Resilience Capacity Assessment of the Traditional Lima Bean (Phaseolus lunatus L.) Landraces Facing Climate Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seed Features
2.2. Germination Assays
2.3. Genetic Assays
2.3.1. Plant Material and DNA Extraction
2.3.2. Molecular Analyses
2.3.3. Phylogenetic Analyses
2.3.4. DNA Polymorphism and Divergence
3. Results
3.1. Seed Features
3.2. Germination Assays
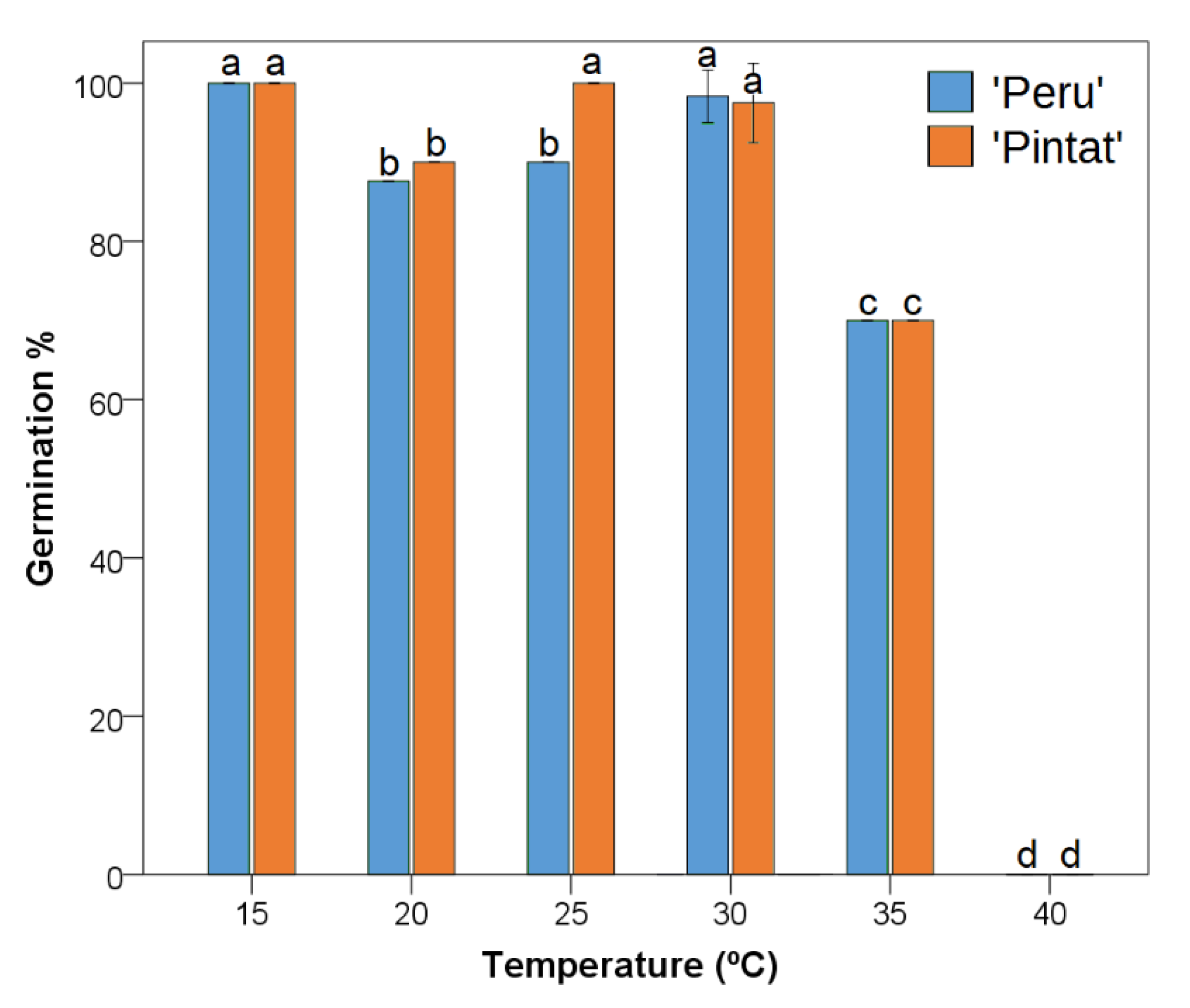
3.2.1. Germination Response to Temperature
3.2.2. Germination Response to Drought Stress
3.3. Genetic Assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crop Production Statistics at Regional Level, EUROSTAT. 2014. Available online: http://ec.europa.eu/eurostat/statistics-explained/index.php/Agricultural_production_-_crops (accessed on 20 December 2019).
