Simulation of Maize Lethal Necrosis (MLN) Damage Using the CERES-Maize Model
Abstract
:1. Introduction
2. Materials and Methods
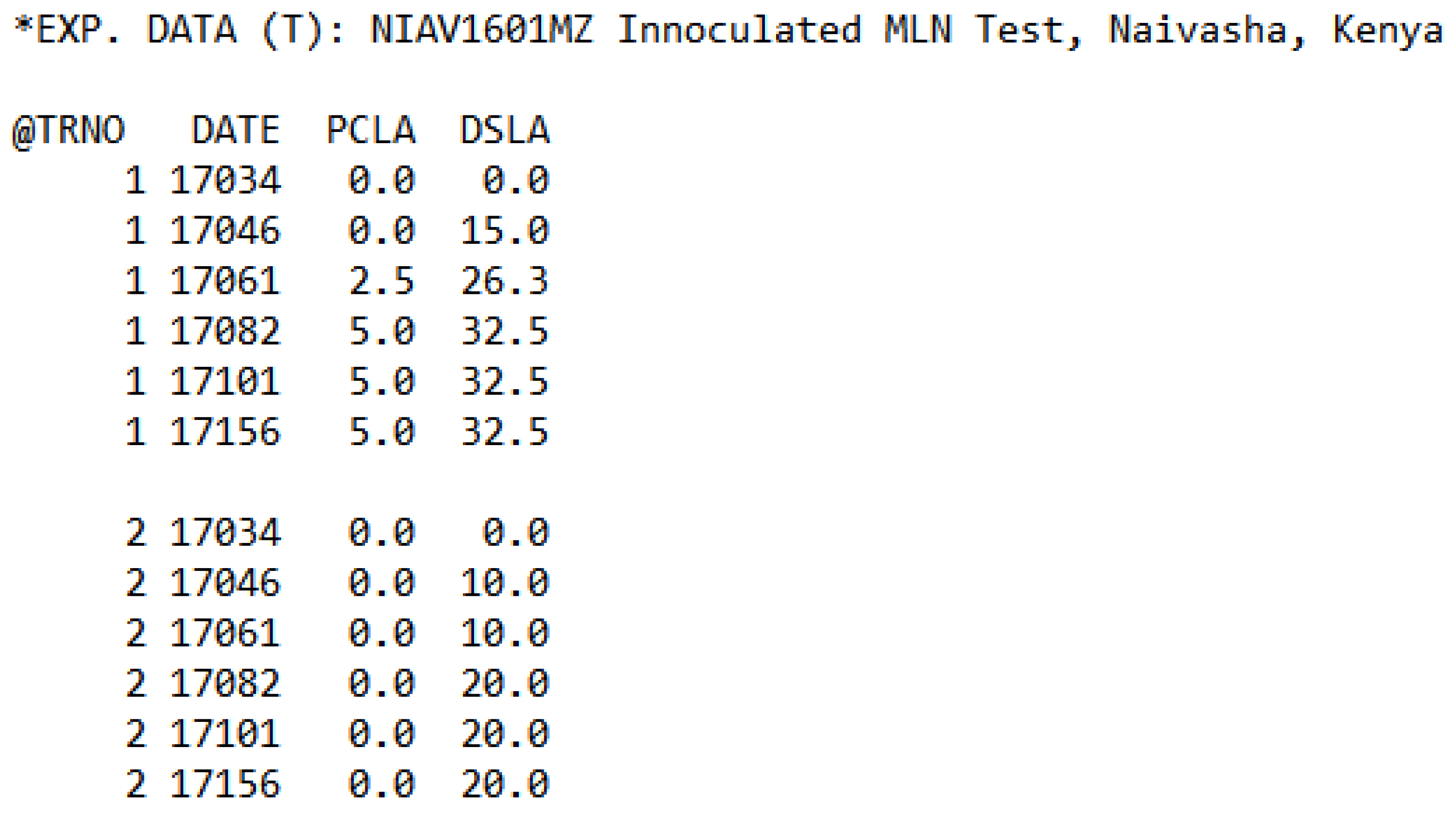
2.1. Field Experiments
2.2. MLN Inoculation Procedure
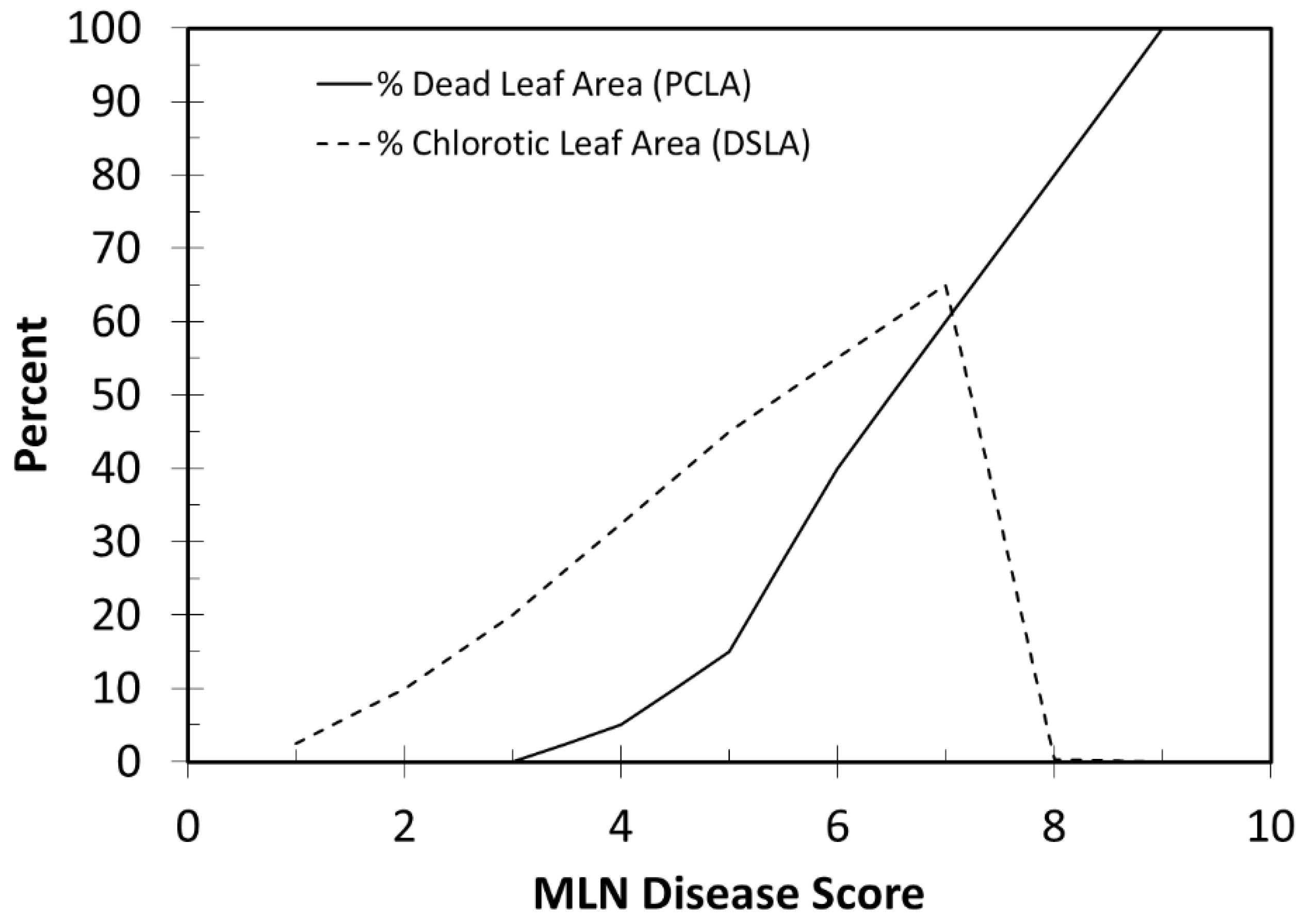
2.3. Converting Disease Scores to Percent Leaf Chlorosis and Percent Dead Leaf Area
2.4. Disease Damage Coupling Points
- WLIDOT = leaf loss due to pests or diseases, g m−2 d−1
- SENLA = leaf senescence today, cm2 pl−1
- LFWT = leaf weight, g pl−1
- PLTPOP = plant population, no. m−2
- DISLA = reduction in photosynthesis due to pests, cm2 pl−1 d−1
2.5. Model Input Files
2.6. Model Calibration and Evaluation
3. Results
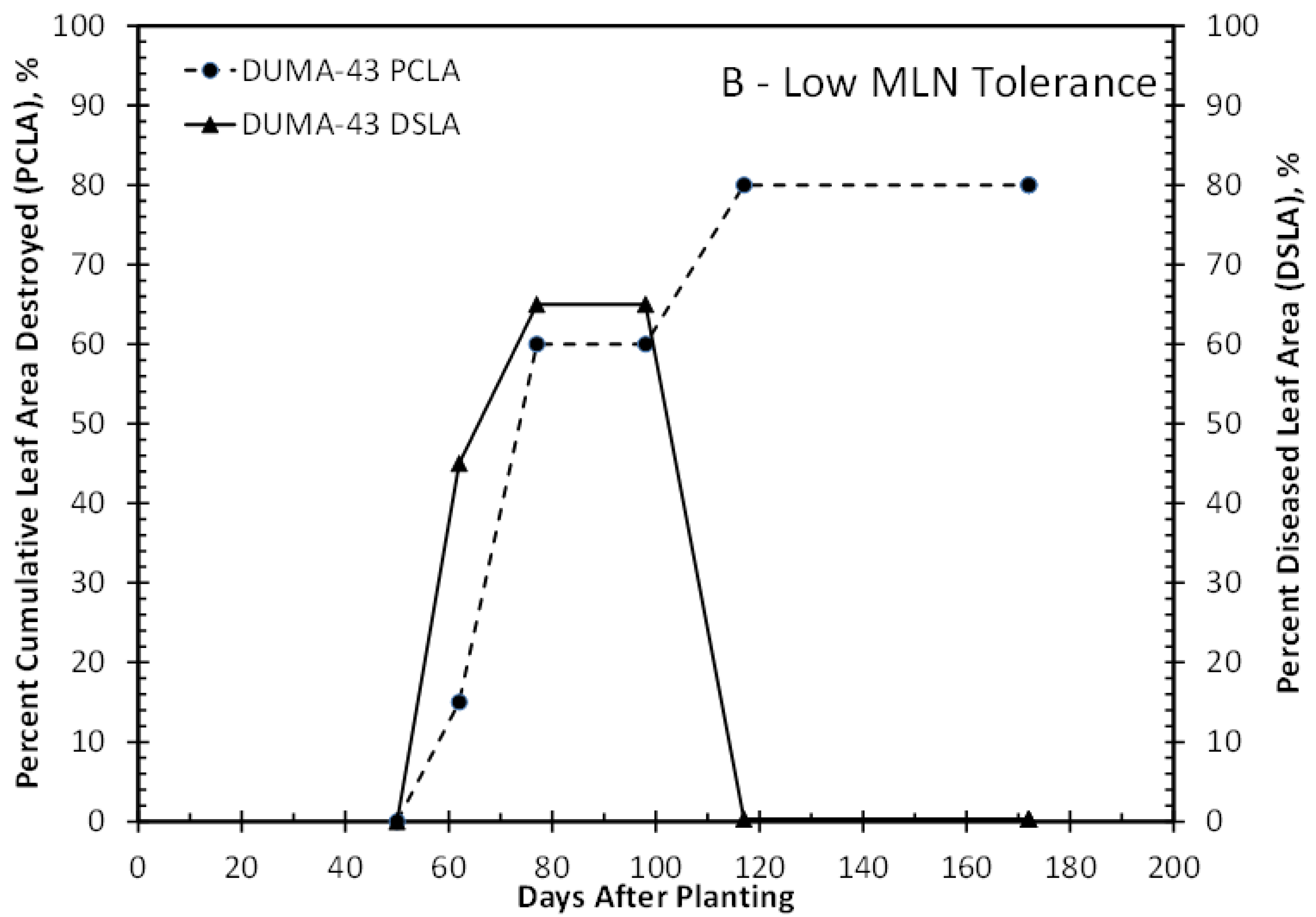
3.1. MLN Disease Severity
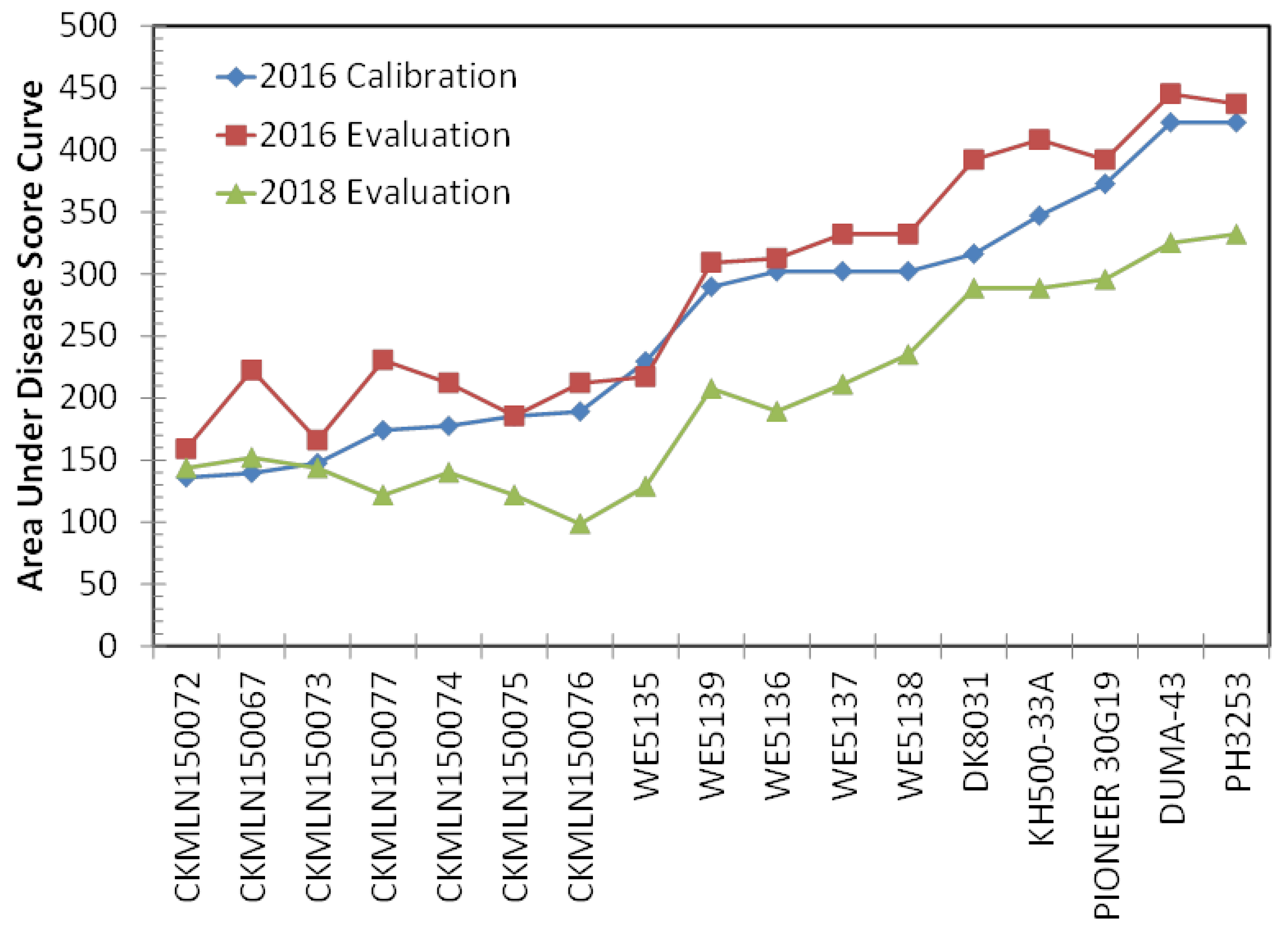
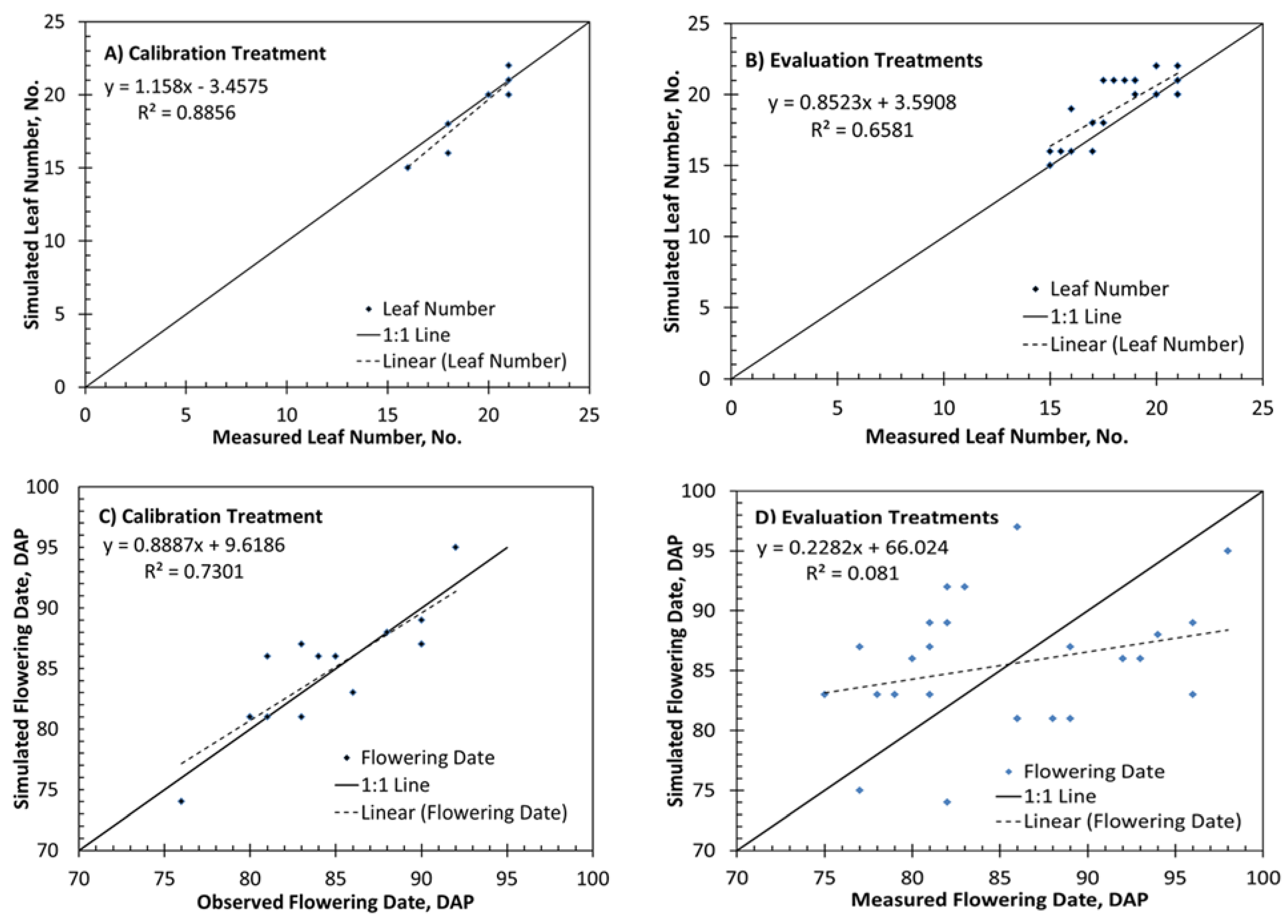
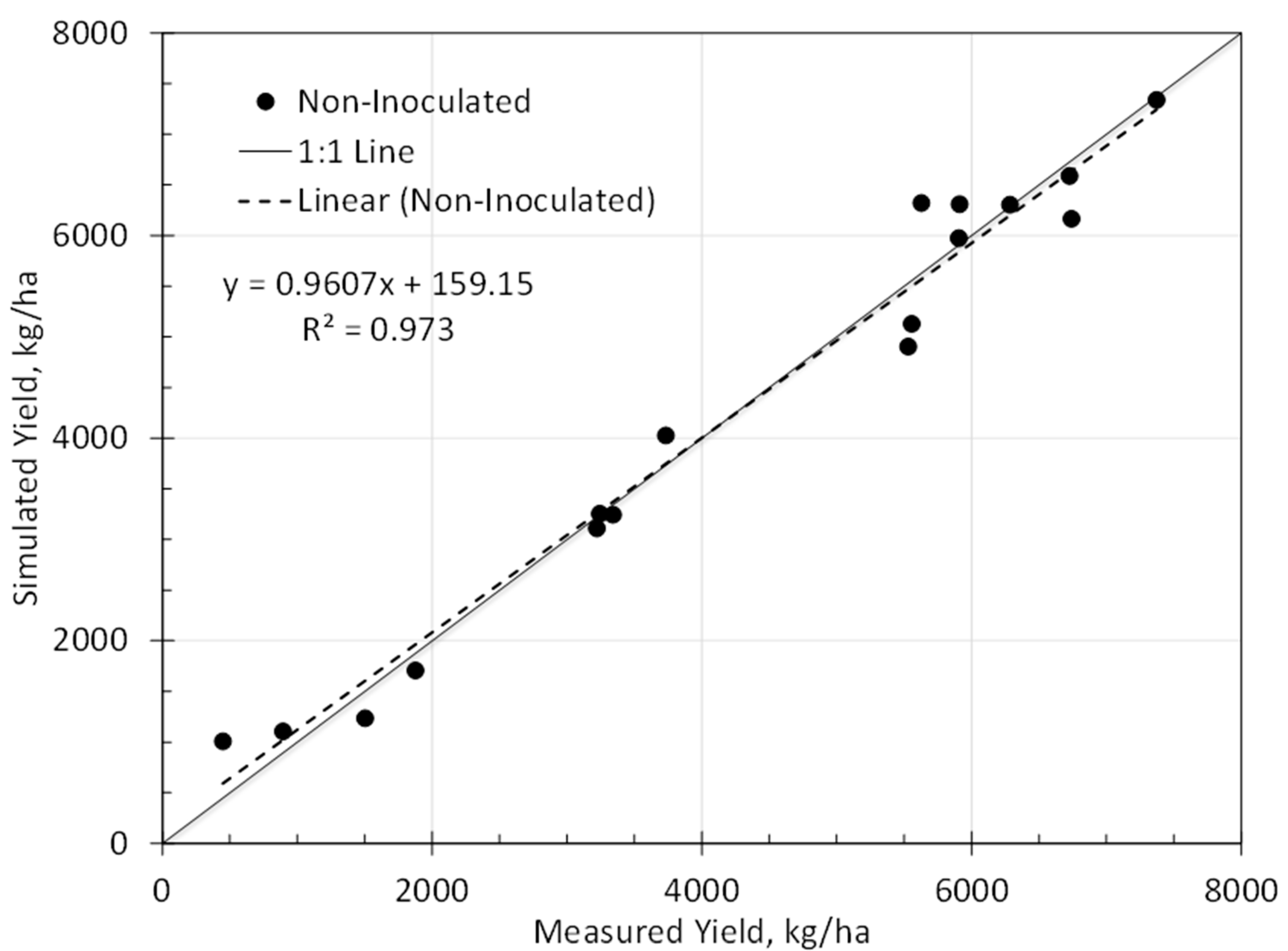
3.2. Model Calibration
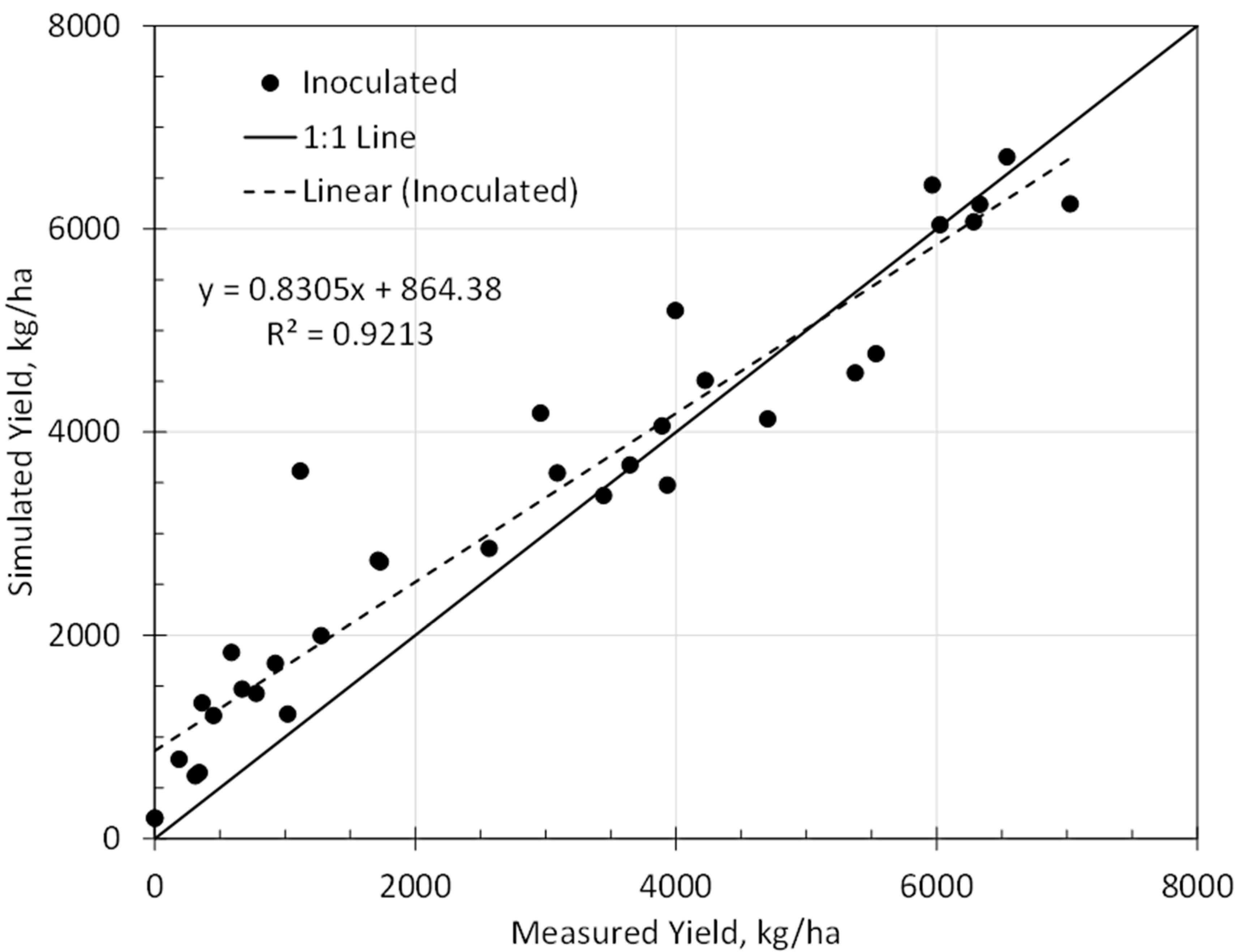
3.3. Model Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahuku, G.; Wangai, A.; Sadessa, K.; Teklewold, A.; Wegary, D.; Ayalneh, D.; Adams, I.; Smith, J.; Bottomley, E.; Bryce, S.; et al. First report of Maize chlorotic mottle virus and Maize Lethal Necrosis on maize in Ethiopia. Plant Dis. 2015, 99, 1870. [Google Scholar] [CrossRef]
