Molecular Characterization and Functional Analysis of Wheat TtLOX Gene Involved in Aphid Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Cultivation and Aphid Rearing
2.2. Experimental Procedure
2.3. Cloning and Bioinformatics Analyses of TtLOX
2.4. Subcellular Localization of TtLOX Fusion Protein in Tobacco
2.5. Gene Silencing Based on BSMV in T. turgidum
2.6. qRT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Isolation and Characteristic Analysis of the TtLOX cDNA Sequence
3.2. Subcellular Localization
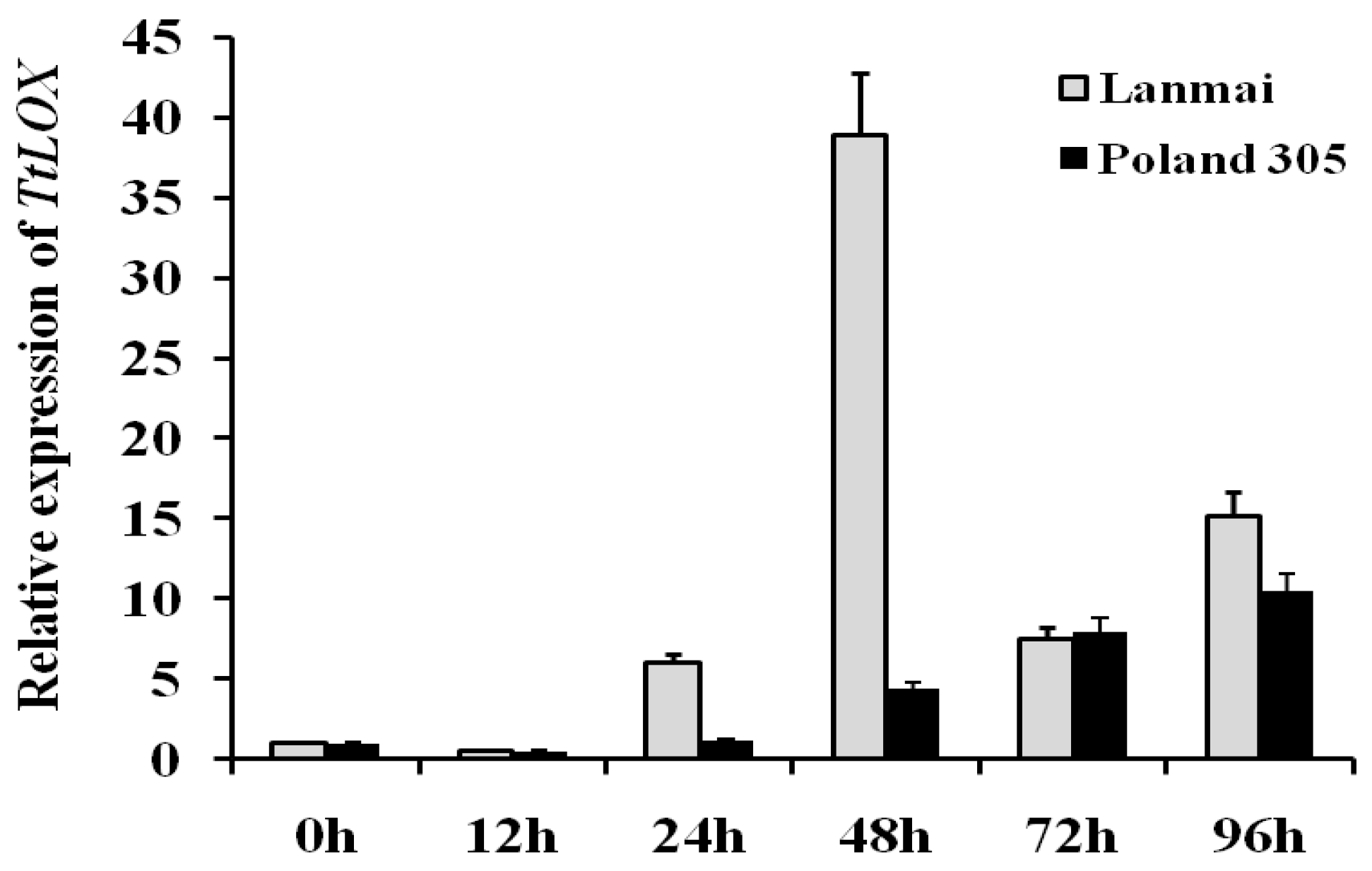
3.3. Expression of TtLOX in Tetraploid Wheat under EGA Stress
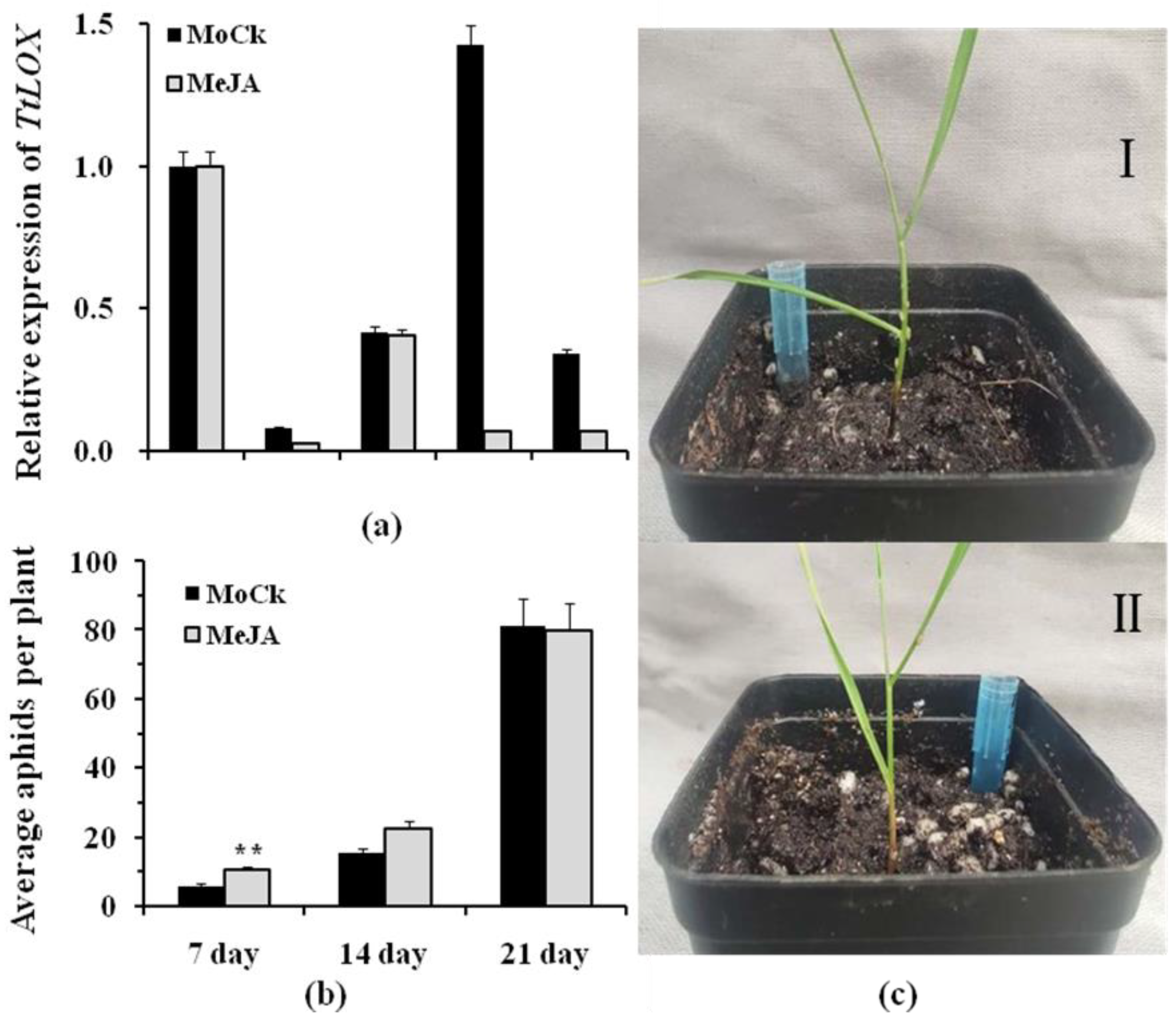
3.4. Changes in TtLOX Expression Levels and EGA Numbers under MeJA Treatment in T. turgidum
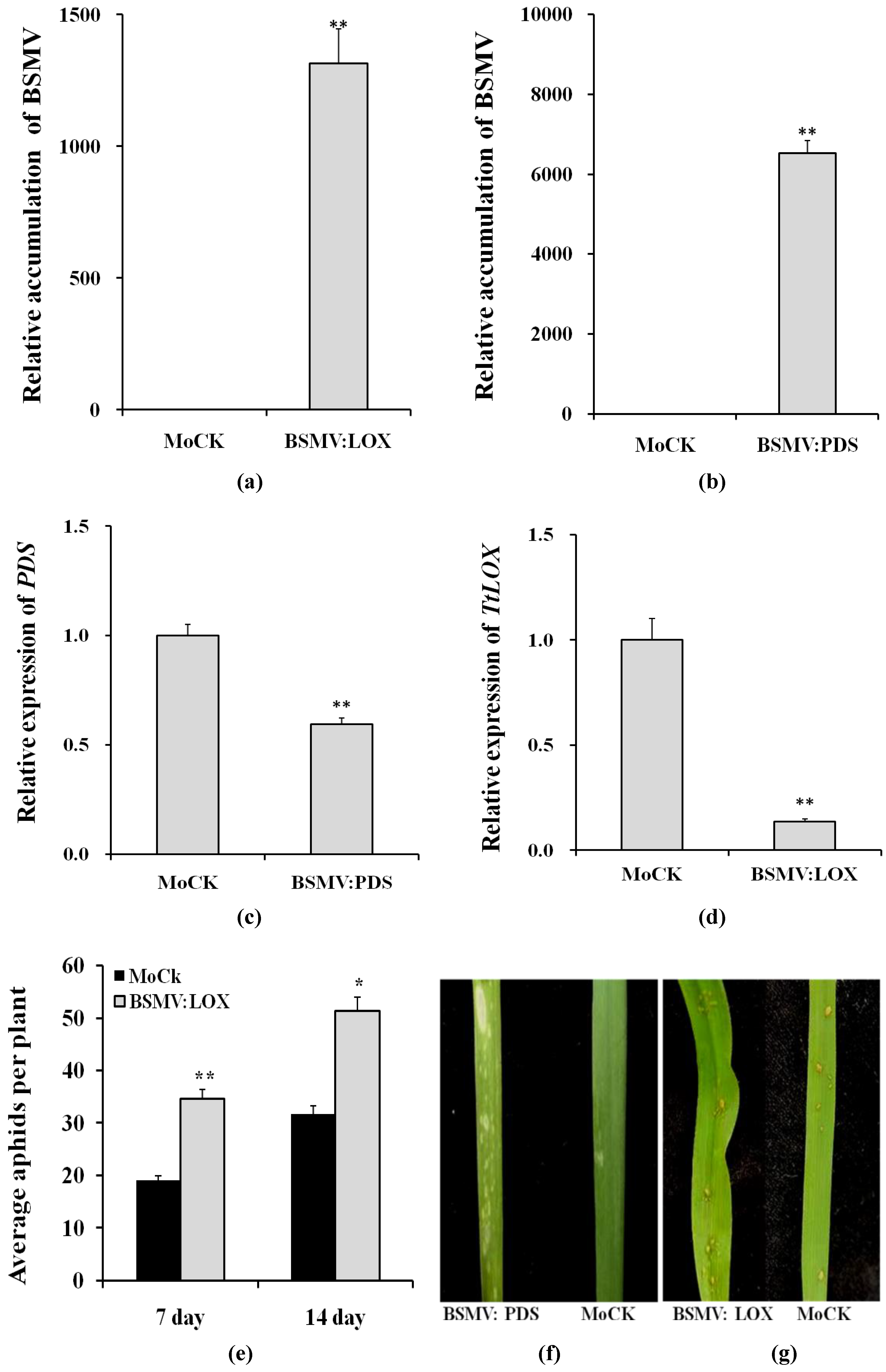
3.5. Gene Silencing and Changes in Aphid Reproduction
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, T.; Aslam, M.; Hussan, M.W.; Iqbal, J. Aphids (Schizaphis graminum R.) infectation on different wheat (Triticum aestivum) varieties and their comparative yields. J. Agric. Res. 2015, 53, 209–216. [Google Scholar]
- Fu, M.; Xu, M.; Zhou, T.; Wang, D.; Tian, S.; Han, L.; Dong, H.; Zhang, C. Transgenic expression of a functional fragment of harpin protein Hpa1 in wheat induces the phloem-based defence against English grain aphid. J. Exp. Bot. 2014, 65, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Honek, A.; Martinkova, Z.; Dixon, A.F.G.; Saska, P. Annual predictions of the peak numbers of Sitobion avenae infesting winter wheat. J. Appl. Entomol. 2017, 141, 352–362. [Google Scholar] [CrossRef]
- He, Y.; Liu, D.; Dai, D.; Wang, D.; Shi, X. Genetic differentiation and structure of Sitobion avenae (Hemiptera Aphididae) populations from moist, semiarid and arid areas in Northwestern China. J. Econ. Entomol. 2018, 111, 603–611. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, Y.; Wang, H.; Yu, Y. lnfluence of honeydew on the wheat yield and nutritive qualities. Chin. Agric. Sci. Bull. 2005, 21, 268–305. [Google Scholar]
