Subalpine Fir (Abies laciocarpa) and Norway Spruce (Picea abies) Seedlings Show Different Growth Responses to Blue Light
Abstract
1. Introduction
2. Materials and Methods
2.1. Germination and Raising of Seedlings of Subalpine Fir and Norway Spruce
2.2. Light Quality Treatments and Climatic Conditions during the Experimental Period
2.3. Recordings of Growth and Morphology
2.4. Recordings of Seedling Transpiration
2.5. Statistical Analyses
3. Results
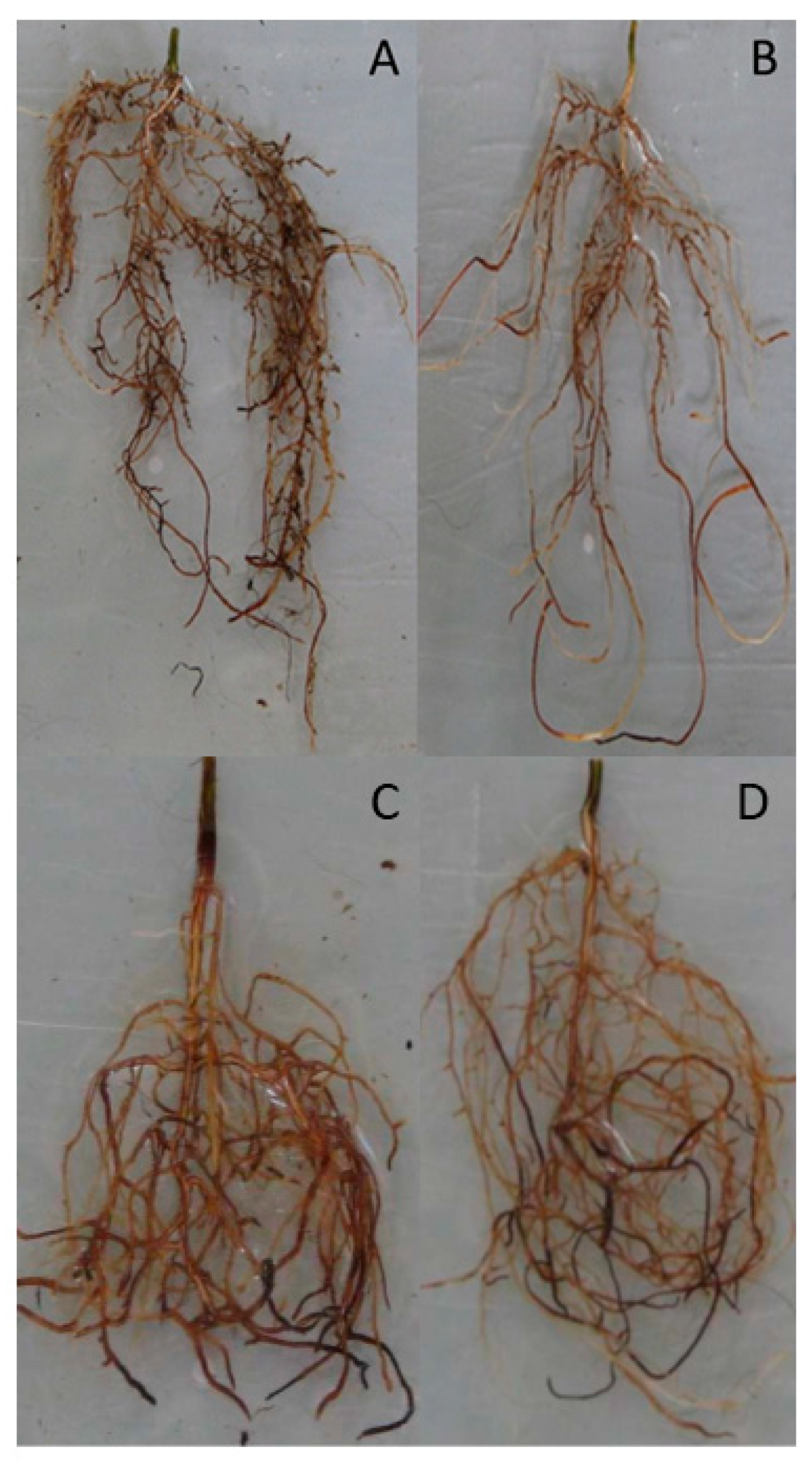
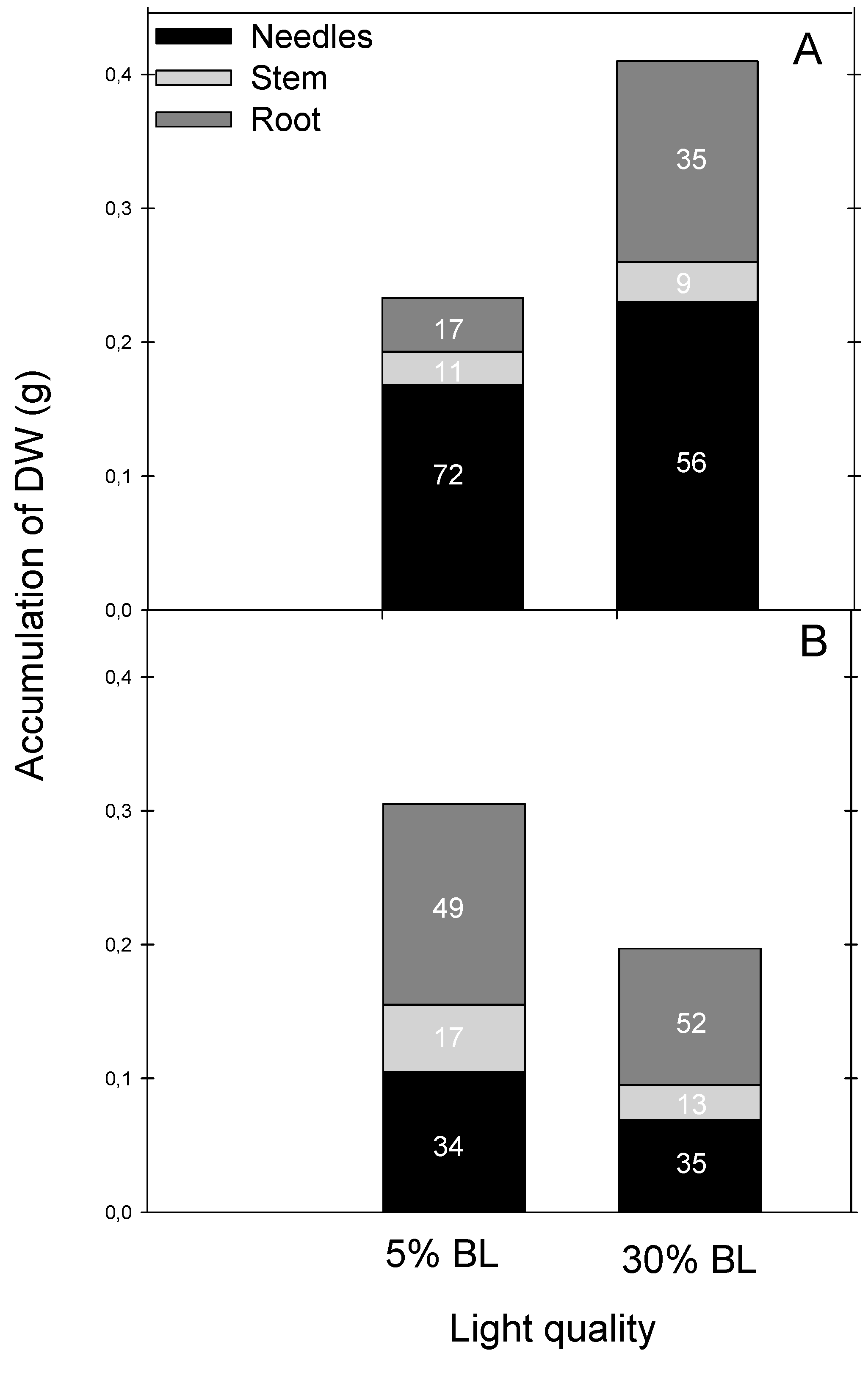
Bud Formation, Morphology and Transpiration Rate of the Seedlings
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why seedlings grow: Influence of plant attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Chiang, C.; Aas, O.T.; Jetmundsen, M.R.; Lee, Y.; Torre, S.; Floistad, I.S.; Olsen, J.E. Day Extension with Far-Red Light Enhances Growth of Subalpine Fir (Abies lasiocarpa (Hooker) Nuttall) Seedlings. Forests 2018, 9, 175. [Google Scholar] [CrossRef]
- Hernandez Velasco, M.; Mattsson, A. Light quality and intensity of light-emitting diodes during pre-cultivation of Picea abies (L.) Karst. and Pinus sylvestris L. seedlings – impact on growth performance, seedling quality and energy consumption. Scand. J. Forest Res. 2019, 34, 159–177. [Google Scholar] [CrossRef]
- Riikonen, J. Efficiency of night interruption treatments with red and far-red light-emitting diodes (LEDs) in preventing bud set in Norway spruce seedlings. Can. J. For. Res. 2018, 48, 1001–1006. [Google Scholar] [CrossRef]
- Riikonen, J. Pre-cultivation of Scots pine and Norway spruce transplant seedlings under four different light spectra did not affect their field performance. New For. 2016, 47, 607–619. [Google Scholar] [CrossRef]
