Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants
Abstract
:1. Introduction
2. Heavy Metals (HMs) and Their Impacts
3. Sources of HMs
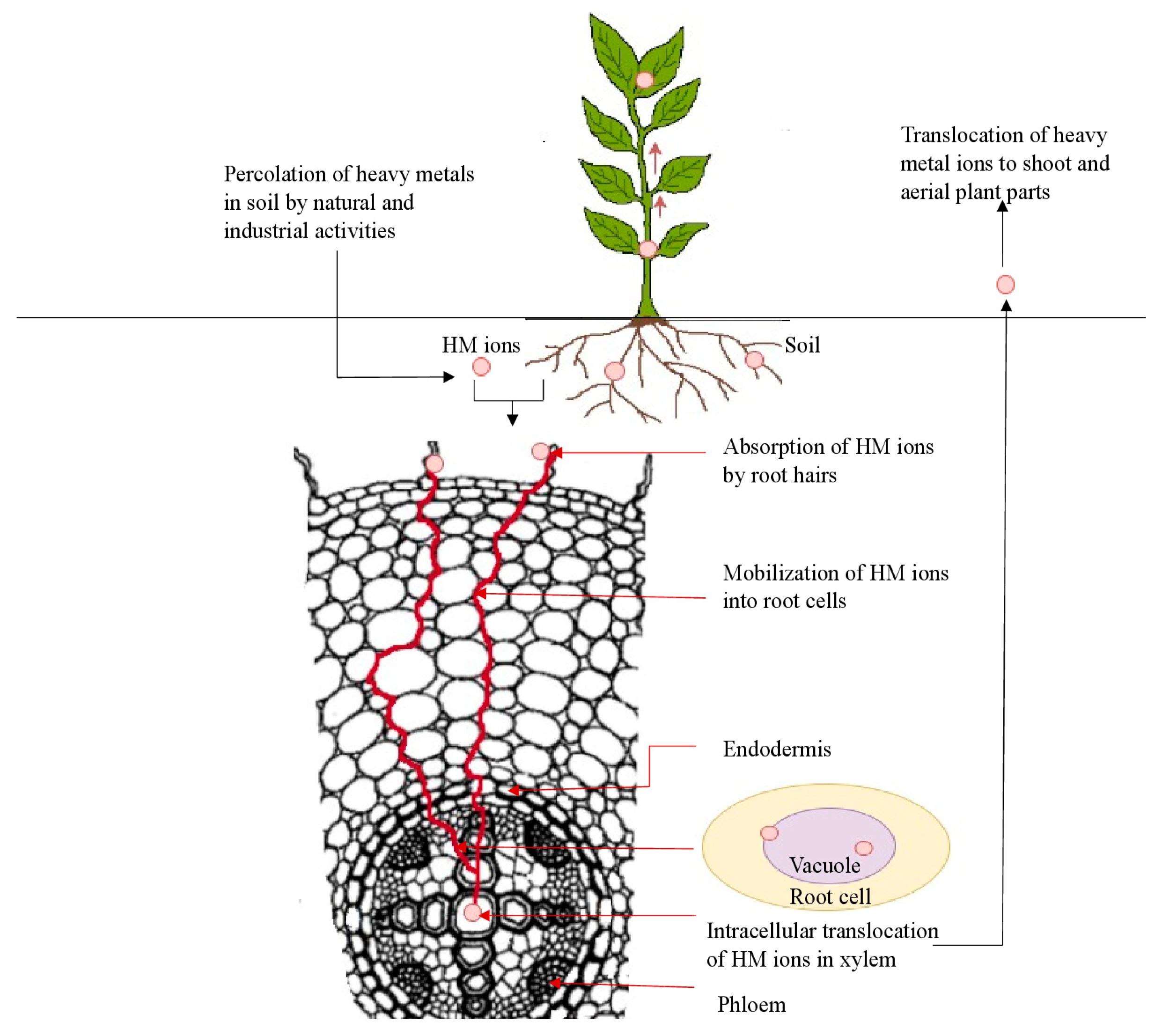
4. Uptake, Translocation and Accumulation of HMs in Plants
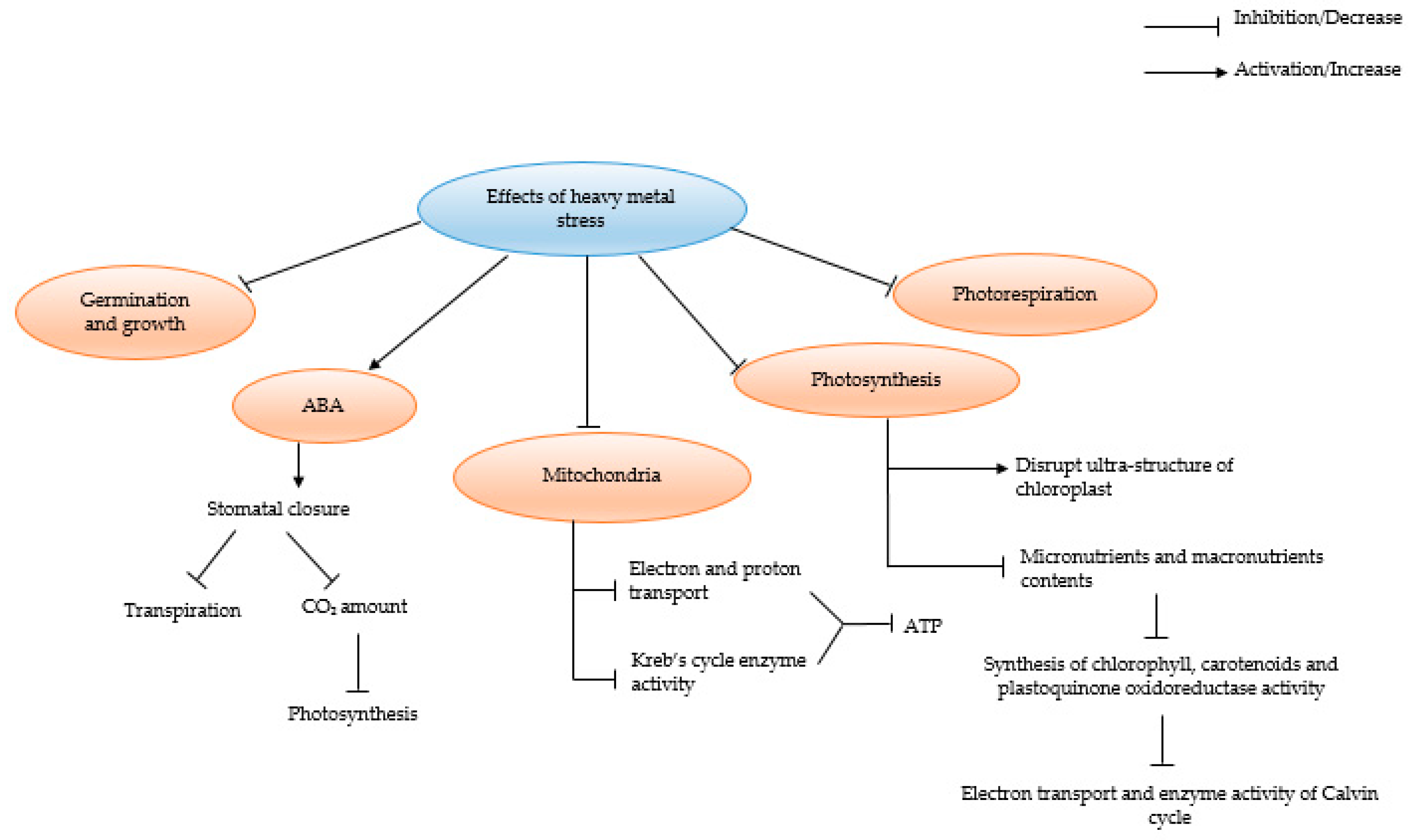
5. Impact of HMs on Plants in the Absence of AMF
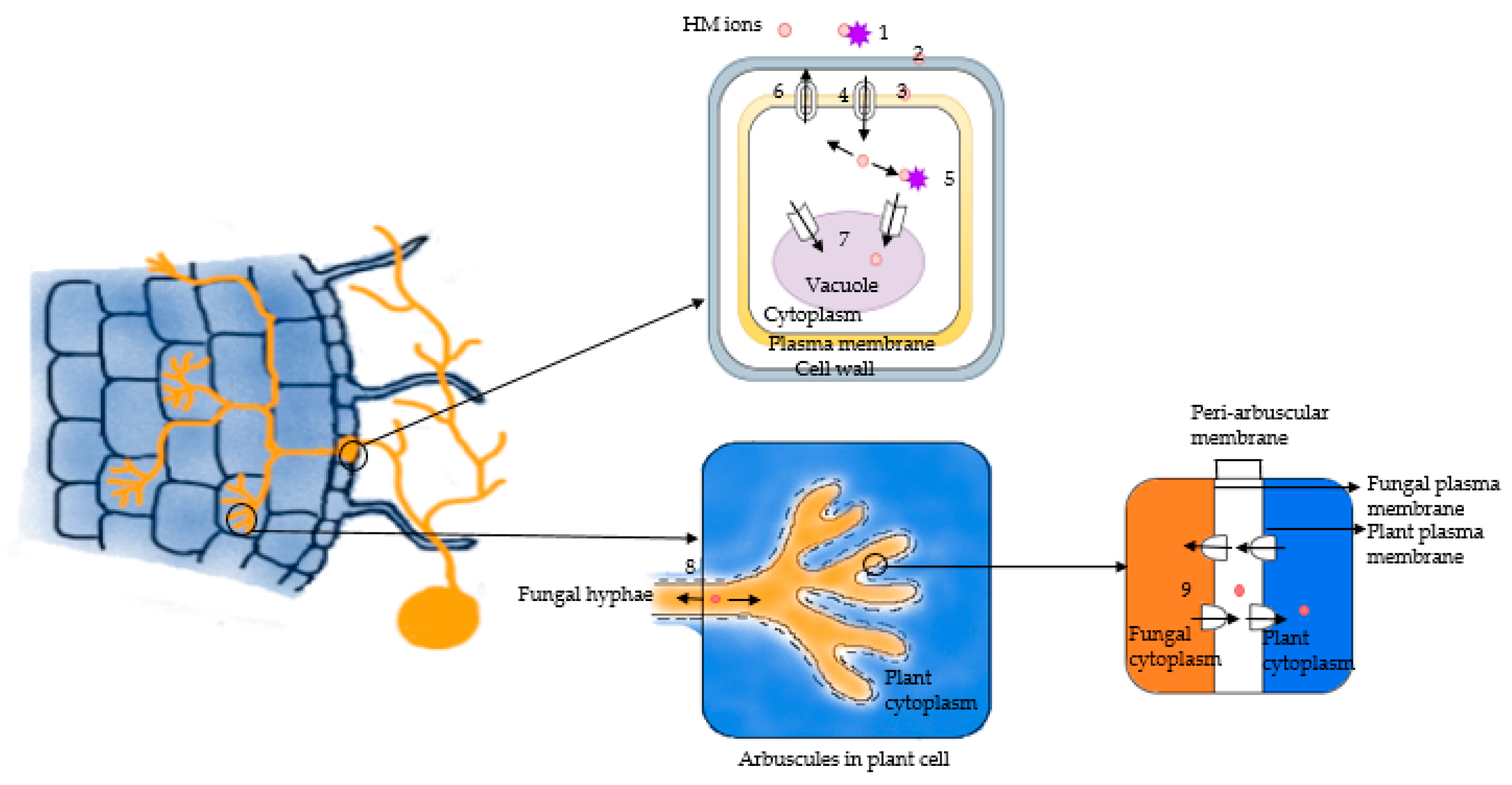
6. Role of AMF in HM Detoxification in Plants
6.1. Retention of HMs in Mycorrhizal Roots and External Hyphae of AMF
6.2. AMF Promotes Nutrient Absorption in Plants
6.3. AMF Sequester HMs in Vacuoles
6.4. AMF Assist in HM Binding on the Fungal Cell Walls
6.5. AMF Protects the PSII Reaction Center and Rectifies the Gas Exchange Capacity
6.6. Heavy Metals Enhance the Antioxidant Responses of Plants
6.7. AMF-Assisted HM Chelation
6.8. Glomalin-Induced Soil Metal Complexes
6.9. AMF-Assisted Phytoremediation of HMs
7. Impact of AMF-Induced Genes on Metal Toxicity
8. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Morkunas, I.; Woźniak, A.; Mai, V.C.; Rucińska-Sobkowiak, R.; Jeandet, P. The role of heavy metals in plant response to biotic stress. Molecules 2018, 23, 2320. [Google Scholar] [CrossRef] [Green Version]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Hadi, P.; Gao, P.; Barford, J.P.; McKay, G. Novel application of the nonmetallic fraction of the recycled printed circuit boards as a toxic heavy metal adsorbent. J. Hazard. Mater. 2013, 252–253, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Dago, A.; Gonzalez, I.; Arino, C.; Diaz-Cruz, J.; Esteban, M. Chemometrics applied to the analysis of induced phytochelatins in Hordeum vulgare plants stressed with various toxic non-essential metals and metalloids. Talanta 2014, 118, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Khan, D.K.; Santra, S.C. Determination of public health hazard potential of wastewater reuse in crop production. World Rev. Sci. Technol. Sustain. Dev. 2010, 7, 328–340. [Google Scholar] [CrossRef]
