Genetic Analysis of Leaf Traits in Small-Flower Chrysanthemum (Chrysanthemum × morifolium Ramat.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phenotyping
2.3. Statistical Analysis and Heterosis Analysis
2.4. Mixed Inheritance Analyses and QTL Analyses
3. Results
3.1. Statistical Analysis of Leaf Characters of Hybrid Offspring
3.2. Classification of Leaf Morphology of Hybrid Offspring
3.3. Heterosis of Chrysanthemum Leaf Characters
3.4. The Major Gene and Polygene Mixed Genetic Model for Leaf Traits
3.5. The Estimation of Genetic Parameters of the Optimal Genetic Model for Leaf Traits
3.5.1. The Estimation of Genetic Parameters of Optimal Genetic Model of Leaf Basic Shape
3.5.2. The Estimation of Genetic Parameters in an Optimal Genetic Model of Leaf Lobe Shape
3.5.3. The Estimation of Genetic Parameters in an Optimal Genetic Model of Leaf Petiole Shape
3.6. QTL Analysis of Leaf Traits
3.6.1. Variation of Leaf Traits in Chrysanthemum
3.6.2. QTLs Related to Leaf Traits Located Based on Genetic Map
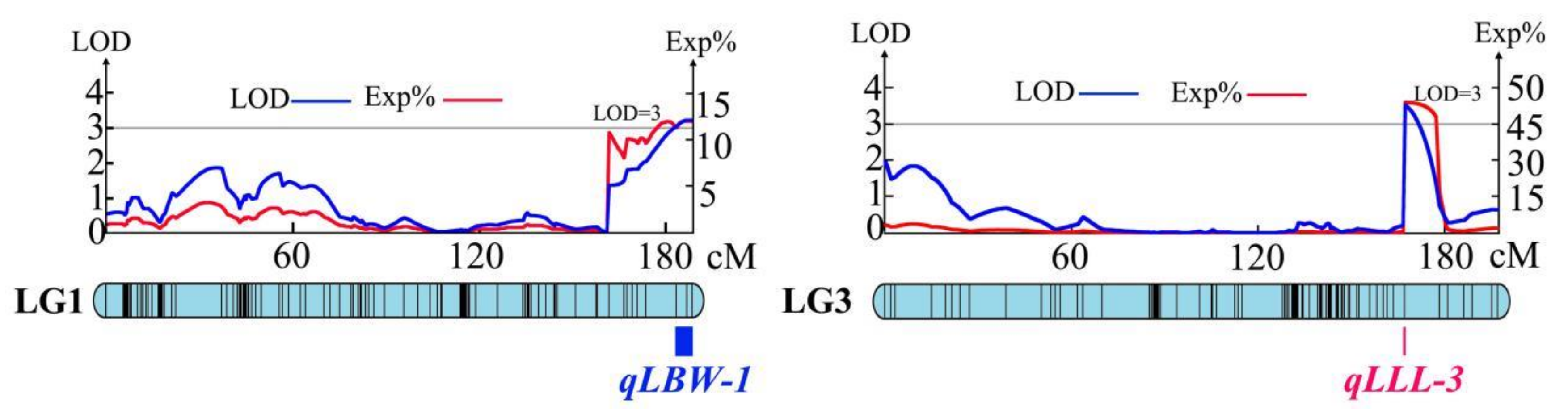
3.6.3. Major QTLs Related to Leaf Trait
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, S.L. Chinese chrysanthemums and world horticulture. J. Hebei Norm. Univ. Sci. Technol. 2004, 2, 1–5. (In Chinese) [Google Scholar]
- Dai, S.L.; Song, X.B.; Deng, C.Y.; Gao, K.; Li, M.L.; Ma, C.F.; Zhang, M.M. Comprehensive approach and molecular tools for breeding and production of ornamental crops. Acta Hortic. 2019, 1263, 1–16. [Google Scholar] [CrossRef]
- Zhang, X.C. Study on the Separation and Variation of Leaf Traits in F1 Generation of Hybrid Apple and Its Application in Early Selection. Master’s Thesis, Northwest Agriculture & Forestry University, Yang ling, China, 2009. [Google Scholar]
- Zhang, B.B.; Yu, M.L.; Cai, Z.X.; Xu, J.L.; Ma, R.J. Variation of leaf traits of hybrid offspring (F1) of narrow leaf peach and common leaf peach. Jiangsu J. Agric. Sci. 2011, 27, 848–852. (In Chinese) [Google Scholar]
- Diao, S.F.; Li, F.D.; Duan, W.; Han, W.J.; Sun, P.; Fu, J.M. Phenotypic genetic diversity of hybrid F1 leaves of persimmon. J. China Agric. Univ. 2017, 22, 32–44. (In Chinese) [Google Scholar]
- Jiang, X.B.; Zhang, P.S.; Yang, L.; Wu, Q.; Wu, C.L.; Wu, X.Y.; Gong, B.C.L.J.S. Study on leaf phenotype and genetic variation of Chinese chestnut and castanopsis chinensis interspecific hybrid F1 generation. Acta Hortic. Sin. 2019, 46, 2129–2142. (In Chinese) [Google Scholar]
- Rowland, S.D.; Zumstein, K.; Nakayama, H.; Cheng, Z.; Flores, A.M.; Chitwood, D.H.; Maloof, J.N.; Sinha, N.R. Leaf shape is a predictor of fruit quality and cultivar performance in tomato. N. Phytol. 2020, 226, 851–865. [Google Scholar] [CrossRef] [Green Version]
