Fatty Acid Composition Dynamics of Rye (Secale cereale L.) and Wheat (Triticum aestivum L.) Forages under Cattle Grazing
Abstract
1. Introduction
2. Materials and Methods
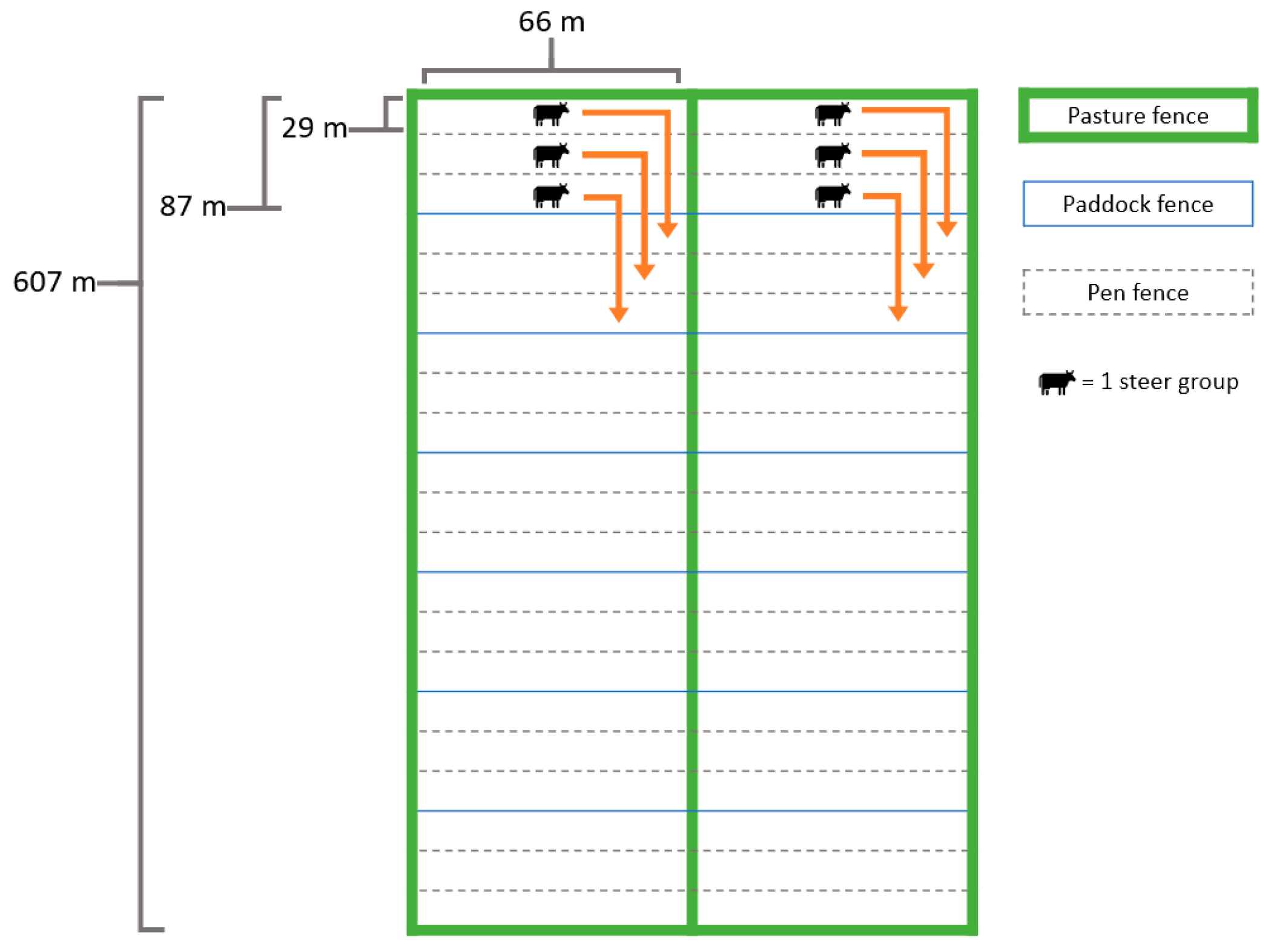
2.1. Experimental Design
2.2. Statistical Analysis
3. Results
3.1. Weather
3.2. Fatty Acids
3.2.1. Alpha-Linolenic Acid (C18:3n-3)
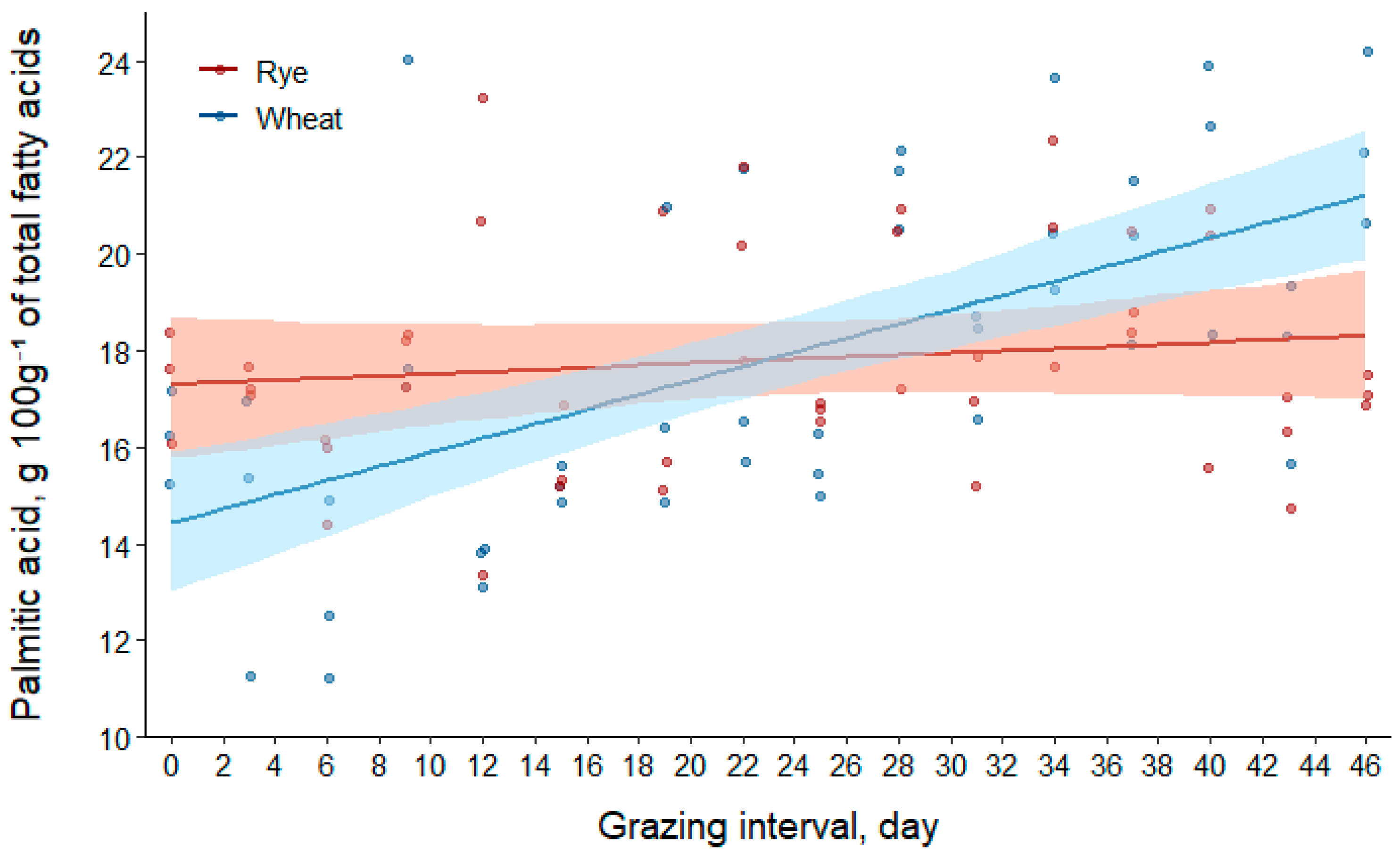
3.2.2. Palmitic Acid (C16:0)
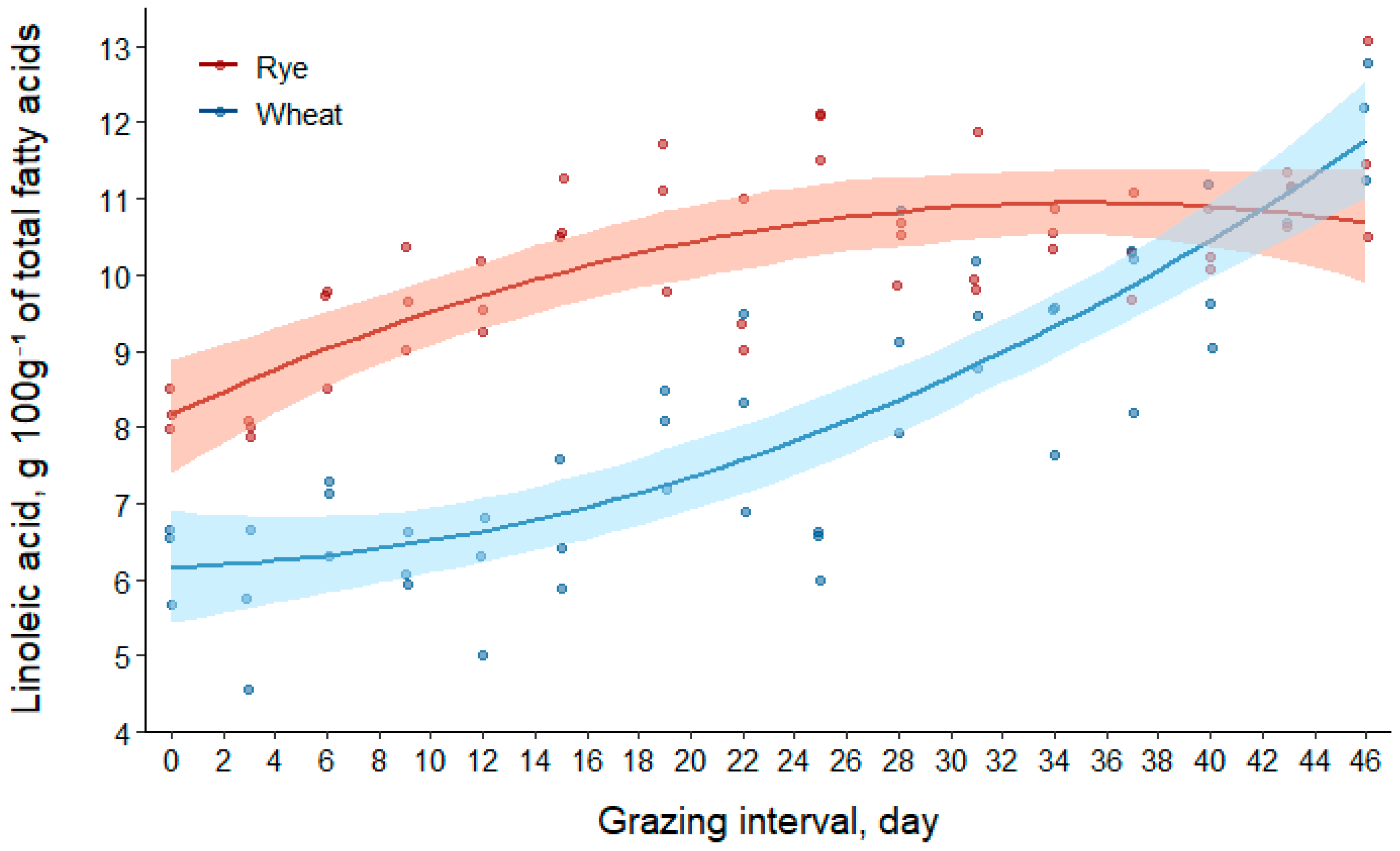
3.2.3. Linoleic Acid (C18:2n-6)
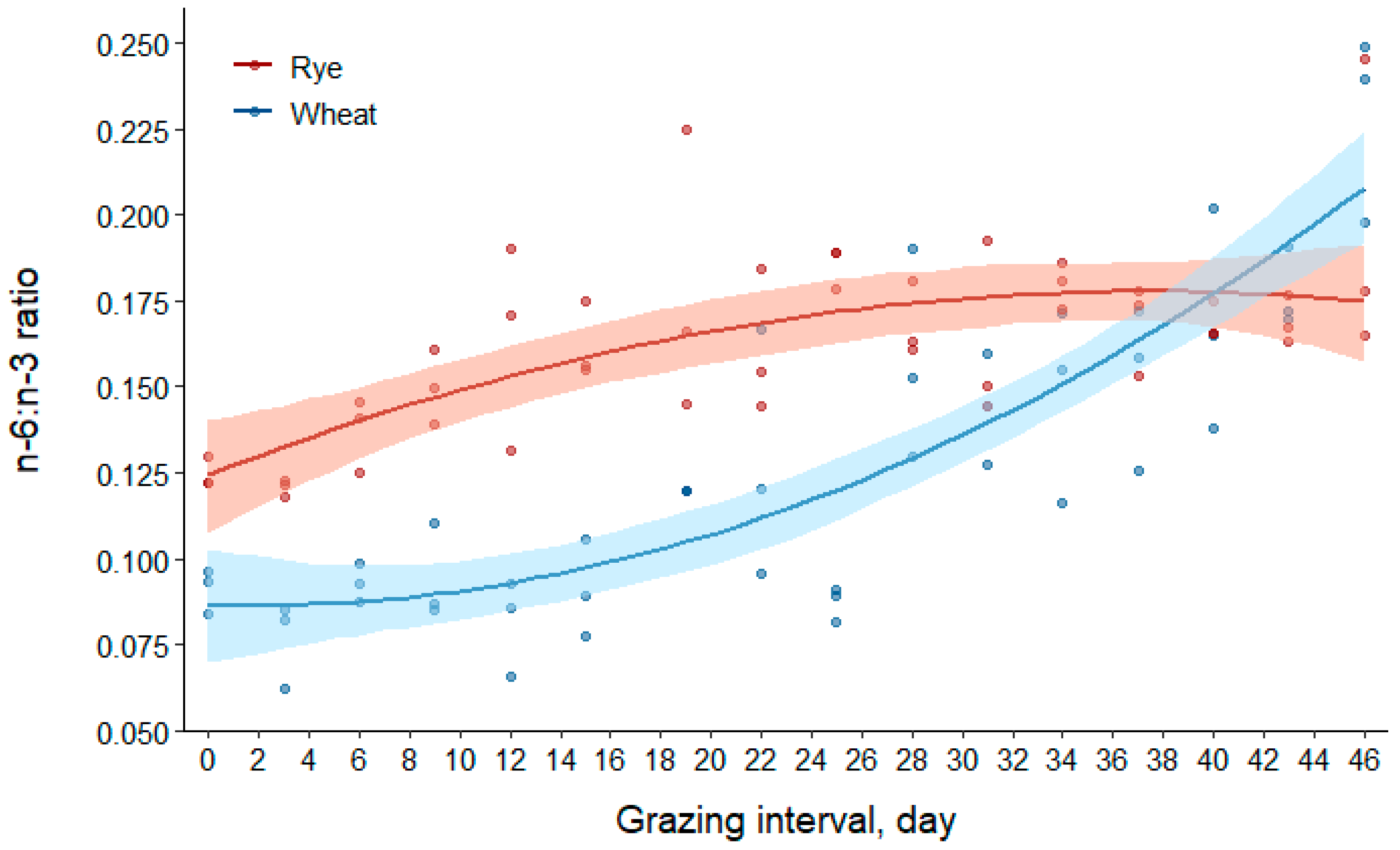
3.2.4. Omega-6 to Omega-3 (n-6:n-3) Fatty Acid Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Greene, C.; McBride, W. Consumer Demand for Organic Milk