- Molmann, J.A.; Junttila, O.; Johnsen, O.; Olsen, J.E. Effects of red, far-red and blue light in maintaining growth in latitudinal populations of Norway spruce (Picea abies). Plant Cell Environ. 2006, 29, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Opseth, L.; Holefors, A.; Rosnes, A.K.R.; Lee, Y.; Olsen, J.E. FTL2 expression preceding bud set corresponds with timing of bud set in Norway spruce under different light quality treatments. Environ. Exp. Bot. 2016, 121, 121–131. [Google Scholar] [CrossRef]
- Olsen, J.E. Light and temperature sensing and signaling in induction of bud dormancy in woody plants. Plant Mol. Biol. 2010, 73, 37–47. [Google Scholar] [CrossRef]
- Chiang, C.; Olsen, J.E.; Basler, D.; Bankestad, D.; Hoch, G. Latitude and Weather Influences on Sun Light Quality and the Relationship to Tree Growth. Forests 2019, 10, 610. [Google Scholar] [CrossRef]
- Taulavuori, K.; Sarala, M.; Taulavuori, E. Growth responses of trees to arctic light environment. In Progress in Botany; Lüttge, U., Beyschlag, W., Büdel, B., Francis, D., Eds.; Springer-Verlag: Berlin, Germany, 2010; Volume 71, pp. 156–168. [Google Scholar]
- Huche-Thelier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Ranade, S.S.; Gil, M.R.G. Application of monochromatic blue light during germination and hypocotyl development improves outplanted Scots pine (Pinus sylvestris L.) trees performance. For. Ecol. Manag. 2016, 361, 368–374. [Google Scholar] [CrossRef]
- Lin, C.T. Plant blue-light receptors. Trends Plant Sci. 2000, 5, 337–342. [Google Scholar] [CrossRef]
- Smith, H.; Whitelam, G.C. The shade avoidance syndrome: Multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997, 20, 840–844. [Google Scholar] [CrossRef]
- Takemiya, A.; Inoue, S.; Doi, M.; Kinoshita, T.; Shimazaki, K. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 2005, 17, 1120–1127. [Google Scholar] [CrossRef]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 11. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic effciency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Thomas B, V.-P.D. Photoperiodism in Plants, 2nd ed; Academic Press Inc.: San Diego, CA, USA, 1997. [Google Scholar]