- Tracy, M. Geographic variation and plasticity of leaf shape and size in Begonia dregei and B. homonyma (Begoniaceae). Bot. J. Linnean Soc. 2000, 132, 79–95. [Google Scholar]
- Zhu, J.; Tian, X.J.; Chen, B.; Lu, J.Z. Computer recognition system of plant leaf shape. Bot. Bull. 2005, 22, 89–94. (In Chinese) [Google Scholar]
- Klingenberg, C.P. Evolution and development of shape: Integrating quantitative approaches. Nat. Rev. Genet. 2010, 11, 623–635. [Google Scholar] [CrossRef]
- Khadivi-khub, A.; Zamani, Z.; Fatahi, M.R. Multivariate analysis of Prunus subgen. Cerasus germplasm in Iran using morphological variables. Genet. Resour. Crop Evol. 2012, 59, 909–926. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.P. Overview of plant leaf recognition technology based on image analysis. Comput. Appl. Res. 2011, 28, 4001–4007. (In Chinese) [Google Scholar]
- Tang, J.; Deng, L.M.; Chen, H.; Luan, T.; Ma, W.J. Feature recognition of transmitted image of maize leaves based on machine vision. Sci. Agric. Sin. 2014, 47, 431–440. (In Chinese) [Google Scholar]
- Wang, L.J.; Huai, Y.J.; Peng, Y.C. Species identification of foliage plants based on multi-feature fusion of leaf images. J. Beijing For. Univ. 2015, 37, 55–61. (In Chinese) [Google Scholar]
- Chen, M.S.; Jin, Z.X.; Ke, S.X. Determination and analysis of carpinus leaf shape changes in different light environments. For. Sci. 2008, 54, 54–63. (In Chinese) [Google Scholar]
- Chatrou, L. Studies in Annonaceae XXVII. Macromorphological variation of recent invaders in northern Central America: The case of Malmea (Annonaceae). Am. J. Bot. 1997, 84, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.L.; Zhou, Q.Y.; Liu, S.J.; Li, J. Numerical analysis of germplasm relationship of grape by leaf shape structure. Fruit Tree S. China 2005, 3, 64–66. (In Chinese) [Google Scholar]
- Zhang, C. Study on the Correlation between Leaf Shape Structure and Variety Identification of Grape. Master’s Thesis, Southwest University, Chong Qing, China, 2006. (In Chinese). [Google Scholar]
- Huang, W.J.; Li, Z.J.; Yang, Z.P.; Bai, G.Z. Structural characters and their relationship in heteromorphic leaves of euphratica populus. Acta Ecol. Sin. 2010, 30, 4636–4642. (In Chinese) [Google Scholar]
- Zuo, L.H.; Zhang, W.L.; Qiu, T.; Zhang, J.; Yang, M.S. Leaf shape variation of wild apple in xinjiang and its correlation with SSR markers. Acta Hortic. Sin. 2015, 42, 759–768. (In Chinese) [Google Scholar]
- Liu, W.Q.; Lu, Y.Y.; Kang, S.; Yan, H.; Wei, F.; Ma, S.C. Comparative study on leaf structure and identification method of different leaf shape mandala. J. Pharm. Anal. 2015, 35, 1092–1098. (In Chinese) [Google Scholar]
- He, J. Study on Morphological Characters and SCoT of Genetic Relationship of 40 Guava Germplasm Resources. Master’s Thesis, Guangxi University, Guangxi, China, 2017. [Google Scholar]
