1. Introduction
Alfalfa (
Medicago sativa L.) is a perennial, productive, high-quality and highly adaptable legume that is widely grown as forage for livestock. It has natural advantages in improving the ecological environment and soil and water conservation due to its deep rooting system. The species is distributed in North America, South America, Asia, Europe, Africa, and Oceania and has been widely sown in pastures. Alfalfa has also been widely planted as a forage crop in China, which contains the largest planting area of this species [
1]. Ongoing climate change, characterized by an increase in the frequency and severity of extreme climatic events and adverse weather conditions, threatens global crop production. As a consequence, drought and water shortage have become the most important factors limiting the growth and productivity of major crop species [
2].
Plant roots are an important component of the soil ecosystem [
3] due to their roles in the absorption, transformation, and storage of water, minerals, and carbohydrates by plants, and the growth and development of the root systems are affected by genetics and environment [
4]. Under drought stress, the roots are the first plant organ to sense soil water deficit and respond accordingly, which leads to the production of a larger number of secondary and/or tertiary lateral roots. These meristematic features are effective for the uptake of water that is not uniformly distributed in the soil because roots with smaller diameter have a strong penetration ability and can absorb water in the deeper soil profile. On the other hand, newly produced lateral roots can effectively transport water to the taproot in dry soil.
Compared to other crops, alfalfa has a stronger root system that can efficiently absorb and utilize the moisture in deep soil. This feature of alfalfa is considered one of the main causes of pasture drought resistance [
5]. The root system is closely related to aboveground growth and morphological characters that have been analyzed under drought stress [
6,
7]. The morphological traits of root systems, such as root length, root volume, root diameter, and root dry matter, can reflect the health of the root system and can be used to assess drought resistance in pastures [
8,
9,
10,
11]. After exposure to drought stress, plant roots undergo a series of changes in growth and development, morphology/anatomy, biomass, and physiological and biochemical metabolism in order to adapt to the dry conditions.
Root system plasticity is the ability of an individual organism to alter its root morphology in response to changes in environmental conditions [
12]. Root system architectural change is one of the main forms of plasticity and is affected by environmental factors and genetic factors [
13]. Root system architecture refers to the spatial structure and distribution of roots in the growth medium and is known to be a major determinant of root functions in the acquisition of soil resources such as nutrients and water [
14,
15]. Studies of root system architecture have focused on root geometry and topological parameters. The geometric parameters primarily include root length, root diameter, root biomass, and branch angle. The branching status of roots is reflected by the number of links, root branching ratio and spatial distribution of roots in the soil layer. Recently, root topology and root system architecture have attracted increasing research interests [
16]. Some studies [
17,
18,
19] have proposed that root topology has two extreme branching patterns, namely, dichotomous and herringbone branching, and the topological index (TI) is used to show the different branching patterns of plant root systems. Moreover, the nutritional competitiveness of different branching patterns was assessed, and dichotomous branching was found to be more suitable for poor habitats [
20].
Fractal geometry is widely applied to assess root system architecture and the distribution of root systems in soils [
4,
21]. Fractal dimension (FD) is correlated with root topology [
16] and root systems architecture [
22]. The characterization of root system architecture would assist in better understanding the functional and growth strategies of plant root systems when they are faced with an insufficient supply of soil water [
23]. FD is closely related to the branching pattern of roots, while the fractal abundance (FA) is associated with the volume of space explored by roots [
24].
Water deficit has significant effects on plants at the physiological and molecular levels [
25]. This stress can disrupt the dynamic equilibration of reactive oxygen species (ROS), which results in the suppression of plant growth, changes in morphology, and reductions in yield [
26]. ROS, such as superoxide (O
2−), hydrogen peroxide (H
2O
2), and hydroxyl radical (OH
−), can decrease cellular structural integrity and physiological and biochemical processes as a result of its effects on phosphatide peroxidation, protein degradation, and DNA fragmentation, and thereby cause substantial cellular damage [
27]. To mitigate and cope with the deleterious effects of ROS, plants have developed a complex antioxidant defense system [
28]. The enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), glutathione reductase (GR), and ascorbate peroxidase (APX) [
29]. H
2O
2 is scavenged by CAT, APX, and GPX, but the functions of APX and GPX must rely on existing non-enzymatic antioxidants such as ascorbate (AsA) and glutathione (GSH) [
30].