- Knox, J.; Daccache, A.; Hess, T.; Haro, D. Meta-analysis of climate impacts and uncertainty on crop yields in Europe. Environ. Res. Lett. 2016, 11, 113004. [Google Scholar] [CrossRef]
- Climate Change and Agriculture. Houses of Parliament. POSTnote 600. Available online: http://researchbriefings.files.parliament.uk/documents/POST-PN-0600/POST-PN-0600.pdf (accessed on 18 December 2019).
- Arteaga, S.; Al Hassan, M.; Chaminda Bandara, W.; Yabor, L.; Llinares, J.; Boscaiu, M.; Vicente, O. Screening for salt tolerance in four local varieties of Phaseolus lunatus from Spain. Agriculture 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- van de Wouw, M.; Kik, C.; van Hintum, T.; van Treuren, R.; Visser, B. Genetic erosion in crops: Concept, research results and challenges. Plant Genet. Resour. 2010, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- What is agrobiodiversity? Food and Agricultural Organization of the UN; FAO: Rome, Italy, 2004; Available online: http://www.fao.org/3/a-y5609e.pdf (accessed on 18 December 2019).
- Seidu, K.T.; Osundahunsi, O.F.; Osamudiamen, P.M. Nutrients assessment of some lima bean varieties grown in southwest Nigeria. Int. Food Res. J. 2018, 25, 848–853. [Google Scholar]
- Seidu, K.T.; Osundahunsi, O.F.; Olaleye, M.T.; Oluwalana, I.B. Amino acid composition, mineral contents and protein solubility of some lima bean (Phaseolus lunatus L. Walp) seeds coat. Food Res. Int. 2015, 73, 130–134. [Google Scholar] [CrossRef]
- López-Alcocer, J.J.; Lépiz-Ildefonso, R.; González-Eguiarte, D.R.; Rodríguez-Macías, R.; López-Alcocer, E. Morphological variability of wild Phaseolus lunatus from the western region of Mexico. Rev. Fitotec. Mex. 2016, 39, 49–58. [Google Scholar]
- Cuny, M.A.; La Forgia, D.; Desurmont, G.A.; Glauser, G.; Benrey, B. Role of cyanogenic glycosides in the seeds of wild lima bean, Phaseolus lunatus: Defense, plant nutrition or both? Planta 2019, 250, 1281–1292. [Google Scholar] [CrossRef]
- Gleadow, R.M.; Woodrow, I.E. Constraints on effectiveness of cyanogenic glycosides in herbivore defense. J. Chem. Ecol. 2002, 28, 1301–1313. [Google Scholar] [CrossRef]
- Pesantes-Vera, M.F.; León-Alcántara, E.; De La Cruz-Araujo, E.; Rodríguez-Soto, J.C. Variabilidad morfo-agronómica en poblaciones de pallar, Phaseolus lunatus, cultivado en condiciones de Costa de la Provincia de Trujillo (Perú). REBIOL 2015, 35, 29–38. [Google Scholar]
- Debouck, D.G. Notes sur Les Différents Taxons de Phaseolus à Partir des Herbiers. Cahiers de Phaséologie—Section Paniculati; International Center for Tropical Agriculture (CIAT): Cali, Colombia, 2008; p. 233. Available online: http://www.ciat.cgiar.org/urg (accessed on 11 February 2019).