- Liu, X.F.; Hu, X.S.; Keller, M.A.; Zhao, H.Y.; Wu, Y.F.; Liu, T.X. Tripartite interactions of barley yellow dwarf virus, Sitobion avenae and wheat varieties. PLoS ONE 2014, 9, e106639. [Google Scholar] [CrossRef]
- Aziz, M.A.; Ahmad, M.; Nasir, M.F.; Naeem, M. Efficacy of different neem (Azadirachta indica) products in comparison with imidacloprid against English Grain Aphid (Sitobion avenae) on Wheat. Int. J. Agric. Biol. 2013, 15, 279–284. [Google Scholar]
- Tétard-Jones, C.; Leifert, C. Plasticity of yield components of winter wheat in response to cereal aphids. NJAS-Wagen. J. Life Sci. 2011, 58, 139–143. [Google Scholar] [CrossRef][Green Version]
- Hu, X.S.; Zhang, Z.F.; Zhu, T.Y.; Song, Y.; Wu, L.J.; Liu, T.X. Maternal effects of the English grain aphids feeding on the wheat varieties with different resistance traits. Sci. Rep. 2018, 8, 7344. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, C.Y.; Wang, Y.J.; Zhang, H.; Ji, W.Q. Screening and evaluation of different wheat cultivars for resistance to Sitobion avenae at seedling and adult-plant stages. Agric. Sci. Technol. 2015, 16, 1686–1692. [Google Scholar]
- Havlíčková, H.; Cvikrová, M.; Eder, J. Phenolic acids in wheat cultivars in relation to plant suitability for and response to cereal aphids. Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz 1996, 10, 535–542. [Google Scholar]
- Bahlmann, L.; Govender, P.; Botha, A.M. Leaf epicuticular wax ultrastructure and trichome presence on Russian wheat aphid (Diuraphis noxia) resistant and susceptible leaves. Afr. Entomol. 2003, 11, 59–64. [Google Scholar]
- Hao, P.; Liu, C.; Wang, Y.; Chen, R.; Tang, M.; Du, B.; Zhu, L.; He, G. Herbivore-induced callose deposition on the sieve plates of rice: An important mechanism for host resistance. Plant Physiol. 2008, 146, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.H.; Zhang, M.; Zhao, H.; Zhang, Y.; Wang, X.X.; Guo, S.S.; Zhang, Z.F.; Liu, T.X. Deciphering the mechanism of β-aminobutyric acid-induced resistance in wheat to the grain aphid, Sitobion avenae. PLoS ONE 2014, 9, e91768. [Google Scholar] [CrossRef] [PubMed]