Drought and rehydration often act as a continuous process in farming practice. Different varieties of the same plant exhibit different levels of drought tolerance and resilience over the course of drought and rehydration. The response of crops to rehydration involves rapid growth after the stress is relieved to compensate for the partial losses caused by water deficit. Plant resilience plays an important role in crop production after drought and rehydration. Plants undergo a series of changes in morphological, physiological and biochemical aspects under the drought and rehydration process, which requires the synergistic responses of the aboveground leaves and belowground roots. Previous studies focused on plant shoots, examining their morphological, physiological and molecular mechanisms of drought resistance [
5,
31,
32,
33]. In contrast, very few studies [
34] have evaluated the architecture of whole root systems in response to soil drought and rehydration using fractal theory and topological analysis.
In this study, we examined root system architectural and morphological responses of alfalfa to changes in water deficit and rehydration using fractal and topological analyses. The aims of this study were (1) to explore the optimum soil moisture for plant root growth using topological and fractal analyses, (2) to discover whether it is possible to manipulate root system architectural features by altering soil moisture conditions in order to screen alfalfa cultivars based on drought tolerance, and to expand knowledge of the root architectural plasticity of alfalfa in agriculture, and (3) to identify the differential physiological pattern of alfalfa root on enzyme activity in response to various soil moistures, which could help elucidate the spatial traits and physiological mechanisms of the root system in alfalfa grown in arid and semiarid regions.
2. Materials and Methods
2.1. Experimental Location, Description, and Treatments
Two experiments were conducted in a controlled greenhouse at the Institute of Animal Science, Chinese Academy of Agricultural Science from January to May 2019, with 20–25 °C and 60% humidity under a 16 h light/8 h dark photoperiod. The first experiment (Experiment 1) examined root systems architecture and root morphological traits. The second experiment (Experiment 2) examined root reactive oxygen species and antioxidant defense systems. Four soil moisture treatments were imposed in both experiments. They were: (I) soil water content was kept at 75–80% of the water-holding capacity throughout the experiment (control, CK); (II) 60–65% of the water-holding capacity for four weeks and subsequent rehydration to 75–80% of the water-holding capacity for three weeks; (III) 45–50% of the water-holding capacity for four weeks and subsequent rehydration to 60–65% of the water-holding capacity for one week and 75–80% for two weeks; and (IV) 30–35% of the water-holding capacity for four weeks and subsequent rehydration to 45–50% of the water-holding capacity for one week, 60–65% for one week and 75–80% for the final week. Details of the experimental treatments are described below (
Supplemental Table S1).
2.2. Seedling Establishment, Transplanting, and Management
The experiments were conducted using 18-cm-wide, 50-cm-deep cylindrical pots. The potting mix (sterilized) contained 80% nutrient-enriched soil and 20% sand with 65.0% organic matter, 1.6% total nitrogen, 0.1% total P2O5, 0.2% total K2O and 1 kg/m3 bulk density (N:P:K = 14:10:18). Prior to sowing, seed from each alfalfa cultivar was disinfected with sodium hypochlorite (1% NaClO) for 30 min and washed with deionized water four times, and then 100 seeds of each alfalfa cultivar were placed in two petri dishes containing two pieces of filter paper wetted with 4 mL of deionized water. Germinated seedlings were incubated in a growth chamber under a 16 h light and 8 h dark (temperature = 25 °C) cycle for approximately 8 days. The seedlings were transplanted into each pot. A nylon mesh bag was placed in each pot, filled with 3.0 kg of potting mix (mix sieved with 5-mm mesh, pH = 6.0), and planted with one seedling. Nitrogen fertilizer was applied at 10 kg urea/ha (4.6 kg N/ha) prior to planting. All pots were checked and watered regularly to ensure sufficient moisture for plant growth before drought and rehydration treatment. Drought treatments were then imposed for four consecutive weeks and subsequent rehydration for one week with different water deficits. Each pot was weighed, and the loss water was added to maintain the corresponding soil moisture during the period of drought stress and rehydration in Experiment 1 and Experiment 2.
2.3. Determination of Water Holding Capacity
Water holding capacity for each pot was determined by weighing the dry pot, watering until it dripped, letting the pot stand 48 h, and weighing it again. The difference between the dry and next-day weight of each pot was recorded as pot capacity.
At the start of the experiments, pots with dry potting mix were weighed and then watered until they were fully saturated. They were then drained for 48 h, and individual pots were weighed again to determine water holding capacity (moisture content at this point), which were used as the basis to calculate moisture and rehydration levels of the treatments.