- Mattsson, A.; Radoglou, K.; Kostopoulou, P.; Bellarosa, R.; Simeone, M.C.; Schirone, B. Use of innovative technology for the production of high-quality forest regeneration materials. Scand. J. Forest Res. 2010, 25, 3–9. [Google Scholar] [CrossRef]
- Clapham, D.H.; Dormling, I.; Ekberg, I.; Eriksson, G.; Qamaruddin, M.; Vince-Prue, D. Latitudinal cline of requirement for far-red light for the photoperiodic control of budset and extension growth in Picea abies (Norway spruce). Physiol. Plant. 1998, 102, 71–78. [Google Scholar] [CrossRef]
- Alakarppa, E.; Taulavuori, E.; Valledor, L.; Marttila, T.; Jokipii-Lukkari, S.; Karppinen, K.; Nguyen, N.; Taulavuori, K.; Haggman, H. Early growth of Scots pine seedlings is affected by seed origin and light quality. J. Plant Physiol. 2019, 237, 120–128. [Google Scholar] [CrossRef]
- Landis, T.D.; Tinus, R.W.; McDonald, S.E.; Barnett, J.P. Atmospheric environment. In The Container Tree Nursery Manual, Agriculture Handbook 674; USDA Forest Service: Washington, DC, USA, 1992; Volume 3. [Google Scholar]
- Knapp, A.K.; Smith, W.K. Factors influencing understory seedling establishment of engelmann spruce (Picea Engelmannii) and subalpine fir (Abies lasiocarpa) in Southeast Wyoming. Can. J. Bot.-Rev. Can. Bot. 1982, 60, 2753–2761. [Google Scholar] [CrossRef]
- Mitamura, M.; Yamamura, Y.; Nakano, T. Large-scale canopy opening causes decreased photosynthesis in the saplings of shade-tolerant conifer, Abies veitchii. Tree Physiol. 2009, 29, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Klinka, K.; Wang, Q.; Kayahara, G.J.; Carter, R.E.; Blackwell, B.A. LIGHT-GROWTH RESPONSE RELATIONSHIPS IN PACIFIC SILVER FIR (ABIES-AMABILIS) AND SUB-ALPINE FIR (ABIES-LASIOCARPA). Can. J. Bot.-Rev. Can. Bot. 1992, 70, 1919–1930. [Google Scholar] [CrossRef]