- Argandoña, V.H.; Chaman, M.; Cardemil, L.; Munoz, O.; Zuñiga, G.E.; Corcuera, L.J. Ethylene production and peroxidase activity in aphid-infested barley. J. Chem. Ecol. 2001, 27, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.K.; Weng, Y.; Rudd, J.C.; Akhunova, A.; Liu, S. Transcriptomics of induced defense responses to greenbug aphid feeding in near isogenic wheat lines. Plant Sci. 2013, 212, 26–36. [Google Scholar] [CrossRef]
- Moran, P.J.; Thompson, G.A. Molecular responses to aphid feeding in Arabidopsis in relation to plant defense pathways. Plant Physiol. 2001, 125, 1074–1085. [Google Scholar] [CrossRef]
- Losvik, A.; Beste, L.; Glinwood, R.; Ivarson, E.; Stephens, J.; Zhu, L.H.; Jonsson, L. Overexpression and down-regulation of barley lipoxygenase LOX2.2 affects jasmonate-regulated genes and aphid fecundity. Int. J. Mol. Sci. 2017, 18, 2765. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.; Vaughn, K.A.; et al. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef]
- Selig, P.; Keough, S.; Nalam, V.J.; Nachappa, P. Jasmonate-dependent plant defenses mediate soybean thrips and soybean aphid performance on soybean. Arthropod-Plant Int. 2016, 10, 273–282. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Sharma, H.C. Induced resistance to Helicoverpa armigera through exogenous application of jasmonic acid and salicylic acid in groundnut, Arachis hypogaea. Pest Manag Sci. 2015, 71, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenase. physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Allmann, S.; Wu, J.; Baldwin, I.T. Comparisons of LIPOXYGENASE3- and JASMONATE-RESISTANT4/6-silenced plants reveal that jasmonic acid and jasmonic acid-amino acid conjugates play different roles in herbivore resistance of Nicotiana attenuata. Plant Physiol. 