- Grimm, N.B.; Foster, D.; Groffman, P.; Grove, J.M.; Hopkinson, C.S.; Nadelhoffer, K.J.; Pataki, D.E.; Peters, D.P. The changing landscape: Ecosystem responses to urbanization and pollution across climatic and societal gradients. Front. Ecol. Environ. 2008, 6, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Song, Q.; Tang, Y.; Li, W.; Xu, J.; Wu, J.; Wang, F.; Brookes, P.C. Human health risk assessment of heavy metals in soil–vegetable system: A multi-medium analysis. Sci. Total Environ 2013, 463–464, 530–540. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Miransari, M. Soybean production and heavy metal stress. In Abiotic and Biotic Stresses in Soybean Production; Miransari, M., Ed.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2016; pp. 197–216. [Google Scholar]
- Singh, P.C.; Srivastava, S.; Shukla, D.; Bist, V.; Tripathi, P.; Anand, V.; Arkvanshi, S.K.; Kaur, J.; Srivastava, S. Mycoremediation mechanisms for heavy metal resistance/tolerance in plants. In Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Springer: Cham, Switzerland, 2018; pp. 351–381. [Google Scholar]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 1–18. [Google Scholar] [CrossRef]
- Reddy, A.M.; Kumar, S.G.; Jyonthsnakumari, G.; Thimmanaik, S.; Sudhakar, C. Lead induced changes in antioxidant metabolism of horse gram (Macrotyloma uniflorum (Lam.) Verdc.) and Bengal gram (Cicer arietinum L.). Chemosphere 2005, 60, 97–104. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Chen, R.; Peng, Y.; Wen, X.; Gao, L. Accumulation of heavy metals in tea leaves and potential health risk assessment: A case study from Puan County, Guizhou province, China. Int. J. Environ. Res. Public Health 2018, 15, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Li, J.; Xie, H.; Yu, C. Review on remediation technologies of soil contaminated by heavy metals. Procedia Environ. Sci. 2012, 16, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Bhattacharya, A.; Mishra, N. Mycorrhizal symbiosis: An effective tool for metal bioremediation. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–128. [Google Scholar]