- Redinbaugh, M.G.; Stewart, L.R. Maize lethal necrosis: An emerging, synergistic viral disease. Annu. Rev. Virol. 2019, 5, 301–322. [Google Scholar] [CrossRef]
- Stewart, L.R.; Willie, K.; Wijeratne, S.; Redinbaugh, M.G.; Massawe, D.; Niblett, C.L.; Kiggundu, A.; Asiimwe, T. Johnsongrass mosaic virus contributes to maize lethal necrosis in East Africa. Plant Dis. 2017, 101, 1455–1462. [Google Scholar] [CrossRef] [Green Version]
- Niblett, C.L.; Claflin, L.E. Corn lethal necrosis a new virus disease of corn in Kansas. Plant Dis. Rep. 1978, 62, 15–19. [Google Scholar]
- De Groote, H.; Oloo, F.; Tongruksawattana, S.; Das, B. Community-survey based assessment of the geographic distribution and impact of maize lethal necrosis (MLN) disease in Kenya. Crop Prot. 2016, 82, 30–35. [Google Scholar] [CrossRef]
- Marenya, P.P.; Erenstein, O.; Prasanna, B.; Makumbi, D.; Jumbo, M. Maize lethal necrosis disease: Evaluating agronomic and genetic control strategies for Ethiopia and Kenya. Agric. Syst. 2018, 162, 220–228. [Google Scholar] [CrossRef]