2008, 146, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Shi, X.; Pei, L.; Gao, X. Induction of phenylalanine ammonia-lyase and lipoxtgenase in cotton seedlings by mechanical wounding and aphid infestation. Prog. Nat. Sci. 2005, 15, 419–423. [Google Scholar]
- Nalam, V.J.; Keeretaweep, J.; Shah, J. The green peach aphid, Myzus persicae, acquires a LIPOXYGENASE5-derived oxylipin from Arabidopsis thaliana, which promotes colonization of the host plant. Plant Signal. Behav. 2013, 8, e22735. [Google Scholar] [CrossRef][Green Version]
- Escudero-Martinez, C.M.; Morris, J.A.; Hedley, P.E.; Bos, J.I.B. Barley transcriptome analyses upon interaction with different aphid species identify thionins contributing to resistance. Plant Cell Environ. 2017, 40, 2628–2643. [Google Scholar] [CrossRef]
- Delp, G.; Gradin, T.; Ahman, I.; Jonsson, L.M.V. Microarray analysis of the interaction between the aphid Rhopalosiphum padi and host plants reveals both differences and similarities between susceptible and partially resistant barley lines. Mol. Genet. Genom. 2009, 281, 233–248. [Google Scholar] [CrossRef]
- Smith, C.M.; Liu, X.; Wang, L.J.; Liu, X.; Chen, M.S.; Starkey, S.; Bai, J. Aphid feeding activates expression of a transcriptome of oxylipin-based defense signals in wheat involved in resistance to herbivory. J. Chem. Ecol. 2010, 36, 260–276. [Google Scholar] [CrossRef][Green Version]
- Liu, X.L. Identification of Wheat Germplasm Resistance to English grain aphid (Sitobion avenae F.) and Molecular Mapping and Expression of Resistance Gene; Northwest A&F University: Yangling, China, 2012. [Google Scholar]
- Ni, X.; Quisenberry, S.S.; Markwell, J.; Heng-Moss, T.; Higley, L.; Baxendale, F.; Sarath, G.; Klucas, R. In vitro enzymatic chlorophyll catabolism in wheat elicited by cereal aphid feeding. Entomol. Exp. Appl. 2001, 101, 159–166. [Google Scholar] [CrossRef]