2.4. Experiment 1
2.4.1. Materials
Three alfalfa cultivars were used in the experiments. They were cv. Zhaodong (ZD), Golden empress (GE) and Aohan (AH), commonly grown in northern China, which represented the range in drought tolerances of cultivars grown in the region, were selected for the study. ZD is suitable to grow in the semiarid areas with annual rainfall of 350 to 800 mm, annual evaporation of 2534 mm, and annual average temperature of 6.4 °C. GE is an indigenous forage grown in the semiarid regions with annual rainfall of 400 to 600 mm, annual evaporation of 1500 mm, and annual average temperature of 8.7 °C, and AH is an improved cultivar with high yield.
2.4.2. Design
A split-plot design was used, with the three cultivars as the main plots and four soil moisture and rehydration treatments as the subplots. The combined 3 × 4 = 12 treatments were replicated four times.
2.4.3. Root Sampling
At the end of the experiment, the nylon mesh bag in each pot was removed carefully in order to maintain their original spatial distribution and to minimize damage and disturbance to the root systems. The roots were washed thoroughly by hand, rinsed with deionized water, and then put evenly in a clear acrylic tray with 1 cm of water and scanned at 600-dpi resolution with a MICROTEK Scan Maker (i800plus). After scanning, the root samples were oven-dried at 65 °C for at least 48 h.
2.4.4. Measurements
Root Characteristics
Win-RHIZO 2017a (Regent Instruments, Inc., Quebec, QC, Canada) was used to analyze the scanned images. The routines used include thresholding, framing, editing to fix breakpoints and eliminate loops in the image and obtain root diameter, root surface area, root volume, root length, and lateral root number. The TI, FDs, FA and root branching ratio (BR) were also calculated. The details are described below.
Fractal Analysis
Digitized root images were used for fractal analysis following the box-counting method [
35] within Win-RHIZO software. Each root image was first covered with a frame, and the frame was divided into boxes (grids) with side length r. The size of the boxes was designated from 200 to 3200 pixels (0.008–0.127 mm). The number N(r) of boxes that intersected with the image was counted. The number N(r) of boxes gradually increased with decreasing side length r. When plotting N(r) against r on a lg–lg scale, the power-law relationship N(r) = Kr − D was obtained if the image was fractal. The two constants D and lg K were calculated based on the equation lg Nr= −FD lgr + lg K as the fractal parameters. The negative value of the slope of regression line was the FD, and lg K was the FA [
17,
23].
Topological Index
Two extreme patterns of root topology were proposed: herringbone and dichotomous branching [
18,
19] (
Figure 1). With both patterns, topological index (TI) is used to reflect the branching patterns of different plant roots, and is defined as log altitude (A)/log magnitude (M) (A is the total number of internal links in the root system and M is the total number of external links in the root system). Most studies [
15,
36] have shown that the majority of plant root topologies are between dichotomous and herringbone branching patterns. The root system tends to exhibit herringbone branching when TI is close to 1, and dichotomous branching when TI is close to 0.5.
Branching Ratio
Plant root orders were assessed and determined from the outside to the inside of the root crown: the first group of small roots in the outer layer of the root crown was classified as the first-level roots, the interlinks between two first-level roots were the second-level roots, the interlinks between two second-level roots were the third-level roots, and so on. If there were roots from two or more different levels, the level of the root group was determined as the roots with the highest level. Then, the root coefficient (Ni) of each level (i) was calculated. The level (i) was plotted on the x-axis, and lg Ni was plotted on the y-axis. The two constants i and lg Ni were calculated using regression analysis. The inverse logarithm of the slope of the regression line was defined as the root system’s total branching ratio.
2.5. Experiment 2
2.5.1. Material, Treatment, Design and Root Sampling
One alfalfa cultivar cv ZD was used in Experiment 2. The four soil moisture treatments identical to that in Experiment 1 were arranged in a randomized complete block (RCB) design with three replicates. At the end of Experiment 2, the nylon mesh bags were removed from each pot after completely soaking in the water for about 5–10 min and then plant roots were washed by hand to avoid damage to the plant root. Root samples were collected and frozen in liquid nitrogen, and then stored at −80 °C until they were processed.