Continues to Expand—Can the US Dairy Sector Catch up? Available online: http://www.choicesmagazine.org/ (accessed on 15 March 2020).
- Umberger, W.J.; Boxall, P.C.; Lacy, R.C. Role of credence and health information in determining us consumers’ willingness-to-pay for grass-finished beef. Aust. J. Agric. Resour. Econ. 2009, 53, 603–623. [Google Scholar] [CrossRef]
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef]
- Leaf, A.; Weber, P. Cardiovascular effects of n-3 fattys acids. N. Engl. J. Med. 1998, 318, 549–557. [Google Scholar] [CrossRef] [PubMed]
- McGuire, M.A.; McGuire, M.K. Conjugated linoleic acid (CLA): A ruminant fatty acid with beneficial effects on human health. J. Anim. Sci. 2000, 77, 1. [Google Scholar] [CrossRef]
- Tapiero, H.; Ba, G.N.; Couvreur, P.; Tew, K.D. Polyunsaturated fatty acids (PUFA) and eicosanoids in human health and pathologies. Biomed. Pharmacother. 2002, 56, 215–222. [Google Scholar] [CrossRef]
- Weill, P.; Schmitt, B.; Chesneau, G.; Daniel, N.; Safraou, F.; Legrand, P. Effects of introducing linseed in livestock diet on blood fatty acid composition of consumers of animal products. Ann. Nutr. Metab. 2002, 46, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.L.; Kolver, E.S.; Bauman, D.E.; Van Amburgh, M.E.; Muller, L.D. Effect of intake of pasture on concentrations of conjugated linoleic acid in milk of lactating cows. J. Dairy Sci. 1998, 81, 1630–1636. [Google Scholar] [CrossRef]
- Dhiman, T.R.; Anand, G.R.; Satter, L.D.; Pariza, M.W. Conjugated linoleic acid content of milk from cows fed different diets. J. Dairy Sci. 1999, 82, 2146–2156. [Google Scholar] [CrossRef]
- French, P.; Stanton, C.; Lawless, F.; O’Riordan, E.G.; Monahan, F.J.; Caffrey, P.J.; Moloney, A.P. Fatty acid composition, including conjugated linoleic acid, of intramuscular fat from steers offered grazed grass, grass silage, or concentrate-based diets. J. Anim. Sci. 2000, 78, 2849–2855. [Google Scholar] [CrossRef]
- Electronic Code of Federal Regulations. Available online: https://www.ecfr.gov/ (accessed on 19 March 2020).