- Sembdner, G. The biochemistry and the physiological and molecular actions of jasmonates. Annu. Rev. Plant Biol. 1993, 44, 569–589. [Google Scholar] [CrossRef]
- Bailey, B.A.; Strem, M.D.; Bae, H.; Mayolo, G.A.D. Gene expression in leaves of Theobroma cacao in response to mechanical wounding, ethylene, and/or methyl jasmonate. Plant Sci. 2005, 168, 1247–1258. [Google Scholar] [CrossRef]
- Sotherton, N.W.; Emden, H.F.V. Laboratory assessment of resistance to the aphids Sitobion avenae and Metopolophium dirhodum in three Triticum species and two modern wheat cultivars. Ann. Appl. Biol. 2008, 101, 99–107. [Google Scholar] [CrossRef]
- Zhai, Y.; Li, P.; Mei, Y.; Chen, M.Y.; Chen, X.C.; Xu, H.; Zhou, X.; Dong, H.S.; Zhang, C.L.; Jiang, W.H. Three MYB genes co-regulate the phloem-based defence against English grain aphid in wheat. J. Exp. Bot. 2017, 68, 4153–4169. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Woodcock, C.M.; Powers, S.J.; Caulfield, J.C.; Pickett, J.A.; Birkett, M.A. Cis-iasmone elicits aphid-induced stress signalling in potatoes. J. Chem. Ecol. 2017, 43, 39–52. [Google Scholar] [CrossRef]
- Andreou, A.; Feussner, I. Lipoxygenases–structure and reaction mechanism. Phytochemistry 2009, 70, 504–1510. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, M.M.; Sun, H.; Ullah, A.; Zhu, L. Genome-wide identification of lipoxygenase gene family in cotton and functional characterization in response to abiotic stresses. BMC Genom. 2018, 19, 599. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef]
- Bachmann, A.; Hause, B.; Maucher, H.; Garbe, E.; Vörös, K.; Weichert, H.; Wasternack, C.; Feussner, I. Jasmonate-induced lipid peroxidation in barley leaves initiated by distinct 13-LOX forms of chloroplasts. Biol. Chem. 2002, 383, 1645–1657. [Google Scholar] [CrossRef]
- Bell, E.; Creelman, R.A.; Mullet, J.E. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc. Natl. Acad. Sci. USA 1995, 92, 8675–8679. [Google Scholar] [CrossRef]