2.5.2. Measurements
Detection of H2O2 and O2−
The level of hydrogen peroxide (H
2O
2) was determined with spectrophotometry–colorimetry at 415 nm as described by Willekens, et al. [
37]. Roots (0.1 g) were homogenized in 4 mL 0.1% (
w/
v) TCA in an ice bath and the extract was centrifuged at 12,000×
g for 10 min at 4 °C. The rate of O
2− generation was measured following Elstner and Heupel [
38]. Roots (0.1 g) were homogenized in 4 mL, 65 mM phosphate buffer (pH 7.8) and centrifuged at 5000×
g for 15 min at 4 °C. The absorbance of the supernatant was read at 530 nm after the addition of an equal volume of ether and centrifugation at 3000×
g for 3 min.
Determination of Malondialdehyde (MDA)
The MDA concentration as the marker of lipid peroxidation was determined using the modified technology described by Heath and Packer [
39]. Root samples (0.5 g) were powdered with liquid nitrogen and homogenized with 5 mL of 20% TCA. Absorbance was measured at 450 nm, 532 nm, and 600 nm using a spectrophotometer.
Estimation of Ascorbic Acid (AsA) and Reduced Glutathione (GSH)
Root tissue was extracted with 6% TCA and centrifuged at 15,000×
g for 5 min (4 °C), and the supernatant was used for AsA and GSH assays [
40]. The content of AsA was determined following a previously established method [
40]. The supernatant (0.2 mL) was neutralized with 0.5 mL of 0.2 M K-phosphate buffer (pH 7.4) and 0.1 mL dithiothreitol (DTT; reducing DHA to AsA). The reaction mixture was incubated at 30 °C for 90 min, and the concentration was determined spectrophotometrically at 534 nm.
GSH content was determined following the method of Griffith [
41]. Supernatant (0.2 mL) was neutralized with 1.4 mL of 50 Mm K-phosphate buffer (pH 7.5). Based on enzymatic recycling, glutathione was oxidized by 2-nitrobenzoic acid (DTNB) and reduced by NADPH in the presence of GR, and total GSH content was evaluated by the rate of absorption changes at 412 nm.
Antioxidant Enzyme Extraction and Activity Assays
Root samples of 0.5 g were ground with a pestle and a pre-chilled mortar in liquid nitrogen. Root tissue (0.5 g) was homogenized with 5 mL of ice-cold extraction buffer, 100 mM potassium phosphate-buffered saline (PBS), and 0.1 mM Na
2EDTA with pH = 7.0–7.4. The homogenate was filtered through four layers of muslin cloth and centrifuged at 16,000×
g for 20 min at 4 °C. The supernatant was used as an enzyme extract for enzyme activity assays [