- Benbrook, C.M.; Davis, D.R.; Heins, B.J.; Latif, M.A.; Leifert, C.; Peterman, L.; Butler, G.; Faergeman, O.; Abel-Caines, S.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018, 6, 681–700. [Google Scholar] [CrossRef]
- Bjorklund, E.A.; Heins, B.J.; Dicostanzo, A.; Chester-Jones, H. Fatty acid profiles, meat quality, and sensory attributes of organic versus conventional dairy beef steers. J. Dairy Sci. 2014, 97, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.; Hocquette, J.-F.; Nuernberg, K.; Dannenberger, D.; Richardson, I.; Moloney, A. Innovations in beef production systems that enhance the nutritional and health value of beef lipids and their relationship with meat quality. Meat Sci. 2006, 74, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Santos, G.; O’Donnell, A.M.; Vicini, J.L.; Hartnell, G.F.; Bauman, D.E. Hot topic: Enhancing omega-3 fatty acids in milk fat of dairy cows by using stearidonic acid-enriched soybean oil from genetically modified soybeans. J. Dairy Sci. 2010, 93, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Griinari, J.; Bauman, D. Biosynthesis of conjugated linoleic acid and its incorporation into meat and milk in ruminants. In Advances in Conjugated Linoleic Acid Research; Yurawecz, M., Mossoba, M., Kramer, J., Pariza, M., Nelson, G., Eds.; AOCS Press: Champaign, IL, USA, 1999; Volume 1, pp. 180–200. ISBN 1-893997-02-2. [Google Scholar]
- Mir, P.S.; McAllister, T.A.; Scott, S.; Aalhus, J.; Baron, V.; McCartney, D.; Charmley, E.; Goonewardene, L.; Basarab, J.; Okine, E.; et al. Conjugated linoleic acid–enriched beef production. Am. J. Clin. Nutr. 2004, 79, 1207S–1211S. [Google Scholar] [CrossRef]
- MacRae, J.; O’Reilly, L.; Morgan, P. Desirable characteristics of animal products from a human health perspective. Livest. Prod. Sci. 2005, 94, 95–103. [Google Scholar] [CrossRef]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Glasser, F.; Doreau, M.; Maxin, G.; Baumont, R. Fat and fatty acid content and composition of forages: A meta-analysis. Anim. Feed Sci. Technol. 2013, 185, 19–34. [Google Scholar] [CrossRef]
- Boufaïed, H.; Chouinard, P.Y.; Tremblay, G.F.; Petit, H.V.; Michaud, R.; Bélanger, G. Fatty acids in forages. I. Factors affecting concentrations. Can. J. Anim. Sci. 2003, 83, 501–511. [Google Scholar] [CrossRef]
- Clapham, W.M.; Foster, J.G.; Neel, J.P.S.; Fedders, J.M. Fatty acid composition of traditional and novel forages. J. Agric. Food Chem. 2005, 53, 10068–10073. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Scollan, N.D.; Youell, S.J.; Tweed, J.K.S.; Humphreys, M.O. Influence of species, cutting date and cutting interval on the fatty acid composition of grasses. Grass Forage Sci. 2001, 56, 68–74. [Google Scholar] [CrossRef]
- Meľuchová, B.; Blaško, J.; Kubinec, R.; Górová, R.; Dubravská, J.; Margetín, M.; Soják, L. Seasonal variations in fatty acid composition of pasture forage plants and CLA content in ewe milk fat. Small Rumin. Res. 2008, 78, 56–65. [Google Scholar] [CrossRef]
- Sulc, R.M.; Tracy, B.F. Integrated crop-livestock systems in the U.S. corn belt. Agron. J. 2007, 99, 335–345. [Google Scholar] [CrossRef]
- Hayden, J.; Rocker, S.; Phillips, H.; Heins, B.; Smith, A.; Delate, K. The importance of social support and communities of practice: Farmer perceptions of the challenges and opportunities of integrated crop-livestock systems on organically managed farms in the Northern US. Sustainability 2018, 10, 4606. [Google Scholar] [CrossRef]