- Li, X.L.; Li, X.H.; Jiang, D.M.; Liu, Z.M.; Wang, H.M.; Ji, L.Z. Leaf morphology of 22 compositae herbaceous plants in horqin sandy land. J. Ecol. 2005, 12, 1397–1401. (In Chinese) [Google Scholar]
- He, W.Q. Taxonomic Study of Pterocypsela Shih (Compositae—Endive). Master’s Thesis, Zhengzhou University, Zhengzhou, China, 2012. (In Chinese). [Google Scholar]
- Zhen, L.P.; Yang, J.; Yu, N.J. Study on the characteristics of the lower leaf surface of wild chrysanthemum plants in anhui. Chin. J. Plant Sci. 2013, 31, 99–106. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, S.L.; Dai, S.L. Chinese Chrysanthemum Book; China Forestry Press: Beijing, China, 2013; pp. 1–5. [Google Scholar]
- Li, N.N. Breeding New Cultivars of Chrysanthemum with Single Head. Master’s Thesis, Beijing Forestry University, Beijing, China, 2012. [Google Scholar]
- Wang, J.M.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Guan, Z.Y.; Tang, H.Y. Identification of cut chrysanthemum based on leaf shape. Acta Bot. Sin. 2013, 48, 608–615. (In Chinese) [Google Scholar]
- Zhang, Y. Identification and Classification of Chinese Traditional Chrysanthemum Based on Three Markers. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2014. [Google Scholar]
- Xue, S.J. Chinese Chrysanthemum Atlas; China Forestry Press: Beijing, China, 2004; pp. 1–20. [Google Scholar]
- Song, X.B. Quantitative Definition and Genetic Analysis of Phenotypic Traits of Chrysanthemum Varieties. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2018. [Google Scholar]
- Richard, F. Introduction to quantitative genetics. Trends Genet. 1996, 12, 280. [Google Scholar]
- Gai, J.Y.; Zhang, Y.M. Genetic System of Quantitative Traits in Plants; Science Press: Beijing, China, 2003; pp. 1–20. (In Chinese) [Google Scholar]
- Elston, R.C.; Stewart, J. The analysis of quantitative traits for simple genetic models from parental, F1 and backcross data. Genetics 1973, 73, 695–711. [Google Scholar] [PubMed]
- Morton, N.E.; MacLean, C.J. Analysis of family resemblance 3 Complex segregation of quantitative traits. Am. J. Human Genet. 1974, 26, 489. (In Chinese) [Google Scholar]
- Elkind, Y.; Cahaner, A. A mixed model for the effects of single gene, polygenes and their interaction on quantitative traits. Theor. Appl. Genet. 1986, 72, 377–383. [Google Scholar] [CrossRef]
- Mo, H.D. The quantity and quality traits of genetic analysis I. Genetic composition and the major gene genotype identification. J. Crops 1993, 1, 1–6. (In Chinese) [Google Scholar]
- Mo, H.D. The quantity and quality traits of genetic analysis II. Generation mean and genetic variance. J. Crops 1993, 3, 193–200. (In Chinese) [Google Scholar]
- Loisel, P.; Goffinet, B.; Monod, H.; Montes, O.G. Detecting a major gene in an F2 population. Biometrics 1994, 50, 512–516. [Google Scholar] [CrossRef]