- Baudet, J.C. The taxonomic status of the cultivated types of Lima bean (Phaseolus lunatus L.). Trop. Grain Legume Bull. 1977, 7, 29–30. [Google Scholar]
- Checa, O.; Ceballos, H.; Blair, M.W. Generation means analysis of climbing ability in common bean (Phaseolus vulgaris L.). J. Hered. 2006, 97, 456–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madesis, P.; Ganopoulos, I.; Ralli, P.; Tsaftaris, A. Barcoding the major Mediterranean leguminous crops by combining universal chloroplast and nuclear DNA sequence targets. Genet. Mol. Res 2012, 11, 2548–2558. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.; Alù, M.; Corradini, B.; Beduschi, G. Forensic botany: Species identification of botanical trace evidence using a multigene barcoding approach. Int. J. Legal Med. 2009, 123, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.; Lickey, E.B.; Beck, J.T.; Farmer, S.B.; Liu, W.; Miller, J.; Siripun, K.C.; Winder, C.T.; Schilling, E.E.; Small, R.L. The tortoise and the hare II: Relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. Am. J. Bot. 2005, 92, 142–166. [Google Scholar] [CrossRef] [Green Version]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Diniz, A.L.; Zucchi, M.I.; Santini, L.; Benchimol-Reis, L.L.; Fungaro, M.H.P.; Vieira, M.L.C. Nucleotide diversity based on phaseolin and iron reductase genes in common bean accessions of different geographical origins. Genome 2014, 57, 69–77. [Google Scholar] [CrossRef]
- Serrano-Serrano, M.L.; Hernández-Torres, J.; Castillo-Villamizar, G.; Debouck, D.G.; Sánchez, M.I.C. Gene pools in wild Lima bean (Phaseolus lunatus L.) from the Americas: Evidences for an Andean origin and past migrations. Mol. Phylogenet. Evol. 2010, 54, 76–87. [Google Scholar] [CrossRef]
- Nicolè, S.; Erickson, D.L.; Ambrosi, D.; Bellucci, E.; Lucchin, M.; Papa, R.; Kress, W.J.; Barcaccia, G. Biodiversity studies in Phaseolus species by DNA barcoding. Genome 2011, 54, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Rasband, W.S. ImageJ, US National Institutes of Health, Bethesda, Maryland, USA, 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 16 September 2019).
- Villela, F.A.; Doni Filho, L.; Sequeira, E.L. Tabela de potencial osmótico em função da concentração de polietileno glicol 6000 e da temperatura. Pesqui. Agropecu. Bras. 1991, 26, 1957–1968. [Google Scholar]
- García-Huidobro, J.; Monteith, J.L.; Squire, G.R. Time, temperature and germination of pearl millet. J. Exp. Bot. 1982, 33, 288–296. [Google Scholar] [CrossRef]
- Trudgill, D.L. Why do tropical poikilothermic organisms tend to have higher threshold temperatures for development than temperate ones? Funct. Ecol. 1995, 9, 136–137. [Google Scholar]
- Kebreab, E.; Murdoch, A.J. Modelling the effects of water stress and temperature on germination rate of Orobanche aegyptiaca seeds. J. Exp. Bot. 1999, 50, 655–664. [Google Scholar] [CrossRef]
- Bradford, K.J. A water relations analysis of seed germination rates. Plant Physiol. 1990, 94, 840–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, J.J.; Doyle, J.L. A rapid procedure for DNA purification from small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Chiang, T.Y.; Schaal, B.A.; Peng, C.I. Universal primers for amplification and sequencing a noncoding spacer between the atpB and rbcL genes of chloroplast DNA. Bot. Bull. Acad. Sin. 1998, 39, 245–250. [Google Scholar]