- Lemaire, G.; Franzluebbers, A.; de Faccio Carvalho, P.C.; Dedieu, B. Integrated crop-livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Allen, V.G.; Batello, C.; Berretta, E.J.; Hodgson, J.; Kothmann, M.; Li, X.; McIvor, J.; Milne, J.; Morris, C.; Peeters, A.; et al. An international terminology for grazing lands and grazing animals. Grass Forage Sci. 2011, 66, 2–28. [Google Scholar] [CrossRef]
- Watson, M.E.; Brown, J.R. pH and lime requirement. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; Volume 221, pp. 13–16. [Google Scholar]
- Combs, S.M.; Nathan, M.V. Soil organic matter. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; Volume 221, pp. 53–58. [Google Scholar]
- Warncke, D.; Brown, J.R. Potassium and other basic cations. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; Volume 221, pp. 31–34. [Google Scholar]
- Frank, K.; Beegle, D.; Denning, J. Phosphorus. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; Volume 221, pp. 21–29. [Google Scholar]
- Nelson, D.W. Determination of ammonium in KCl extracts of soils by the salicylate method. Commun. Soil Sci. Plant Anal. 1983, 14, 1051–1062. [Google Scholar] [CrossRef]
- Gelderman, R.H.; Beegle, D. Nitrate-nitrogen. In Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R., Ed.; Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; Volume 221, pp. 17–20. [Google Scholar]
- Phillips, H.N.; Heins, B.J.; Delate, K.; Turnbull, R. Impact of grazing dairy steers on winter rye (Secale cereale) versus winter wheat (Triticum aestivum) and effects on meat quality, fatty acid and amino acid profiles, and consumer acceptability of organic beef. PLoS ONE 2017, 12, e0187686. [Google Scholar] [CrossRef]
- Nazareth, J.; Shaw, A.; Delate, K.; Turnbull, R. Food safety considerations in integrated organic crop-livestock systems: Prevalence of Salmonella spp. and E. coli O157:H7 in organically raised cattle and organic feed. Renew. Agric. Food Syst. 2019, 1–9. [Google Scholar] [CrossRef]
- Murison, R.; Scott, J.M. Statistical methodologies for drawing causal inference from an unreplicated farmlet experiment conducted by the Cicerone Project. Anim. Prod. Sci. 2013, 53, 643–648. [Google Scholar] [CrossRef][Green Version]
- Fisher, D. Defining the experimental unit in grazing trials. J. Anim. Sci. 2000, 77, 1–5. [Google Scholar] [CrossRef]
- Boissy, A.; Le Neindre, P. Behavioral, cardiac and cortisol responses to brief peer separation and reunion in cattle. Physiol. Behav. 1997, 61, 693–699. [Google Scholar] [CrossRef]
- Schmidt, J.R.; Miller, M.C.; Andrae, J.G.; Ellis, S.E.; Duckett, S.K. Effect of summer forage species grazed during finishing on animal performance, carcass quality, and meat quality. J. Anim. Sci. 2013, 91, 4451–4461. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment For Statistical Computing. Available online: http://203.187.160.132:9011/finzi.psych.upenn.edu/c3pr90ntc0td/R/library/dplR/doc/intro-dplR.pdf (accessed on 1 June 2020).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (multi-level/mixed) Regression Models. Available online: https://cran.r-project.org/package=DHARMa (accessed on 27 May 2020).