42]. All extracts were prepared at 4 °C, and the measurements of enzymatic activity were performed at 25 °C.
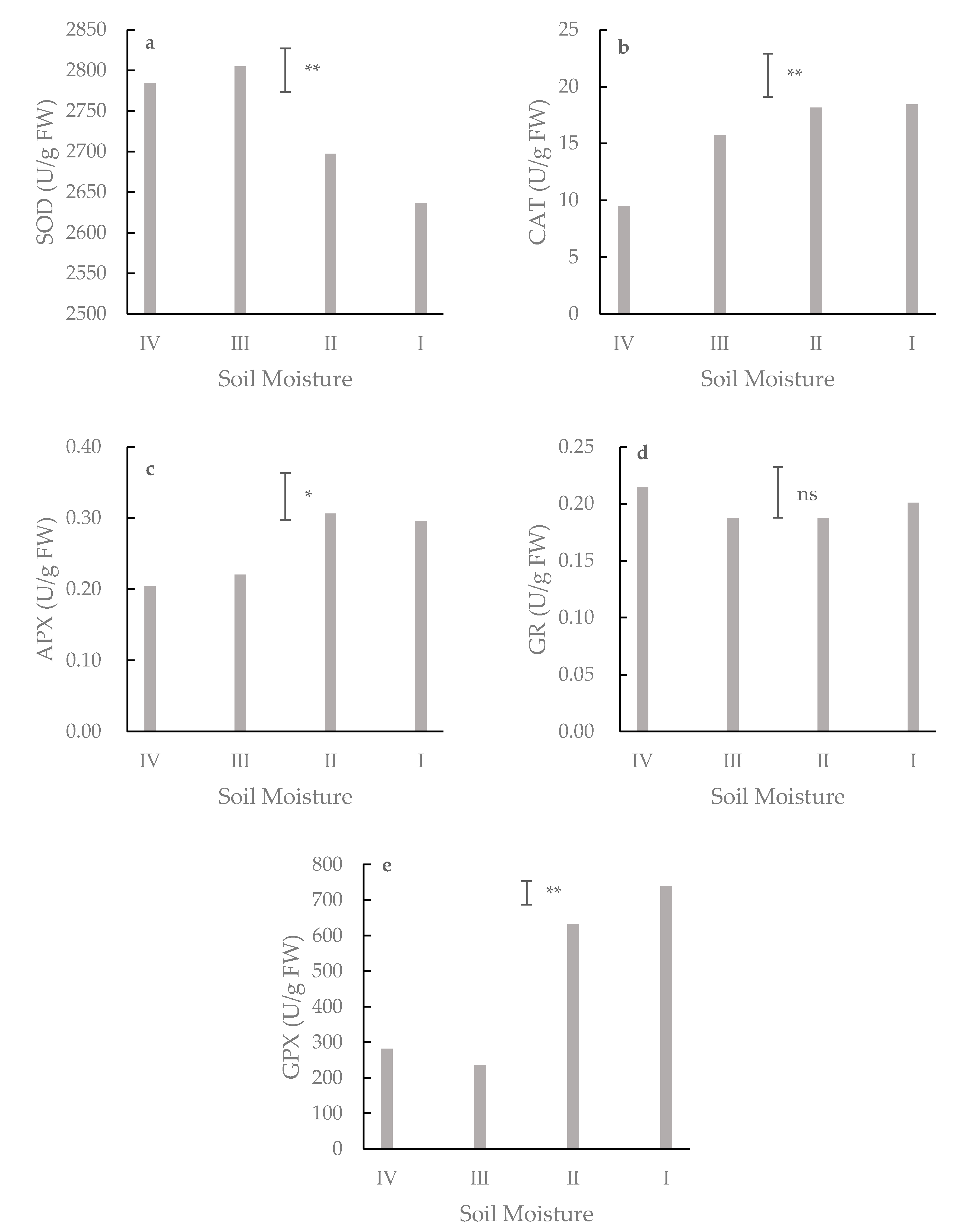
In the superoxide dismutase (SOD) activity [
43], the enzyme extract (50 μL) was added into 3 mL mixture (2.7 mL of 14.5 mM methionine, 90 μL of 50 mM PBS, 100 μL of 60 μM riboflavin, 100 μL of 2.25 mM nitro-blue tetrazolium, 10 μL of 30 μM EDTA), then reacted in an illumination incubator (4000 l×) for 20 min, and the absorbance was recorded at 560 nm.
In the catalase (CAT) activity [
44], the enzyme extract (100 μL) was mixed with 3 mL of 0.15 M PBS and 5 μL of 0.1 M H
2O
2. The absorbance was recorded at 240 nm.
In the ascorbate peroxidase (APX) activity [
44], the reaction mixture contained 50 mM PBS (pH 7.0), 0.5 mM AsA, 0.1 mM EDTA, 0.1 mM H
2O
2, and 30 μL of enzyme extract in a final volume of 2 mL. The activity was calculated from the recorded decrease in absorbance at 290 nm for 1 min. One unit of APX was defined as the amount of enzyme that oxidizes 1 µmol of AsA per minute at room temperature.
In the glutathione peroxidase (GPX) activity [
45], the reaction mixture contained 100 mM PBS (pH 7.0), 1 mM EDTA, 1 mM NaN
3, 0.12 mM NADPH, 2 mM GSH, 1 μ GR, 0.6 mM H
2O
2 (as a substrate), and 30 μL of enzyme extract. The reaction was initiated with H
2O
2. The change in absorbance was recorded at 340 nm.
In the glutathione reductase (GR) activity [
46], the reaction mixture consisted of 0.1 M PBS (pH 7.0), 1 mM EDTA, 1 mM GSSG, 0.2 mM NADPH, and 50 μL of enzyme extract. The reaction was started by the addition of GSSG. A decrease in absorbance was recorded at 340 nm.
2.6. Statistical Analyses
All data were analyzed using the general analysis of variance (ANOVA) procedure in GENSTAT release 18.0 (VSN International 2009). A split-plot model with repeated measures was used to analyze the main effects of water treatments and alfalfa cultivars, and their interaction on topology, fractal features, the branching ratio and root morphological traits for Experiment 1. An RCB model was used to analyze MDA, reactive oxygen species (O2−, H2O2, ASA, and GSH) and antioxidant enzyme activity (SOD, CAT, APX, GR, and GPX) for Experiment 2. A significant difference was quoted at p < 0.05 or 0.01 and no significant difference between treatments indicates the probability was greater than 5% (p > 0.05).