- Claveau, Y.; Messier, C.; Comeau, P.G.; Coates, K.D. Growth and crown morphological responses of boreal conifer seedlings and saplings with contrasting shade tolerance to a gradient of light and height. Can. J. For. Res.-Rev. Can. Rech. For. 2002, 32, 458–468. [Google Scholar] [CrossRef]
- Terfa, M.T.; Solhaug, K.A.; Gislerod, H.R.; Olsen, J.E.; Torre, S. A high proportion of blue light increases the photosynthesis capacity and leaf formation rate of Rosa x hybrida but does not affect time to flower opening. Physiol. Plant. 2013, 148, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Devireddy, A.R.; Zandalinas, S.I.; Gomez-Cadenas, A.; Blumwald, E.; Mittler, R. Coordinating the overall stomatal response of plants: Rapid leaf-to-leaf communication during light stress. Sci. Signal. 2018, 11, 9. [Google Scholar] [CrossRef]
- Riikonen, J.; Kettunen, N.; Gritsevich, M.; Hakala, T.; Särkkä, L.; Tahvonen, R. Growth and development of Norway spruce and Scots pine seedlings under different light spectra. Environ. Exp. Bot. 2016, 121, 112–120. [Google Scholar] [CrossRef]
- Vanneste, S.; Maes, L.; De Smet, I.; Himanen, K.; Naudts, M.; Inze, D.; Beeckman, T. Auxin regulation of cell cycle and its role during lateral root initiation. Physiol. Plant. 2005, 123, 139–146. [Google Scholar] [CrossRef]
- Mattsson, A. Predicting field performance using seedling quality assessment. New For. 1997, 13, 227–252. [Google Scholar] [CrossRef]
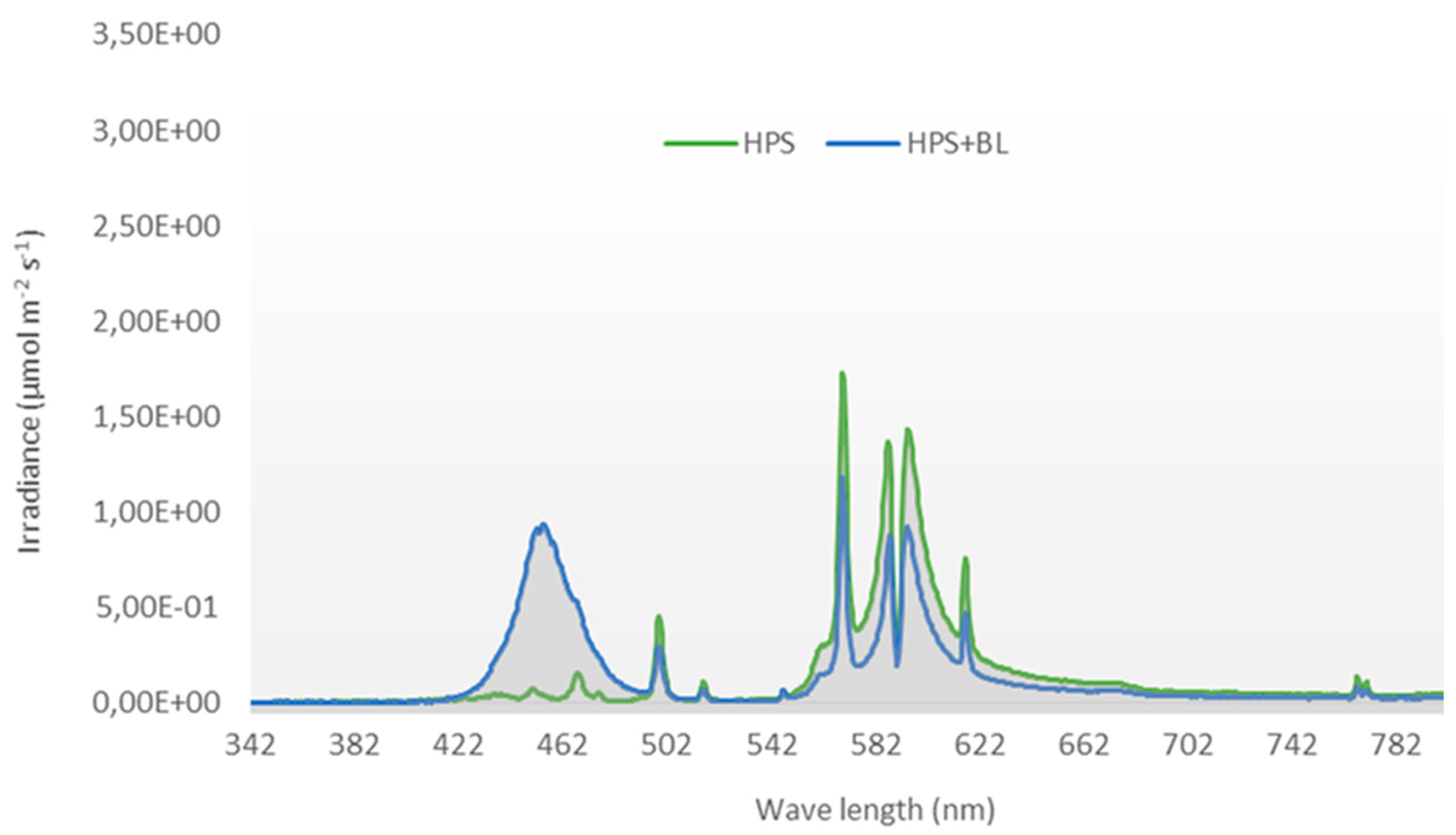
| Blue Light * | Photosynthetic Active Radiation (µmol m−2 s−1) | Total PAR | R/FR | PPS ** | Plant Temperature (°C) *** | |
|---|---|---|---|---|---|---|
| HPS | Blue LED | |||||
| 5% | 300 | - | 300 | 3.5 | 0.70 | 22 |
| 30% | 225 | 75 | 300 | 3.5 | 0.70 | 22 |