- Chen, S.; Zhao, H.; Zou, C.; Li, Y.; Chen, Y.; Wang, Z.; Jiang, Y.; Liu, A.; Zhao, P.; Wang, M.; et al. Combined inoculation with multiple arbuscular mycorrhizal fungi improves growth, nutrient uptake and photosynthesis in cucumber seedlings. Front. Microbiol. 2017, 8, 2516. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Uniyal, N.; Panneerselvam, P.; Senapati, A.; Ganeshamurthy, A.N. Role of mycorrhiza and its associated bacteria on plant growth promotion and nutrient management in sustainable agriculture. Int. J. Life Sci. Appl. Sci. 2019, 1, 1–10. [Google Scholar]
- Verma, R.; Tapwal, A.; Kumar, D.; Parkash, V.; Puri, S. Vesicular arbuscular mycorrhizal diversity in some important ethnomedicinal plants of Western Himalaya. Med. Plants 2019, 11, 279–285. [Google Scholar] [CrossRef]
- Schubler, A. Molecular phylogeny, taxonomy, and evolution of Geosiphon pyriformis and arbuscular mycorrhizal fungi. Plant Soil 2001, 244, 75–83. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). CERCLA Priority List. Available online: https://www.atsdr.cdc.gov/spl/resources/ATSDR_2017_SPL_Support_Document.pdf (accessed on 2 March 2019).
- Borzsonyi, M.; Bereczky, A.; Rudnai, P.; Csanady, M.; Horvath, A. Epidemiological studies on human subjects exposed to arsenic in drinking water in southeast Hungary. Arch. Toxicol. 1992, 66, 77–78. [Google Scholar] [CrossRef]
- Hopenhayn-Rich, C.; Browning, S.R.; Hertz-Picciotto, I.; Ferreccio, C.; Peralta, C.; Gibb, H. Chronic arsenic exposure and risk of infant mortality in two areas of Chile. Environ. Health Perspect. 2000, 108, 667–673. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Sayed, M.H.; Barua, S.; Khan, M.H.; Faruquee, M.H.; Jalil, A.; Hadi, S.A.; Talukder, H.K. Arsenic in drinking water and pregnancy outcomes. Environ. Health Perspect. 2001, 109, 629–631. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chang, C.C.; Tsai, S.S.; Chuang, H.Y.; Ho, C.K.; Wu, T.N. Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ. Res. 2003, 91, 29–34. [Google Scholar] [CrossRef]