- Jiang, C.J.; Mo, H.D. Genetic analysis IV of quantitative traits and quantity traits. The application of the maximum likelihood method. J. Crops 1995, 6, 641–648. (In Chinese) [Google Scholar]
- Jiang, C.; Pan, X.; Gu, M. The use of mixture models to detect effects of major genes on quantitative characters in a plant breeding experiment. Genetics 1994, 136, 383–394. (In Chinese) [Google Scholar] [PubMed]
- Zhang, F.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Li, F.T. Heterosis and hybrid genetic analysis of chrysanthemum floral organ traits. Sci. Agric. Sin. 2010, 43, 2953–2961. (In Chinese) [Google Scholar]
- Zhang, F.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Li, F.T. Heterosis and hybrid genetic analysis of major gene and multi-gene in chrysanthemum nutritional traits. For. Sci. 2011, 47, 46–52. (In Chinese) [Google Scholar]
- Zhang, F.; Chen, F.D.; Fang, W.M.; Chen, S.M.; Liu, P.S.; Yin, D.M. Heterosis and hybrid genetic analysis of chrysanthemum flowering traits. J. Nanjing Agric. Univ. 2011, 34, 31–36. (In Chinese) [Google Scholar]
- Xu, L.L.; Chen, F.D.; Chen, S.M.; Fang, W.M.; Zhu, W.Y.; Wang, H.B.; Li, P.L. Genetic analysis of salt tolerance of hybrid progeny between chrysanthemum and genera. Acta Ecol. Sin. 2013, 33, 4902–4908. (In Chinese) [Google Scholar]
- Peng, H.; Chen, F.D.; Fang, W.M.; Jiang, J.F.; Chen, S.M.; Guan, Z.Y.; Liao, Y. Heterosis and genetic analysis of branching traits of chrysanthemum. Acta Hortic. Sin. 2013, 40, 1327–1336. [Google Scholar]
- Tang, H.Q.; Zhang, F.; Chen, F.D.; Fang, W.M.; Wang, C.C.; Chen, S.M. Heterosis and hybrid genetic analysis of characteristics of chrysanthemum floral organ of anemone type. Acta Hortic. Sin. 2015, 42, 907–916. [Google Scholar]
- Song, X.B.; Zhao, X.G.; Fan, G.X.; Gao, K.; Dai, S.L.; Zhang, M.M. Genetic analysis of the corolla tube merged degree and the relative number of ray florets in chrysanthemum (Chrysanthemum×morifolium Ramat.). Sci. Hortic. 2018, 242, 214–224. [Google Scholar] [CrossRef]
- Fazio, G.; Staub, J.E.; Stevens, M.R. Genetic mapping and QTL analysis of horticultural traits in cucumber (Cucumis sativus L.) using recombinant inbred lines. Theor. Appl. Genet. 2003, 107, 864–874. [Google Scholar] [CrossRef]
- Zhang, W.K.; Wang, Y.J.; Luo, G.Z.; Zhang, J.S.; He, C.Y.; Wu, X.L.; Gai, J.Y.; Chen, S.Y. QTL mapping of ten agronomic traits on the soybean (Glycine max L. Merr.) genetic map and their association with EST markers. Theor. Appl. Genet. 2004, 108, 1131–1139. [Google Scholar] [CrossRef]
- He, D.H.; Lin, Z.X.; Zhang, X.L.; Nie, Y.C.; Guo, X.P.; Zhang, Y.X.; Li, W. QTL mapping for economic traits based on a dense genetic map of cotton with PCR-based markers using the interspecific cross of Gossypium hirsutum× Gossypium barbadense. Euphytica 2007, 153, 181–197. [Google Scholar] [CrossRef]
