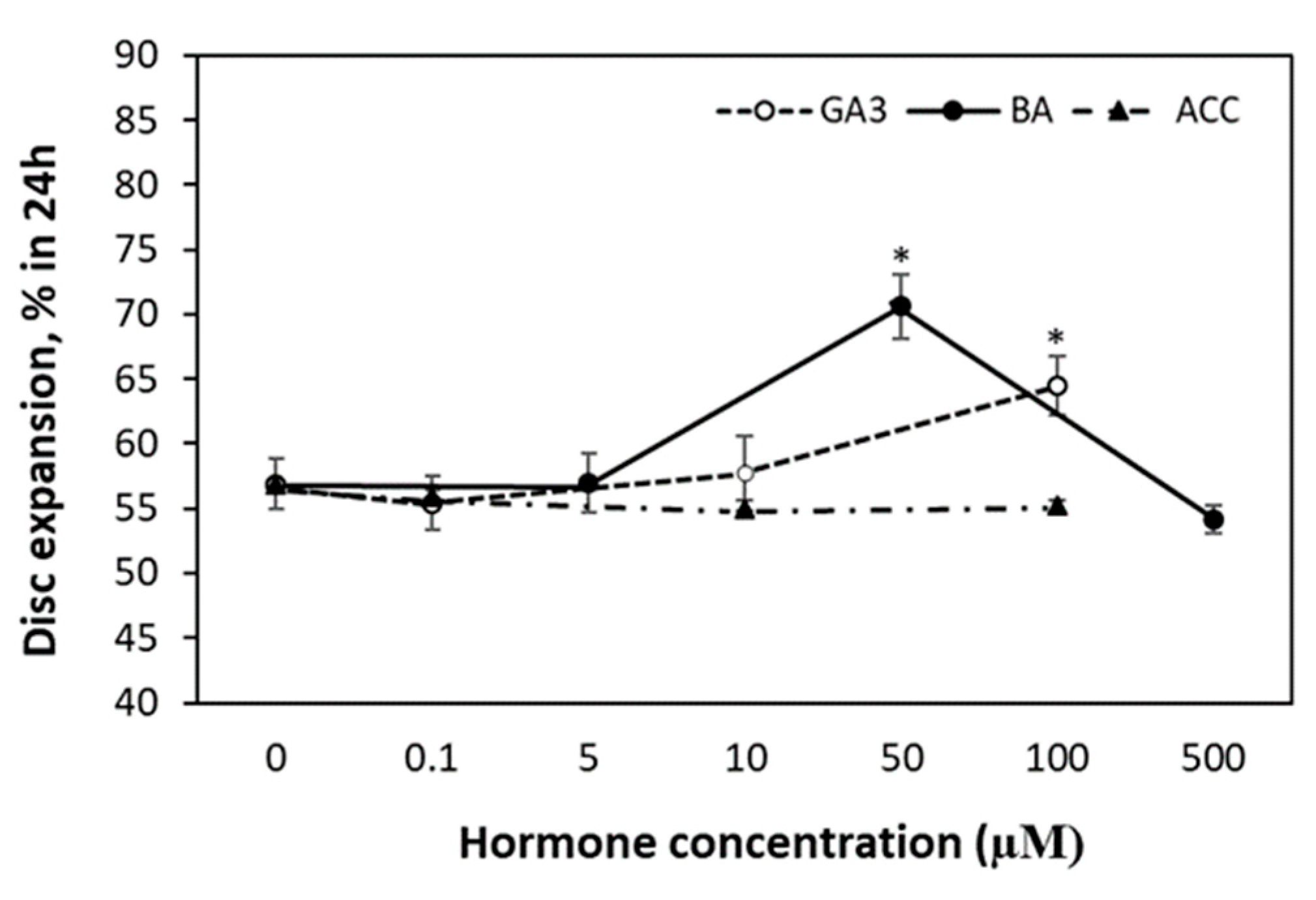

| Treatment | Shoot Fresh Weight (g) ± SE | Shoot Dry Weight (g) ± SE | Total Leaf Area (cm2) ± SE | Root Dry Weight (g) ± SE | |
|---|---|---|---|---|---|
| Experiment 1 Lettuce | Control | 71.30 ± 4.28 | 3.40 ± 0.21 | nd | 0.65 ± 0.17 |
| RZ CO2 | 86.86 ± 2.56 ** | 4.17 ± 0.12 ** | nd | 0.56 ± 0.11 | |
| Experiment 2 Pepper | Control | 9.52 ± 0.38 | 1.10 ± 0.05 | 215 ± 7 | 0.64 ± 0.17 |
| RZ CO2 | 9.44 ± 0.39 | 1.10 ± 0.03 | 215 ± 9 | 0.54 ± 0.07 | |
| Experiment 3 Pepper | Control | 10.50 ± 0.75 | 1.26 ± 0.10 | 276 ± 21 | nd |
| RZ CO2 | 8.47 ± 0.76 | 1.01 ± 0.10 | 219 ± 16 | nd |
| Treatment | Shoot Fresh Weight (g) ± SE | Shoot Dry Weight (g) ± SE | Total Leaf Area (cm2) ± SE |
|---|---|---|---|
| ACC | 79.93 ± 2.63 a | 8.95 ± 0.15 a | 858 ± 49 c |
| GA3 | 90.05 ± 6.93 a | 9.83 ± 0.65 a | 1354 ± 126 a |
| BA | 85.95 ± 2.55 a | 10.13 ± 0.41 a | 1129 ± 39 abc |
| Control | 84.75 ± 4.48 a | 9.94 ± 0.40 a | 1161 ± 85 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leibar-Porcel, E.; McAinsh, M.R.; Dodd, I.C. Phytohormone Profiles of Lettuce and Pepper Grown Aeroponically with Elevated Root-Zone Carbon Dioxide Concentrations. Agronomy 2020, 10, 665. https://doi.org/10.3390/agronomy10050665
Leibar-Porcel E, McAinsh MR, Dodd IC. Phytohormone Profiles of Lettuce and Pepper Grown Aeroponically with Elevated Root-Zone Carbon Dioxide Concentrations. Agronomy. 2020; 10(5):665. https://doi.org/10.3390/agronomy10050665
Chicago/Turabian StyleLeibar-Porcel, Estibaliz, Martin R. McAinsh, and Ian C. Dodd. 2020. "Phytohormone Profiles of Lettuce and Pepper Grown Aeroponically with Elevated Root-Zone Carbon Dioxide Concentrations" Agronomy 10, no. 5: 665. https://doi.org/10.3390/agronomy10050665
APA StyleLeibar-Porcel, E., McAinsh, M. R., & Dodd, I. C. (2020). Phytohormone Profiles of Lettuce and Pepper Grown Aeroponically with Elevated Root-Zone Carbon Dioxide Concentrations. Agronomy, 10(5), 665. https://doi.org/10.3390/agronomy10050665