- Prasanna, B.M.; Suresh, L.M.; Mwatuni, F.; Beyene, Y.; Makumbi, D.; Gowda, M.; Olsen, M.; Hodson, D.; Worku, M.; Mezzalama, M.; et al. Maize lethal necrosis (MLN): Efforts toward containing the spread and impact of a devastating transboundary disease in sub-Saharan Africa. Virus Res. 2020, 282, 187943. [Google Scholar] [CrossRef]
- Isabiry, B.E.; Rwomushana, I. Current and future potential distribution of maize chlorotic mottle virus and risk of maize lethal necrosis disease in Africa. J. Crop Prot. 2016, 5, 215–228. [Google Scholar] [CrossRef]
- Tesfaye, K.; Gbegbelegbe, S.; Cairns, J.E.; Shiferaw, B.; Prasanna, B.M.; Sonder, K.; Boote, K.J.; Makumbi, D.; Robertson, R. Maize systems under climate change in sub-Saharan Africa. Int. J. Clim. Chang. Strateg. Manag. 2015, 7, 247–271. [Google Scholar] [CrossRef]
- Shiferaw, B.; Tesfaye, K.; Kassie, M.; Abate, T.; Prasanna, B.M.; Menkir, A. Managing vulnerability to drought and enhancing livelihood resilience in sub-Saharan Africa. Weather Clim. Extrem. 2014, 3, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Tesfaye, K.; Gbegbelegbe, S.; Cairns, J.E.; Shiferaw, B.; Prasanna, B.M.; Boote, K.J.; Sonder, K.; Makumbi, D.; Robertson, R. Maize systems under climate change in sub-Saharan Africa: Potential impacts on production and food security. Int. J. Clim. Chang. Strateg. Manag. 2014, 7, 247–271. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sonder, K.; Zaidi, P.H.; Verhulst, N.; Mahuku, G.; Babu, R.; Nair, S.K.; Das, B.; Govaerts, B.; Vinayan, M.T.; et al. Maize production in a changing climate. Adv. Agron. 2012, 144, 1–58. [Google Scholar]
- Nair, S.K.; Babu, R.; Magorokosho, C.; Mahuku, G.; Semagn, K.; Beyene, Y.; Das, B.; Makumbi, D.; Kumar, P.L.; Olsen, M.; et al. Fine mapping of Msv1, a major QTL for resistance to Maize Streak Virus leads to development of production markers for breeding pipelines. Theor. Appl. Genet. 2015, 128, 1839–1854. [Google Scholar] [CrossRef] [PubMed]
- Worku, M.; Makumbi, D.; Beyene, Y.; Das, B.; Mugo, S.; Pixley, K.; Banziger, M.; Owino, F.; Olsen, M.; Asea, G.; et al. Grain yield performance and flowering synchrony of CIMMYT’s tropical maize (Zea mays L.) parental inbred lines and single crosses. Euphytica 2016, 211, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Beyene, Y.; Gowda, M.; Suresh, L.M.; Mugo, S.; Olsen, M.; Oikeh, S.O.; Juma, C.; Tarekegne, A.; Prasanna, B.M. Genetic analysis of tropical maize inbred lines for resistance to maize lethal necrosis disease. Euphytica 2017, 213, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.W.; Hoogenboom, G.; Porter, C.