- Moran, P.A.P. Notes on continuous stochastic phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. Available online: https://cran.r-project.org/package=MuMIn (accessed on 30 March 2020).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Mayland, H.F.; Molloy, L.F.; Collie, T.W. Higher fatty acid composition of immature forages as affected by N fertilization. Agron. J. 1976, 68, 979–982. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Shingfield, K.J.; Lee, M.R.F.; Scollan, N.D. Increasing the concentrations of beneficial polyunsaturated fatty acids in milk produced by dairy cows in high-forage systems. Anim. Feed Sci. Technol. 2006, 131, 168–206. [Google Scholar] [CrossRef]
- Khan, N.A.; Cone, J.W.; Fievez, V.; Hendriks, W.H. Causes of variation in fatty acid content and composition in grass and maize silages. Anim. Feed Sci. Technol. 2012, 174, 36–45. [Google Scholar] [CrossRef]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Buccioni, A.; Decandia, M.; Minieri, S.; Molle, G.; Cabiddu, A. Lipid metabolism in the rumen: New insights on lipolysis and biohydrogenation with an emphasis on the role of endogenous plant factors. Anim. Feed Sci. Technol. 2012, 174, 1–25. [Google Scholar] [CrossRef]
- Dhiman, T.R.; Satter, L.D.; Pariza, M.W.; Galli, M.P.; Albright, K.; Tolosa, M.X. Conjugated linoleic acid (CLA) content of milk from cows offered diets rich in linoleic and linolenic acid. J. Dairy Sci. 2000, 83, 1016–1027. [Google Scholar] [CrossRef]

| Time | Month | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sep | Oct | Nov | Dec | Jan | Feb | Mar | Apr | May | Jun | ||
| Temperature, °C a | |||||||||||
| Mean | |||||||||||
| Study | 14 | 6 | 0 | −6 | −11 | −8 | 0 | 3 | 10 | 16 | 2 |
| Long-term | 15 | 8 | −1 | −9 | −13 | −10 | −3 | 6 | 14 | 19 | 3 |
| Rainfall, mm b | |||||||||||
| Sum | |||||||||||
| Study | 34 | 40 | 47 | 27 | 7 | 17 | 16 | 47 | 51 | 48 | 333 |
| Long-term | 59 | 47 | 25 | 17 | 18 | 18 | 30 | 58 | 76 | 102 | 447 |
| Snowfall, mm b | |||||||||||
| Sum | |||||||||||
| Study | 0 | 0 | 5 | 287 | 112 | 155 | 36 | 36 | 0 | 0 | 630 |
| Long-term | 0 | 18 | 127 | 178 | 178 | 188 | 198 | 84 | 3 | 0 | 973 |
| Fatty Acid | Rye | Wheat | ||
|---|---|---|---|---|
| Day0 | Day46 | Day0 | Day46 | |
| g 100g−1 of total fatty acids | ||||
| Myristic, C14:0 | 0.9 [0.6, 1.3] | 2.5 [2.1, 2.8] | 0.6 [0.3, 1.0] | 1.4 [1.1, 1.8] |
| Palmitic, C16:0 | 17.3 [15.8, 18.7] | 18.3 [17.0, 19.7] | 14.4 [13.0, 15.9] | 21.2 [19.9, 22.6] |
| Stearic, C18:0 | 1.4 [1.1, 1.6] | 1.3 [1.1, 1.5] | 1.2 [1.0, 1.5] | 1.5 [1.3, 1.7] |
| Oleic, C18:1 | 2.0 [1.3, 2.8] | 1.8 [1.0, 2.6] | 2.1 [1.4, 2.8] | 4.6 [3.8, 5.3] |
| Linoleic, C18:2n-6 | 8.2 [7.4, 8.9] | 10.7 [9.9, 11.4] | 6.2 [5.4, 6.9] | 11.8 [11.0, 12.5] |
| α-linolenic, C18:3n-3 | 65.6 [62.8, 68.1] | 61.1 [58.6, 63.5] | 74.1 [71.5, 76.8] | 58.5 [56.0, 61.0] |
| Arachidic, C20:0 | 2.3 [2.0, 2.5] | 1.3 [1.1, 1.6] | 1.3 [1.0, 1.5] | 0.9 [0.6, 1.1] |
| Behenic, C22:0 | 1.0 [0.8, 1.1] | 0.9 [0.7, 1.1] | 1.6 [1.4, 1.8] | 1.4 [1.2, 1.6] |