- Von Ehrenstein, O.S.; Guha-Mazumder, D.N.; Hira-Smith, M.; Ghosh, N.; Yuan, Y.; Windham, G.; Ghosh, A.; Haque, R.; Lahiri, S.; Kalman, D.; et al. Pregnancy outcomes, infant mortality, and arsenic in drinking water in West Bengal, India. Am. J. Epidemiol. 2006, 163, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaw, J.; Marshall, G.; Yuan, Y.; Ferreccio, C.; Steinmaus, C.; Smith, A.H. Increased childhood liver cancer mortality and arsenic in drinking water in Northern Chile. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1982–1987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.H.; Goycolea, M.; Haque, R.; Biggs, M.L. Marked increase in bladder and lung cancer mortality in a region of Northern Chile due to arsenic in drinking water. Am. J. Epidemiol. 1998, 147, 660–669. [Google Scholar] [CrossRef]
- Parvez, F.; Chen, Y.; Brandt-Rauf, P.W.; Slavkovich, V.; Islam, T.; Ahmed, A.; Argos, M.; Hassan, R.; Yunus, M.; Haque, S.E.; et al. A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: Findings from the health effects of arsenic longitudinal study (HEALS). Thorax 2010, 65, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.Z.; Xia, Y.J.; Wu, K.G.; Sun, T.Z.; Mumford, J.L. Human exposure to arsenic and health effects in Bayingnormen, inner Mongolia. In Proceedings of the Third International Conference on Arsenic Exposure and Health Effects, San Diego, CA, USA, 12–15 July 1998; pp. 127–131. [Google Scholar]
- Wilberforce, J.O.; Nwabue, F.I. Heavy metals effect due to contamination of vegetables from Enyigba lead mine in Ebonyi State, Nigeria. Environ. Pollut. 2013, 2, 19. [Google Scholar]
- Fang, Y.; Sun, X.; Yang, W.; Ma, N.; Xin, Z.; Fu, J.; Liu, X.; Liu, M.; Mariga, A.M.; Zhu, X.; et al. Concentrations and health risks of lead, cadmium, arsenic, and mercury in rice and edible mushrooms in China. Food Chem. 2014, 147, 147–151. [Google Scholar] [CrossRef]