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.; Singh, U.; Gijsman, A.; Ritchie, J.T. DSSAT cropping system model. Spec. Ed. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Batchelor, W.D.; Jones, J.W.; Boote, K.J.; Pinnschmidt, H.O. Extending the use of crop models to study pest damage. Trans. ASAE 1993, 36, 551–558. [Google Scholar] [CrossRef]
- Fallick, J.B.; Batchelor, W.D.; Tylka, G.; Niblack, T.; Paz, J.O. Coupling soybean cyst nematode damage to CROPGRO-Soybean. Trans. ASAE 2002, 45, 433–441. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.; et al. Decision Support System for Agrotechnology Transfer (DSSAT) Version 4.7 (www.DSSAT.net); DSSAT Foundation: Gainesville, FL, USA, 2017. [Google Scholar]
- Masuka, B.; Atlin, G.N.; Olsen, M.; Magorokosho, C.; Labuschagne, M.; Crossa, J.; Bänziger, M.; Pixley, K.; Vivek, B.; von Biljon, A.; et al. Gains in maize genetic improvement in Eastern and Southern Africa i) CIMMYT hybrid breeding pipeline. Crop Sci. 2016, 57, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Masuka, B.; Magorokosho, C.; Olsen, M.; Atlin, G.N.; Bänziger, M.; Pixley, K.V.; Vivek, B.S.; Labuschagne, M.; Matemba-Mutasa, R.; Burgenõ, J.; et al. Gains in maize genetic improvement in Eastern and Southern Africa ii) CIMMYT