- Fang, X.; Dong, K.; Wang, X.; Liu, T.; He, J.; Ren, R.; Zhang, L.; Liu, R.; Liu, X.; Li, M.; et al. A high density genetic map and QTL for agronomic and yield traits in Foxtail millet [Setaria italica (L.) P. Beauv.]. BMC Genom. 2016, 17, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, A.H.; Lander, E.S.; Hewitt, J.D.; Peterson, S.; Lincoln, S.E.; Tanksley, S.D. Resolution of quantitative traits into Mendelian factors by using a complete linkage map of restriction fragment length polymorphisms. Nature 1988, 335, 721–726. [Google Scholar] [CrossRef]
- Simmonds, J.; Scott, P.; Leverington-Waite, M.; Turner, A.S.; Brinton, J.; Korzun, V.; Snape, J.; Uauy, C.J. Identification and independent validation of a stable yield and thousand grain weight QTL on chromosome 6A of hexaploid wheat (Triticum aestivum L.). BMC Plant Biol. 2014, 14, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, N.F.; Shi, S.L.; Shi, H.; Khanzada, H.; Wassan, G.M.; Zhu, C.L.; Peng, X.S.; Yu, Q.Y.; Chen, X.R.; He, X.P.; et al. Mapping QTL for seed germinability under low temperature using a new high-density genetic map of rice. Front. Plant Sci. 2017, 8, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.H.; Huang, C.L.; Wu, D.; Qiao, F.; Li, W.Q.; Duan, L.F.; Wang, K.; Xiao, Y.J.; Chen, G.X.; Liu, Q.; et al. High-throughput phenotyping and QTL mapping reveals the genetic architecture of maize plant growth. Plant. Physiol. 2017, 173, 1554–1564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, W.; Baenziger, P.S.; Belamkar, V.; Guttieri, M.J.; Venegas, J.P.; Easterly, A.; Sallam, A.; Poland, J. Genotyping-by-sequencing derived high-density linkage map and its application to QTL mapping of flag leaf traits in bread wheat. Sci. Rep. 2017, 7, 16394. [Google Scholar] [CrossRef]
- Murakami, N.; Hara, N.; Onodera, H.; Kashiwagi, T.; Ujiie, K.; Shimizu, B.; Onishi, A.; Miyagawa, H.; Katoh, E. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 2013, 45, 707–711. [Google Scholar]
- Wu, Y.; Wang, Y.; Mi, X.F.; Shan, J.X.; Li, X.M.; Xu, J.L.; Lin, H.X. The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet. 2016, 12, e1006386. [Google Scholar] [CrossRef]
- Yano, M.; Katayose, Y.; Ashikari, M.; Yamanouchi, U.; Monna, L.; Fuse, T.; Baba, T.; Yamamoto, K.; Umehara, Y.; Nagamura, Y.; et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 2000, 12, 2473–2483. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.H.; Wang, P.; Chen, H.X.; Zhou, H.J.; Li, Q.P.; Wang, C.R.; Ding, Z.H.; Zhang, Y.S.; Yu, S.B.; Xing, Y.Z.; et al. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol. Plant 2011, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jin, M.N.; Zheng, X.M.; Chen, J.; Yuan, D.Y.; Xin, Y.Y.; Wang, M.Q.; Huang, D.Y.; Zhang, Z.; Zhou, K.N.; et al. Days to heading 7, a major quantitative locus determining photoperiod sensitivity and regional adaptation in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 16337–16342. [Google Scholar] [CrossRef] [Green Version]