- Martorell, I.; Perelló, G.; Martí-Cid, R.; Llobet, J.M.; Castell, V.; Domingo, J.L. Human exposure to arsenic, cadmium, mercury, and lead from foods in Catalonia, Spain: Temporal trend. Biol. Trace Elem. Res. 2011, 142, 309–322. [Google Scholar] [CrossRef]
- Swaddiwudhipong, W.; Nguntra, P.; Kaewnate, Y.; Mahasakpan, P.; Limpatanachote, P.; Aunjai, T.; Jeekeeree, W.; Punta, B.; Funkhiew, T.; Phopueng, I. Human health effects from cadmium exposure: Comparison between persons living in cadmium-contaminated and non-contaminated areas in northwestern Thailand. Southeast Asian J. Trop. Med. Public Health 2015, 46, 133–142. [Google Scholar]
- Akesson, A.; Lundh, T.; Vahter, M.; Bjellerup, P.; Lidfeldt, J.; Nerbrand, C.; Samsioe, G.; Strömberg, U.; Skerfving, S. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ. Health Perspect. 2005, 113, 1627–1631. [Google Scholar] [CrossRef]
- Interdonato, M.; Pizzino1, G.; Bitto, A.; Galfo, F.; Irrera, N.; Mecchio, A.; Pallio, G.; Ramistella, V.; Luca, D.F.; Santamaria, A.; et al. Cadmium delays puberty onset and testis growth in adolescents. Clin. Endocrinol. (Oxford) 2015, 83, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Türkdoğan, M.K.; Kilicel, F.; Kara, K.; Tuncer, I.; Uygan, I. Heavy metals in soil, vegetables and fruits in the endemic upper gastrointestinal cancer region of Turkey. Environ. Toxicol. Pharmacol. 2003, 13, 175–179. [Google Scholar] [CrossRef]
- Wang, X.; Sato, T.; Xing, B.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.T.; Kong, Q.; Wang, Z.; Li, P.; Lundstrom, N.G.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 2002, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bihari, V.; Agarwal, S.K.; Verma, V.; Kesavachandran, C.N.; Pangtey, B.S.; Mathur, N.; Singh, K.P.; Srivastava, M.; Goel, S.K. Groundwater contaminated with hexavalent chromium [Cr (VI)]: A health survey and clinical examination of community inhabitants (Kanpur, India). PLoS ONE 2012, 7, e47877. [Google Scholar] [CrossRef] [Green Version]