open pollinated varieties (OPVs) breeding pipeline. Crop Sci. 2017, 57, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Sitonik, C.; Suresh, L.M.; Beyene, Y.; Olsen, M.S.; Makumbi, D.; Kiplagat, O.; Das, B.; Jumbo, M.B.; Mugo, S.; Crossa, J.; et al. Genetic architecture of maize chlorotic mottle virus and maize lethal necrosis through GWAS, linkage analysis and genomic prediction in tropical germplasm. Theor. Appl. Genet. 2019, 132, 2381–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, G.Y.; Uehara, G.; Balas, S. (Eds.) DSSAT v3; University of Hawaii: Honolulu, HI, USA, 1994; Volume 2. [Google Scholar]


| Hybrid | MLN Resistance Level 1 | P1 2 | P2 3 | P5 4 | G2 5 | G3 6 | PHINT 7 |
|---|---|---|---|---|---|---|---|
| CKMLN150072 | R | 170 | 0.27 | 700 | 610 | 6.5 | 30 |
| CKMLN150067 | R | 220 | 0.27 | 660 | 590 | 6.0 | 35 |
| CKMLN150073 | R | 192 | 0.27 | 700 | 500 | 6.0 | 32 |
| CKMLN150077 | R | 220 | 0.27 | 700 | 630 | 6.5 | 32 |
| CKMLN150074 | R | 192 | 0.27 | 700 | 590 | 6.5 | 32 |
| CKMLN150075 | R | 192 | 0.27 | 700 | 500 | 6.0 | 32 |
| CKMLN150076 | R | 192 | 0.27 | 700 | 630 | 6.5 | 32 |
| WE5135 | T | 220 | 0.27 | 700 | 630 | 6.5 | 32 |
| WE5139 | T | 221 | 0.27 | 750 | 650 | 6.0 | 36 |