- Liu, D.; Cheng, Y.; Gong, M.; Zhao, Q.; Jiang, C.; Cheng, L.; Ren, M.; Wang, Y.; Yang, A. Comparative transcriptome analysis reveals differential gene expression in resistant and susceptible tobacco cultivars in response to infection by cucumber mosaic virus. J. Crop Sci. Engl. Ed. 2019, 3, 307–321. [Google Scholar]
- Müller, V.; Amé, M.V.; Carrari, V.; Gieco, J.; Asis, R. Lipoxygenase activation in peanut seed cultivars resistant and susceptible to Aspergillus parasiticus colonization. Phytopatholog 2014, 104, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, D.F.; Brown, G.C.; Jackson, D.M.; Hamilton-Kemp, T.R. Effects of some leaf-emitted volatile compounds on aphid population increase. J. Chem. Ecol. 1993, 19, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ye, J.; Li, S.; Niinemets, Ü. Methyl jasmonate-induced emission of biogenic volatiles is biphasic in cucumber: A high-resolution analysis of dose dependence. J. Exp. Bot. 2017, 68, 4679–4694. [Google Scholar] [CrossRef] [PubMed]
- Gális, I.; Gaquerel, E.; Pandey, S.P.; Baldwin, I.T. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant Cell Environ. 2009, 32, 617–627. [Google Scholar] [CrossRef]
- Glowacz, M.; Bill, M.; Tinyane, P.P.; Sivakumar, D. Maintaining postharvest quality of cold stored ‘Hass’ avocados by altering the fatty acids content and composition with the use of natural volatile compounds-methyl jasmonate and methyl salicylate. J. Sci. Food Agric. 2017, 97, 5186–5193. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Yan, H.; Li, W.; Cai, R.; Xiang, Y. The lipoxygenase gene family in poplar: Identification, classification, and expression in response to MeJA treatment. PLoS ONE 2015, 10, e0125526. [Google Scholar] [CrossRef]

| Primer Name | Primer Sequence 5′→3′ |
|---|---|
| TtLOX-f | CTATAAATAGGATCCAGGGC |
| TtLOX-r | CCTTTTATTAGCATTTCCGT |
| GFP-TtLOX-f | TAGCCATGGTAGATCTGATGCTAACGGCGACGAAGTC |
| GFP-TtLOX-r | ACAGGCCTTACGTATCAAATGGAGATGCTGTTGG |