- Sang, T.; Crawford, D.J.; Stuessy, T.F. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120–1136. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Castresana, J. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 2000, 17, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Teslenko, M.; Van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Müller, K. Seqstate: Primer design and sequences statistics for phylogenetic DNA data sets. Appl. Bioinf. 2005, 4, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2006, 23, 127–128. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; IEEE: New York, NY, USA, 2010; pp. 45–52. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Fita, A.; Rodríguez-Burruezo, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Breeding and domesticating crops adapted to drought and salinity: A new paradigm for increasing food production. Front. Plant Sci. 2015, 6, 978. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Haghparast, R.; Sadeghzadeh, B.; Ahmadi, H.; Solimani, K.; Amri, A. Adaptation patterns and yield stability of durum wheat landraces to highland cold rainfed areas of Iran. Crop Sci. 2014, 54, 944–954. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Ceccarelli, S.; Blair, M.W.; Upadhyaya, H.D.; Are, A.K.; Ortiz, R. Landrace germplasm for improving yield and abiotic stress adaptation. Trends Plant Sci. 2016, 21, 31–42. [Google Scholar] [CrossRef]
- Lopes, M.S.; El-Basyoni, I.; Baenziger, P.S.; Singh, S.; Royo, C.; Ozbek, K.; Aktas, H.; Ozer, E.; Ozdemir, F.; Manickavelu, A.; et al. Exploiting genetic diversity from landraces in wheat breeding for adaptation to climate change. J. Exp. Bot. 2015, 66, 3477–3486. [Google Scholar] [CrossRef]
- Baudoin, J.P. Genetic Resources, Domestication and Evolution of Lima Bean, Phaseolus lunatus. In Genetic Resources of Phaseolus Beans. Current Plant Science and Biotechnology in Agriculture; Gepts, P., Ed.; Springer: Dordrecht, The Netherlands, 1988; Volume 6, pp. 393–407. [Google Scholar]
- Castiñeiras, L.; Guzmán, F.A.; Duque, M.C.; Shagarodsky, T.; Cristóbal, R.; De Vicente, M.C. AFLPs and morphological diversity of Phaseolus lunatus L. in Cuban home gardens: Approaches to recovering the lost ex situ collection. Biodivers. Conserv. 2007, 16, 2847–2865. [Google Scholar] [CrossRef]
- Wahua, T.A.T.; Tariah, N.M. Seed treatment to enhance germination of coloured lima bean (Phaseolus lunatus L.). Field Crops Res. 1984, 8, 361–369. [Google Scholar] [CrossRef]
- Pollock, B.M.; Toole, V.K. Imbibition period as the critical temperature sensitive stage in germination of lima bean seeds. Plant Physiol. 1966, 41, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollock, B.M. Imbibition temperature sensitivity of lima bean seeds controlled by initial seed moisture. Plant Physiol. 1969, 44, 907–911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues Do Nascimento, M.G.; Ursulino Alves, E.; Mauricio da Silva, M.L.; Marques Rodrigues, C. Lima bean (Phaseolus lunatus L.) seeds exposed to different salt concentrations and temperatures. Rev. Caatinga 2017, 30, 738–747. [Google Scholar] [CrossRef] [Green Version]
- Sedlar, A.; Kidrič, M.; Šuštar-Vozlič, J.; Pipan, B.; Zadražnik, T.; Meglič, V. Drought Stress Response in Agricultural Plants: A Case Study of Common Bean (Phaseolus vulgaris L.). In Drought-Detection and Solutions; Ondrasek, G., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/drought-detection-and-solutions/drought-stress-response-in-agricultural-plants-a-case-study-of-common-bean-em-phaseolus-vulgaris-em- (accessed on 21 November 2019).
- da Silva, E.C.; de Albuquerque, M.B.; de Azevedo Neto, A.D.; da Silva Junior, C.D. Drought and its consequences to plants—from individuals to ecosystem. In Responses of Organisms to Water Stress; Akinci, S., Ed.; Intechopen: London, UK, 2013; Available online: https://www.intechopen.com/books/responsesof-organisms-to-water-stress/drought-and-its-consequences-toplants-from-individual-to-ecosystem (accessed on 10 September 2019).
- Cardoso, V.J.M.; Bianconi, A. Hydrotime model can describe the response of common bean (Phaseolus vulgaris L.) seeds to temperature and reduced water potential. Acta Sci. Biol. Sci. 2013, 35, 255–261. [Google Scholar] [CrossRef]
- Chacón-Sánchez, M.I.; Martínez-Castillo, J. Testing domestication scenarios of lima bean (Phaseolus lunatus L.) in Mesoamerica: Insights from genome-wide genetic markers. Front. Plant Sci. 2017, 8, 1551. [Google Scholar] [CrossRef] [Green Version]