- Debener, T.; Mattiesch, L. Construction of a genetic linkage map for roses using RAPD and AFLP markers. Theor. Appl. Genet. 1999, 99, 891–899. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.M.; Chen, F.D.; Fang, W.M.; Chen, Y.; Li, F.T. SRAP-based mapping and QTL detection for inflorescence-related traits in chrysanthemum (Dendranthema morifolium). Mol. Breed. 2011, 27, 11–23. [Google Scholar] [CrossRef]
- Tang, H.Q. Genetic Analysis and QTL Mapping of Floral Organ Traits in Chrysanthemum Flowers of Osmanthus Type. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Van, G.G.; Bourke, P.M.; Voorrips, R.E.; Marasek, A.; Liao, Y.L.; Post, A.; Van, M.U.; Visser, R.G.; Maliepaard, C.; Arens, P. An ultra-dense integrated linkage map for hexaploid chrysanthemum enables multi-allelic QTL analysis. Theor. Appl. Genet. 2017, 130, 2527–2541. [Google Scholar]
- Van, O.J.W. Multipoint maximum likelihood mapping in a full-sib family of an outbreeding species. Genet. Res. 2011, 93, 343–349. [Google Scholar]
- McCouch, S.R.; Chen, X.; Panaud, O.; Temnykh, S.; Xu, Y.; Cho, Y.G.; Huang, N.; Ishii, T.; Blair, M. Microsatellite marker development, mapping and applications in rice genetics and breeding. Plant Mol. Biol. 1997, 35, 89–99. [Google Scholar] [CrossRef]





| Abbreviation | Leaf Traits |
|---|---|
| LBL | leaf blade length |
| LBW | leaf blade width |
| LBS | leaf blade shape (LBL/LBW) |
| LWL | leaf at widest part length |
| WLL | widest part length/leaf length |
| LPL | leaf petiole length |
| LPS | leaf petiole shape (LPL/LBL) |
| LVL-RUL | leaf vein length of right upper lobe |
| LLL-RUL | leaf lobe length of right upper lobe |
| LLW-RUL | leaf lobe width of right upper lobe |
| LCD-RUL | leaf crack depth of right upper lobe |
| LLS-RUL | leaf lobe size of right upper lobe |
| LVL-RLL | leaf vein length of right lower lobe |
| LLL-RLL | leaf lobe length of right lower lobe |
| LLW-RLL | leaf lobe width of right lower lobe |
| LCD-RLL | leaf crack depth of right lower lobe |
| LLS-RLL | leaf lobe size of right lower lobe |
| Traits | Minimum | Maximum | Mean | Standard Deviation | C.V.% | p-Value | |
|---|---|---|---|---|---|---|---|
| Between the Strain | In the Strain | ||||||
| LBL | 1.04 | 8.29 | 3.97 | 1.18 | 29.77 | 0.00 ** | 0.00 ** |
| LBW | 0.05 | 4.73 | 1.56 | 0.49 | 31.20 | 0.00 ** | 0.00 ** |
| LBS | 0.92 | 7.50 | 2.93 | 0.84 | 28.52 | 0.00 ** | 0.26 |
| LWL | 0.75 | 5.29 | 2.71 | 0.87 | 32.02 | 0.00 ** | 0.00 ** |
| WLL | 0.38 | 0.92 | 0.68 | 0.09 | 12.99 | 0.00 ** | 0.63 |