- Hensawang, S.; Chanpiwat, P. Health impact assessment of arsenic and cadmium intake via rice consumption in Bangkok, Thailand. Environ. Monit. Assess. 2017, 189, 599. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Huang, Z.; Li, R.; Song, Y.; Lan, Z.; Ma, S.; Wu, Y.; Chen, J.; Zhang, L. Dietary cadmium exposure assessment in rural areas of Southwest China. PLoS ONE 2018, 13, e0201454. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Cheng, H.; Liu, X.; Giubilato, E.; Critto, A.; Sun, H.; Zhang, L. Cadmium exposure and early renal effects in the children and adults living in a tungsten-molybdenum mining areas of South China. Environ. Sci. Pollut. Res. 2018, 25, 15089–15101. [Google Scholar] [CrossRef]
- Gibb, H.J.; Lees, P.S.J.; Pinsky, P.F.; Rooney, B.C. Clinical findings of irritation among chromium chemical production workers. Am. J. Ind. Med. 2000, 38, 127–131. [Google Scholar] [CrossRef]
- Pizarro, F.; Olivares, M.; Uauy, C.; Contreras, P.; Rebelo, A.; Gidi, V. Acute gastrointestinal effects of graded levels of copper in drinking water. Environ. Health Perspect. 2001, 107, 117–121. [Google Scholar] [CrossRef]
- Sarvestani, R.A.; Aghasi, M. Health risk assessment of heavy metals exposure (lead, cadmium, and copper) through drinking water consumption in Kerman city, Iran. Environ. Earth Sci. 2019, 78, 714. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Habibullah-Al-Mamun, M. Determination of heavy metals in fish and vegetables in Bangladesh and health implications. Hum. Ecol. Risk Assess. An Int. J. 2015, 21, 986–1006. [Google Scholar] [CrossRef]
- Iwata, T.; Sakamoto, M.; Feng, X.; Yoshida, M.; Liu, X.J.; Dakeishi, M.; Li, P.; Qiu, G.; Jiang, H.; Nakamura, M.; et al. Effects of mercury vapor exposure on neuromotor function in Chinese miners and smelters. Int. Arch. Occup. Environ. Health 2007, 80, 381–387. [Google Scholar] [CrossRef]
- Li, P.; Feng, X.; Qiu, G.; Li, Z.; Fu, X.; Sakamoto, M.; Liu, X.; Wang, D. Mercury exposures and symptoms in smelting workers of artisanal mercury mines in Wuchuan, Guizhou, China. Environ. Res. 2008, 107, 108–114. [Google Scholar] [CrossRef]