| Lignoceric, C24:0 | 0.5 [0.4, 0.7] | 0.7 [0.6, 0.9] | 0.4 [0.3, 0.6] | 0.6 [0.5, 0.8] |
| Others 1 | 0.1 | 2.3 | 0.1 | 0.2 |
| n-6:n-3 2 | 0.12 [0.11, 0.14] | 0.17 [0.16, 0.19] | 0.09 [0.07, 0.10] | 0.21 [0.19, 0.22] |
| Fatty Acid | Rye | Wheat | Source of Variation 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β0 | DayL | DayQ | β0 | DayL | DayQ | D | F | F × D | R2(m) | |
| g 100g−1 of total fatty acids | Probability | |||||||||
| Myristic C14:0 | 0.9 [0.6, 1.3] | 0.03 [0.02, 0.04] | 0.6 [0.3, 0.9] | 0.02 [0.01, 0.03] | < 0.0001 | 0.14 | 0.06 | 0.45 | ||
| Palmitic C16:0 | 17.3 [15.9, 18.6] | 0.0 [−0.03, 0.07] | 14.4 [13.1, 15.7] | 0.15 [0.10, 0.20] | < 0.0001 | 0.009 | 0.0009 | 0.27 | ||
| Stearic C18:0 | 1.4 [1.2, 1.6] | −0.002 [−0.010, 0.006] | 1.2 [1.0, 1.4] | 0.006 [−0.002, 0.014] | 0.52 | 0.37 | 0.17 | 0.03 | ||
| Oleic C18:1 | 2.3 [2.0, 2.6] | −0.72 [−3.34, 1.91] | −2.02 [−4.64, 0.61] | 2.4 [2.1, 2.7] | 7.38 [4.75, 10.00] | 5.02 [2.39, 7.64] | 0.0002 | 0.69 | < 0.0001 | 0.32 |
| Linoleic C18:2n-6 | 10.2 [9.8, 10.5] | 7.67 [5.18, 10.10] | −3.92 [−6.45, −1.00] | 8.2 [7.9, 8.5] | 16.97 [14.56, 19.50] | 4.19 [1.46, 6.70] | < 0.0001 | 0.002 | < 0.0001 | 0.77 |
| α-linolenic C18:3n-3 | 65.6 [63.1, 68.1] | −0.10 [−0.19, −0.01] | 74.1 [71.7, 76.6] | −0.34 [−0.43, −0.25] | < 0.0001 | 0.0002 | 0.0006 | 0.41 | ||
| Arachidic C20:0 | 1.5 [1.4, 1.7] | −2.85 [−3.76, −1.94] | 1.44 [0.49, 2.37] | 0.9 [0.8, 1.0] | −1.19 [−2.10, −0.28] | 0.95 [0.01, 1.89] | <.0001 | 0.009 | 0.04 | 0.59 |
| Behenic C22:0 | 1.0 [0.8, 1.1] | −0.001 [−0.007, 0.004] | 1.6 [1.4, 1.8] | −0.005 [−0.011, 0.001] | 0.14 | 0.0001 | 0.37 | 0.46 | ||
| Lignoceric C24:0 | 0.5 [0.4, 0.7] | 0.004 [0.001, 0.008] | 0.4 [0.3, 0.6] | 0.004 [0.000, 0.008] | 0.003 | 0.32 | 0.94 | 0.13 | ||
| n-6:n-3 2 | 0.16 [0.16, 0.17] | 0.154 [0.095, 0.212] | −0.066 [−0.125, −0.008] | 0.13 [0.12, 0.13] | 0.367 [0.308, 0.425] | 0.107 [0.049, 0.166] | < 0.0001 | 0.02 | < 0.0001 | 0.72 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, H.N.; Heins, B.J.; Delate, K.; Turnbull, R. Fatty Acid Composition Dynamics of Rye (Secale cereale L.) and Wheat (Triticum aestivum L.) Forages under Cattle Grazing. Agronomy 2020, 10, 813. https://doi.org/10.3390/agronomy10060813
Phillips HN, Heins BJ, Delate K, Turnbull R. Fatty Acid Composition Dynamics of Rye (Secale cereale L.) and Wheat (Triticum aestivum L.) Forages under Cattle Grazing. Agronomy. 2020; 10(6):813. https://doi.org/10.3390/agronomy10060813
Chicago/Turabian StylePhillips, Hannah N., Bradley J. Heins, Kathleen Delate, and Robert Turnbull. 2020. "Fatty Acid Composition Dynamics of Rye (Secale cereale L.) and Wheat (Triticum aestivum L.) Forages under Cattle Grazing" Agronomy 10, no. 6: 813. https://doi.org/10.3390/agronomy10060813
APA StylePhillips, H. N., Heins, B. J., Delate, K., & Turnbull, R. (2020). Fatty Acid Composition Dynamics of Rye (Secale cereale L.) and Wheat (Triticum aestivum L.) Forages under Cattle Grazing. Agronomy, 10(6), 813. https://doi.org/10.3390/agronomy10060813