| WE5136 | T | 252 | 0.27 | 700 | 400 | 6.0 | 40 |
| WE5137 | T | 230 | 0.27 | 700 | 400 | 6.0 | 35 |
| WE5138 | T | 215 | 0.27 | 700 | 500 | 6.0 | 35 |
| DK8031 | S | 250 | 0.27 | 650 | 475 | 6.5 | 46 |
| KH500-33A | S | 200 | 0.27 | 700 | 300 | 6.0 | 46 |
| PIONEER 30G19 | S | 200 | 0.27 | 750 | 100 | 6.0 | 37 |
| DUMA-43 | S | 200 | 0.27 | 750 | 100 | 6.0 | 47 |
| PH3253 | S | 200 | 0.27 | 750 | 100 | 6.0 | 47 |
| Layer Depth, cm | Lower Limit, cm3 cm−3 | Drained Upper Limit, cm3 cm−3 | Saturated Water Holding Capacity, cm3 cm−3 | Organic Carbon, % |
|---|---|---|---|---|
| 12 | 0.272 | 0.411 | 0.452 | 1.67 |
| 19 | 0.288 | 0.421 | 0.446 | 1.72 |
| 42 | 0.348 | 0.467 | 0.490 | 1.49 |
| 71 | 0.342 | 0.456 | 0.490 | 1.56 |
| 100 | 0.335 | 0.445 | 0.490 | 1.38 |
| 138 | 0.324 | 0.432 | 0.494 | 1.44 |
| 149 | 0.310 | 0.420 | 0.495 | 1.54 |
| 161 | 0.273 | 0.382 | 0.473 | 1.75 |
| 190 | 0.265 | 0.378 | 0.473 | 1.41 |
| 190 | 0.197 | 0.316 | 0.430 | 1.66 |
| 200 | 0.272 | 0.411 | 0.452 | 1.67 |
| R2 | RMSE | MAE | D-Statistic | |
|---|---|---|---|---|
| Calibration leaf number | 0.89 | 0.84 | 0.59 | 0.95 |
| Evaluation leaf number | 0.65 | 1.5 | 1.1 | 0.86 |
| Calibration flowering date | 0.73 | 2.4 days | 1.9 days | 0.99 |