- Motta-Aldana, J.R.; Serrano-Serrano, M.L.; Hernández-Torres, J.; Castillo-Villamizar, G.; Debouck, D.G. Multiple origins of Lima bean landraces in the Americas: Evidence from chloroplast and nuclear DNA polymorphisms. Crop Sci. 2010, 50, 1773–1787. [Google Scholar] [CrossRef]
- Abu-Zaitoun, S.Y.; Chandrasekhar, K.; Assili, S.; Shtaya, M.J.; Jamous, R.M.; Mallah, O.B.; Nashef, K.; Sela, H.; Distelfeld, A.; Alhajaj, N.; et al. Unlocking the genetic diversity within a Middle-East panel of durum wheat landraces for adaptation to semi-arid climate. Agronomy 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Fortes, A.M.; Gallusci, P. Plant stress responses and phenotypic plasticity in the epigenomics era: Perspectives on the grapevine scenario, a model for perennial crop plants. Front. Plant Sci. 2017, 8, 82. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A. Plant abiotic stress challenges from the changing environment. Front. Plant Sci. 2016, 7, 1123. [Google Scholar] [CrossRef] [Green Version]





| Marker | Primer Names | Tm | Reference |
|---|---|---|---|
| atpB-rbcL | atpB-f | 55 | [31] |
| rbcL-r | |||
| trnL-trnF | trnL (UAA) 3′ exon f | 58 | [19] |
| trnF (GAA) r | |||
| trnL intron + trnL-trnF | trnL (UAA) 5′ exon f | 58 | [19] |
| trnF (GAA) r | |||
| rpoB-trnC | rpoB-f | 55 | [18] |
| trnC-r | |||
| psbA-trnH | psba-f | 56 | [32] |
| trnH-r | |||
| ITS | ITS1-f | 58 | [20] |
| ITS4-r | |||
| Phs7 | Phs7-f | 62 | [21] |
| Phs7-r | |||
| FRO3 | FRO3-f | 62 | [21] |
| FRO3-r |
| ‘Peru’ | ‘Pintat’ | ‘Ull de Perdiu’ | ‘Cella Negra’ | |
|---|---|---|---|---|
| L (mm) | 25.3 ± 0.21 b | 26.4 ± 0.13 a | 24.8 ± 0.17 b | 25.0 ± 0.18 b |
| W (mm) | 15.5 ± 0.14 b | 17.3 ± 0.09 a | 15.7 ± 0.12 b | 15.9 ± 0.14 b |
| L/W | 1.63 ± 0.16 a | 1.53 ± 0.10 b | 1.58 ± 0.14 ab | 1.58 ± 0.21 ab |
| Thickness (mm) | 6.72 ± 0.45 a | 5.60 ± 0.42 c | 5.96 ± 0.81 bc | 6.37 ± 0.72 ab |
| Weight (g) | 1.82 ± 0.07 a | 1.81 ± 0.10 a | 1.75 ± 0.39 ab | 1.54 ± 0.15 b |
| Pigmentation | No | Yes | Yes | Yes |
| 15 °C | 20 °C | 25 °C | 30 °C | 35 °C | 40 °C | |
|---|---|---|---|---|---|---|
| ‘Peru’ | 5.6 ± 0.2 b | 5.9 ± 0.5 b | 5.0 ± 0.3 b | 3.9 ± 0.6 a | 5.1 ± 0.6 b | - |
| ‘Pintat’ | 6.1 ± 0.2 cd | 5.6 ± 0.2 c | 4.6 ± 0.2 b | 3.6 ± 0.4 a | 6.3 ± 0.5 d | - |
| Osmotic Potential (Bar) | |||||
|---|---|---|---|---|---|
| 0 | −1 | −2 | −3 | −4 | |
| ’Peru’ | 3.9 ± 0.6 a | 4.2 ± 0.4 ab | 4.8 ± 1.2 ab | 5.5 ± 0.6 abc | 6.5 ± 0.6 bc |
| ‘Pintat’ | 3.6 ± 0.4 a | 3.9 ± 0.8 a | 3.8 ± 0.2 a | 5.1 ± 0.2 ab | 5.2 ± 0.6 ab |
| N | n | n’ | S | H | Π (s.d.) | Hd (s.d.) | Kst | |
|---|---|---|---|---|---|---|---|---|
| Valencian cultivars | 29 | 4080 | 4080 | 4 | 9 | 3.8· × 10−4 (4 × 10−5) | 0.862 (0.035) | 0.022 n.s. |
| Valencian + S. American Accessions | 67 | 1800 | 1413 | 44 | 37 | 3.9· × 10−3 (2.5 × 10−4) | 0.954 (2 · 10−4) | 0.528 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Nieto, M.I.; Estrelles, E.; Prieto-Mossi, J.; Roselló, J.; Soriano, P. Resilience Capacity Assessment of the Traditional Lima Bean (Phaseolus lunatus L.) Landraces Facing Climate Change. Agronomy 2020, 10, 758. https://doi.org/10.3390/agronomy10060758
Martínez-Nieto MI, Estrelles E, Prieto-Mossi J, Roselló J, Soriano P. Resilience Capacity Assessment of the Traditional Lima Bean (Phaseolus lunatus L.) Landraces Facing Climate Change. Agronomy. 2020; 10(6):758. https://doi.org/10.3390/agronomy10060758
Chicago/Turabian StyleMartínez-Nieto, María Isabel, Elena Estrelles, Josefa Prieto-Mossi, Josep Roselló, and Pilar Soriano. 2020. "Resilience Capacity Assessment of the Traditional Lima Bean (Phaseolus lunatus L.) Landraces Facing Climate Change" Agronomy 10, no. 6: 758. https://doi.org/10.3390/agronomy10060758
APA StyleMartínez-Nieto, M. I., Estrelles, E., Prieto-Mossi, J., Roselló, J., & Soriano, P. (2020). Resilience Capacity Assessment of the Traditional Lima Bean (Phaseolus lunatus L.) Landraces Facing Climate Change. Agronomy, 10(6), 758. https://doi.org/10.3390/agronomy10060758