| LPL | 0.48 | 3.24 | 1.36 | 0.21 | 15.35 | 0.00 ** | 0.00 ** |
| LPS | 0.01 | 0.74 | 0.28 | 0.05 | 18.31 | 0.00 ** | 0.73 |
| LVL-RUL | 0.00 | 5.18 | 2.15 | 0.72 | 33.57 | 0.00 ** | 0.00 ** |
| LLL-RUL | 0.00 | 2.61 | 0.67 | 0.33 | 48.92 | 0.00 ** | 0.02 * |
| LLW-RUL | 0.00 | 1.73 | 0.77 | 0.25 | 32.70 | 0.00 ** | 0.40 |
| LCD-RUL | 0.00 | 0.71 | 0.30 | 0.10 | 33.60 | 0.00 ** | 0.30 |
| LLS-RUL | 0.00 | 3.34 | 0.84 | 0.32 | 37.58 | 0.00 ** | 0.04 * |
| LVL-RLL | 0.00 | 4.54 | 1.78 | 0.79 | 44.12 | 0.00 ** | 0.00 ** |
| LLL-RLL | 0.00 | 2.47 | 0.64 | 0.40 | 62.39 | 0.00 ** | 0.00 ** |
| LLW-RLL | 0.00 | 1.83 | 0.67 | 0.31 | 45.81 | 0.00 ** | 0.30 |
| LCD-RLL | 0.00 | 0.81 | 0.33 | 0.14 | 41.93 | 0.00 ** | 0.21 |
| LLS-RLL | 0.00 | 5.39 | 0.89 | 0.43 | 48.00 | 0.00 ** | 0.29 |
| Cross Number | Traits | P1 (F 1) | P2 (M 2) | Mid-Parents Value | Skewness | Kurtosis | Hm | RHm% | RHb% |
|---|---|---|---|---|---|---|---|---|---|
| CrossI | LBL | 4.00 | 5.39 | 4.69 | −0.05 | −0.11 | 0.29 | 6.22 | −7.41 |
| LBW | 3.13 | 3.37 | 3.25 | 0.27 | 0.54 | 0.34 | 10.42 | 6.57 | |
| LBS | 1.29 | 1.61 | 1.45 | 0.28 | −0.18 | −0.04 | −2.92 | −12.63 | |
| LWL | 2.33 | 3.09 | 2.71 | −0.23 | −0.33 | 0.73 | 0.27 | 0.48 | |
| WLL | 0.40 | 0.76 | 0.58 | −1.17 | 3.10 | 0.11 | 0.18 | 0.17 | |
| LPL | 1.31 | 1.62 | 1.47 | 0.43 | −0.14 | 0.37 | 25.56 | 14.11 | |
| LPS | 0.25 | 0.23 | 0.24 | 0.52 | 1.27 | 0.03 | 13.67 | 10.00 | |
| LVL-RLL | 2.37 | 2.63 | 2.50 | 0.22 | −0.03 | −0.01 | −0.35 | −6.22 | |
| LLL- RLL | 0.72 | 1.10 | 0.91 | 0.52 | 0.30 | 0.11 | 12.65 | −7.32 | |
| LLW-RLL | 1.03 | 0.97 | 1.00 | 0.47 | −0.15 | −0.10 | −10.24 | −13.74 | |
| LCD-RLL | 0.30 | 0.41 | 0.36 | 0.42 | −0.29 | 0.05 | 13.57 | −2.41 | |
| LLS- RLL | 0.72 | 1.11 | 0.91 | 1.42 | 3.30 | 0.26 | 28.00 | 4.74 | |
| CrossII | LBL | 2.85 | 4.32 | 3.59 | 0.31 | 0.39 | −0.08 | −2.12 | −18.78 |
| LBW | 2.60 | 2.86 | 2.73 | 0.28 | 0.36 | −0.10 | −3.58 | −7.93 | |
| LBS | 1.10 | 1.51 | 1.31 | 2.19 | 14.60 | 0.04 | 2.95 | −11.20 | |
| LWL | 1.39 | 2.73 | 2.06 | 0.47 | 1.15 | 0.33 | 15.88 | −12.60 | |
| WLL | 0.49 | 0.63 | 0.56 | −0.53 | −0.07 | 0.12 | 21.81 | 7.70 | |
| LPL | 1.18 | 1.42 | 1.30 | 0.58 | 0.84 | 0.14 | 10.94 | 1.50 | |
| LPS | 0.30 | 0.25 | 0.27 | 1.92 | 16.75 | 0.02 | 6.86 | −2.11 | |
| LVL-RLL | 1.99 | 2.08 | 2.03 | 0.64 | 0.82 | −0.39 | −19.15 | −28.84 | |
| LLL- RLL | 0.68 | 0.54 | 0.61 | 0.69 | 0.33 | −0.07 | −11.75 | −29.33 | |
| LLW-RLL | 0.99 | 0.95 | 0.97 | 0.53 | 0.50 | −0.33 | −33.95 | −41.63 | |
| LCD-RLL | 0.34 | 0.26 | 0.30 | 0.14 | −0.37 | 0.02 | 8.08 | −13.65 | |
| LLS- RLL | 0.69 | 0.56 | 0.62 | 0.59 | 0.42 | 0.22 | 35.05 | 11.64 |
| Traits | Model | Optimum Genetic Model | |
|---|---|---|---|
| Cross I | Cross II | ||
| LBL | A-0 | A-0 | A-0 (Polygenes) |