- Harada, M.; Nakanishi, J.; Yasoda, E.; Pinheiro, M.D.C.N.; Oikawa, T.; Guimarâes, G.D.A.; Cardoso, B.D.S.; Kizaki, T.; Ohno, H. Mercury pollution in the Tapajos River basin, Amazon mercury level of head hair and health effects. Environ. Int. 2001, 27, 285–290. [Google Scholar] [CrossRef]
- Murata, K.; Araki, S.; Yokoyama, K.; Nomiyama, K.; Nomiyama, H.; Tao, Y.X.; Liu, S.J. Autonomic and central nervous system effects of lead in female glass workers in China. Am. J. Ind. Med. 1995, 28, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Gurer-Orhan, H.; Sabır, H.U.; Özgüneş, H. Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology 2004, 195, 147–154. [Google Scholar] [CrossRef]
- Dongre, N.N.; Suryakar, A.N.; Patil, A.J.; Ambekar, J.G.; Rathi, D.P. Biochemical effects of lead exposure on systolic & diastolic blood pressure, heme biosynthesis and hematological parameters in automobile workers of north Karnataka (India). Indian J. Clin. Biochem. 2011, 26, 400–406. [Google Scholar] [PubMed] [Green Version]
- Cui, Y.; Zhu, Y.G.; Zhai, R.; Huang, Y.; Qiu, Y.; Liang, J. Exposure to metal mixtures and human health impacts in a contaminated area in Nanning, China. Environ. Int. 2005, 31, 784–790. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibi, S.; Farid, G. Role of plant nutrients in minimizing cadmium accumulation by plant. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar]
- Chen, L.; Xu, Z.; Liu, M.; Huang, Y.; Fan, R.; Su, Y.; Hu, G.; Peng, X. Lead exposure assessment from study near a lead-acid battery factory in China. Sci. Total Environ. 2012, 429, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Edelstein, M.; Ben-Hur, M. Heavy metals and metalloids: Sources, risks and strategies to reduce their accumulation in horticultural crops. Sci. Hortic. 2018, 234, 431–444. [Google Scholar] [CrossRef]
- Ward, M.; Smith, G.; Tran, Q. This Report Contains Assessments of Commodity and Trade Issues Made by USDA Staff and Not Necessarily Statements of Official US Government Policy; USDA Foreign Agricultural Service: Washington, DC, USA, 2016; Volume 11.