| Norway Spruce | Subalpine Fir | |||
|---|---|---|---|---|
| 5% BL | 30% BL | 5% BL | 30% BL | |
| Plants with terminal buds (%) | 0 | 0 | 9 a | 38.0 b |
| Shoot elongation (cm) | 4.40 ± 0.26 a | 4.03 ± 0.29 a | 1.12.52 ± 0.12 a | 1.22 ± 0.07 a |
| Stem diameter (mm) | 1.61 ± 0.11 a | 1.98 ± 0.11 b | 1.44 ± 0.08 a | 1.50 ± 0.05 a |
| No of branches | 2.70 ± 0.15 a | 4.70 ± 0.36 b | 0 | 0 |
| Length of branches | 1.34 ± 0.09 a | 1.60 ± 0.12 a | - | - |
| Average number of needles per seedling | 179.2 ± 14.7 a | 234.2 ± 17.1 b | 36.2 ± 4.93 a | 51.0 ± 6.66 a |
| Shoot transpiration rate (mg H2O per needle h−1) | 0.0006 ± 0.00 a | 0.0014 ± 0.00 b | 0.004 ± 0.00 a | 0.003 ± 0.00 b |
| Root length (cm) | 16.73 ± 2.28 a | 13.07 ± 1.56 b | 11.32 ± 1.48 a | 8.75 ± 0.79 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navidad, H.; Fløistad, I.S.; Olsen, J.E.; Torre, S. Subalpine Fir (Abies laciocarpa) and Norway Spruce (Picea abies) Seedlings Show Different Growth Responses to Blue Light. Agronomy 2020, 10, 712. https://doi.org/10.3390/agronomy10050712
Navidad H, Fløistad IS, Olsen JE, Torre S. Subalpine Fir (Abies laciocarpa) and Norway Spruce (Picea abies) Seedlings Show Different Growth Responses to Blue Light. Agronomy. 2020; 10(5):712. https://doi.org/10.3390/agronomy10050712
Chicago/Turabian StyleNavidad, Hazel, Inger Sundheim Fløistad, Jorunn E. Olsen, and Sissel Torre. 2020. "Subalpine Fir (Abies laciocarpa) and Norway Spruce (Picea abies) Seedlings Show Different Growth Responses to Blue Light" Agronomy 10, no. 5: 712. https://doi.org/10.3390/agronomy10050712
APA StyleNavidad, H., Fløistad, I. S., Olsen, J. E., & Torre, S. (2020). Subalpine Fir (Abies laciocarpa) and Norway Spruce (Picea abies) Seedlings Show Different Growth Responses to Blue Light. Agronomy, 10(5), 712. https://doi.org/10.3390/agronomy10050712