| VIGS-TtLOX-f | ACGGGCATAAAGACCGCCAA |
| VIGS-TtLOX-r | CTTGAGGACGTATGGCAGCA |
| VIGS-PDS-f | TTCACTGTTCCGTCCGGGTT |
| VIGS-PDS-r | AAGCAGGGTGTTCCTGATCG |
| qPCR-TtLOX-f | AAGTTGGACGAGGCAACCTAC |
| qPCR-TtLOX-r | AGAGCTTCTTGTTAGCCACGG |
| qPCR-PDS-f | TTCACTGTTCCGTCAGGGTTC |
| qPCR-PDS-r | CAGTCTTTGGGTGGTGAGGTC |
| qPCR-BSMV-f | AACTGCCAATCGTGAGTAGGTT |
| qPCR-BSMV-r | CTCCTGTTCAGAACGTTTCAGAAGT |
| Actin-f | TAGGAGGGCAAGTCTGGT |
| Actin-r | CTTTCGCAGTTGTTCGTC |
| Function | Cis-Regulatory Element |
|---|---|
| ABRE | Abscisic acid (ABA) responsiveness |
| AuxRR-core | Auxin responsiveness |
| CGTCA-motifTGACG-motif | MeJA-responsiveness |
| GARE-motif | Gibberellin (GA) -responsive |
| TC-rich repeats | Defense and stress responsiveness |
| TCA-element | Salicylic acid (SA) responsiveness |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ma, X.; Kou, X.; Bai, J.; Zhang, H.; Wang, C.; Wang, Y.; Zhao, J.; Tian, Z.; Ji, W. Molecular Characterization and Functional Analysis of Wheat TtLOX Gene Involved in Aphid Resistance. Agronomy 2020, 10, 780. https://doi.org/10.3390/agronomy10060780
Liu X, Ma X, Kou X, Bai J, Zhang H, Wang C, Wang Y, Zhao J, Tian Z, Ji W. Molecular Characterization and Functional Analysis of Wheat TtLOX Gene Involved in Aphid Resistance. Agronomy. 2020; 10(6):780. https://doi.org/10.3390/agronomy10060780
Chicago/Turabian StyleLiu, Xinlun, Xiaolong Ma, Xudan Kou, Jinfeng Bai, Hong Zhang, Changyou Wang, Yajuan Wang, Jixin Zhao, Zengrong Tian, and Wanquan Ji. 2020. "Molecular Characterization and Functional Analysis of Wheat TtLOX Gene Involved in Aphid Resistance" Agronomy 10, no. 6: 780. https://doi.org/10.3390/agronomy10060780
APA StyleLiu, X., Ma, X., Kou, X., Bai, J., Zhang, H., Wang, C., Wang, Y., Zhao, J., Tian, Z., & Ji, W. (2020). Molecular Characterization and Functional Analysis of Wheat TtLOX Gene Involved in Aphid Resistance. Agronomy, 10(6), 780. https://doi.org/10.3390/agronomy10060780