| LBW | A-0 | A-0 | A-0 (Polygenes) |
| LBS | B-1 | B-1 | B-1 (Two pairs of additive-dominant-epistatic major genes) |
| LWL | A-0 | A-0 | A-0 (Polygenes) |
| WLL | B-1 | B-5 | B-5 (Two pairs of fully dominant major genes) |
| LPL | A-1 | A-1 | A-1 (A pair of additive-dominant major genes) |
| LPS | A-0 | A-0 | A-0 (Polygenes) |
| LVL-RLL | A-0 | A-1 | A-1 (A pair of additive-dominant major genes) |
| LLL-RLL | A-1 | A-1 | A-1 (A pair of additive-dominant major genes) |
| LLW-RLL | A-1 | A-1 | A-1 (A pair of additive-dominant major genes) |
| LCD-RLL | A-1 | A-1 | A-1 (A pair of additive-dominant major genes) |
| LLS-RLL | B-2 | B-2 | B-2 (Two pairs of additive-dominant major genes) |
| Traits | Cross Number | σ2mg | h2mg(%) | σ2p |
|---|---|---|---|---|
| LBS | Cross I | 0.01 | 40.13 | 0.03 |
| Cross II | 0.01 | 33.75 | 0.04 | |
| LPL | Cross I | 0.09 | 50.20 | 0.18 |
| Cross II | 0.06 | 34.24 | 0.17 | |
| WLL | Cross I | 0.003 | 50.50 | 0.01 |
| Cross II | 0.01 | 53.48 | 0.01 | |
| LVL-RLL | Cross I | — | — | — |
| Cross II | 0.06 | 37.23 | 0.16 | |
| LLL-RLL | Cross I | 0.05 | 40.39 | 0.13 |
| Cross II | 0.02 | 51.03 | 0.05 | |
| LLW-RLL | Cross I | 0.04 | 56.63 | 0.07 |
| Cross II | 0.01 | 39.47 | 0.03 | |
| LCD-RLL | Cross I | 0.00 | 49.50 | 0.01 |
| Cross II | 0.00 | 56.55 | 0.01 | |
| LLS-RLL | Cross I | 0.08 | 67.74 | 0.11 |
| Cross II | 0.03 | 45.70 | 0.06 |
| Traits | The Number of QTLs | Linkage Group | The Number of QTL Associated Markers | LOD Threshold | Exp% |
|---|---|---|---|---|---|
| LBL | 1 | LG22 | 4 | 3.21 | 4.90 |
| LBW | 3 | LG1, LG18 | 6 | 3.00–3.23 | 4.40–12.00 |
| LBS | 2 | LG20 | 3 | 3.14–3.93 | 5.80–7.10 |
| LPL | 1 | LG6 | 1 | 4.55 | 6.80 |
| LPS | 8 | LG4, LG6, LG7,LG23, LG25 | 74 | 3.01–3.95 | 4.50–5.80 |
| LLL-RLL | 13 | LG2, LG3, LG10, LG16, LG20, LG22 | 78 | 3.04–4.33 | 5.10–54.00 |
| LLW-RLL | 1 | LG27 | 1 | 4.40 | 7.50 |
| LVL-RLL | 4 | LG2, LG11, LG17, LG20 | 9 | 3.02–3.20 | 5.10–9.00 |
| LLS-RLL | 6 | LG2, LG10, LG16 | 55 | 3.11–3.99 | 5.00–6.30 |
| LCD-RLL | 12 | LG2, LG15, LG16, LG20 | 53 | 3.00–4.22 | 4.9 0–7.10 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, K.; Song, X.; Kong, D.; Dai, S. Genetic Analysis of Leaf Traits in Small-Flower Chrysanthemum (Chrysanthemum × morifolium Ramat.). Agronomy 2020, 10, 697. https://doi.org/10.3390/agronomy10050697
Gao K, Song X, Kong D, Dai S. Genetic Analysis of Leaf Traits in Small-Flower Chrysanthemum (Chrysanthemum × morifolium Ramat.). Agronomy. 2020; 10(5):697. https://doi.org/10.3390/agronomy10050697
Chicago/Turabian StyleGao, Kang, Xuebin Song, Deyuan Kong, and Silan Dai. 2020. "Genetic Analysis of Leaf Traits in Small-Flower Chrysanthemum (Chrysanthemum × morifolium Ramat.)" Agronomy 10, no. 5: 697. https://doi.org/10.3390/agronomy10050697