| Evaluation flowering date | 0.08 | 7.1 days | 6.6 days | 0.55 |
| Calibration yield | 0.97 | 355 kg/ha | 277 kg/ha | 0.99 |
| Evaluation yield | 0.92 | 773 kg/ha | 600 kg/ha | 0.97 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batchelor, W.D.; Suresh, L.M.; Zhen, X.; Beyene, Y.; Wilson, M.; Kruseman, G.; Prasanna, B. Simulation of Maize Lethal Necrosis (MLN) Damage Using the CERES-Maize Model. Agronomy 2020, 10, 710. https://doi.org/10.3390/agronomy10050710
Batchelor WD, Suresh LM, Zhen X, Beyene Y, Wilson M, Kruseman G, Prasanna B. Simulation of Maize Lethal Necrosis (MLN) Damage Using the CERES-Maize Model. Agronomy. 2020; 10(5):710. https://doi.org/10.3390/agronomy10050710
Chicago/Turabian StyleBatchelor, William D., L. M. Suresh, Xiaoxing Zhen, Yoseph Beyene, Mwaura Wilson, Gideon Kruseman, and Boddupalli Prasanna. 2020. "Simulation of Maize Lethal Necrosis (MLN) Damage Using the CERES-Maize Model" Agronomy 10, no. 5: 710. https://doi.org/10.3390/agronomy10050710
APA StyleBatchelor, W. D., Suresh, L. M., Zhen, X., Beyene, Y., Wilson, M., Kruseman, G., & Prasanna, B. (2020). Simulation of Maize Lethal Necrosis (MLN) Damage Using the CERES-Maize Model. Agronomy, 10(5), 710. https://doi.org/10.3390/agronomy10050710