- Chary, N.S.; Kamala, C.T.; Raj, D.S.S. Assessing risk of heavy metals from consuming food grow non sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf. 2008, 69, 513–524. [Google Scholar] [CrossRef]
- Cai, Q.; Long, M.L.; Zhu, M.; Zhou, Q.Z.; Zhang, L.; Liu, J. Food chain transfer of cadmium and lead to cattle in a lead-zinc smelter in Guizhou, China. Environ. Pollut. 2009, 157, 3078–3082. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.A.; Belal, M.H.; Abou-Arab, A.A.K.; Gad, M.F. Monitoring of pesticides and heavy metals in cucumber fruits produced from different farming systems. Chemosphere 2009, 75, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Ma, Y.; Zhang, S.; Wei, D.; Zhu, Y.G. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag. 2009, 90, 2524–2530. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, Y.; Zhang, Z.; Dai, J.; Dai, B.; Zhu, Y. Identifying the origins and spatial distributions of heavy metals in soils of Ju country (Eastern China) using multivariate and geostatistical approach. J. Soils Sediments 2014, 15, 163–178. [Google Scholar] [CrossRef]
- Gall, J.E.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy metals through terrestrial food webs: A review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [Green Version]
- Elgallal, M.; Fletcher, L.; Evans, B. Assessment of potential risks associated with chemicals in waste water used for irrigation in arid and semiarid zones: A review. Agric. Water Manag. 2016, 177, 419–431. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Itanna, F.; Gebrekidan, H. Heavy metal accumulation and health risk assessment in wastewater-irrigated urban vegetable farming sites of Addis Ababa, Ethiopia. Int. J. Food Contam. 2017, 4, 9. [Google Scholar] [CrossRef]
- El-Kady, A.A.; Abdel-Wahhab, M.A. Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci. Technol. 2018, 75, 36–45. [Google Scholar] [CrossRef]
- Waterlot, C.; Pruvot, C.; Marot, F.; Douay, F. Impact of a phosphate amendment on the environmental availability and phytoavailability of Cd and Pb in moderately and highly carbonated kitchen garden soils. Pedosphere 2017, 27, 588–605. [Google Scholar] [CrossRef]
- Wang, H.; Shan, X.; Wen, B.; Owens, G.; Fang, J.; Zhang, S. Effect of indole-3-acetic acid on lead accumulation in maize (Zea mays L.) seedlings and the relevant antioxidant response. Environ. Exp. Bot. 2007, 61, 246–253. [Google Scholar] [CrossRef]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. Biophys. Acta 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Grennan, A.K. Identification of genes involved in metal transport in plants. Plant Physiol. 2009, 149, 1623–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, C.C.; Melo, J.O.; Magalhães, J.V.; Guimarães, C.T. Genetic and molecular mechanisms of aluminum tolerance in plants. Genet. Mol. Res. 2012, 11, 1949–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, S.D.; Martin, E.S. A histochemical investigation of lead uptake in Raphanus sativus. New Phytol. 1977, 79, 281–286. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanov, V.B. Physiological aspects of cadmium and lead toxic effects on higher plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Seregin, I.V.; Shpigun, L.K.; Ivanov, V.B. Distribution and toxic effects of cadmium and lead on maize roots. Russ. J. Plant Physiol. 2004, 51, 525–533. [Google Scholar] [CrossRef]
- Verma, S.; Dubey, R.S. Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Zaray, G.; Fodor, F.; Cseh, E. Study of interaction of iron and lead during their uptake process in wheat roots by total-reflection X-ray fluorescence spectrometry. Spectrochim. Acta Part B 1997, 52, 1027–1032. [Google Scholar] [CrossRef]
- Lopez, M.L.; Peralta-Videa, J.R.; Parsons, J.G.; Gardea-Torresdey, J.L.; Duarte-Gardea, M. Effect of indole-3-acetic acid, kinetin, and ethylene diamine tetra acetic acid on plant growth and uptake and translocation of lead, micronutrients, and macronutrients in alfalfa plants. Int. J. Phytoremediation 2009, 11, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.C.; Gardea-Torresdey, J.L.; Parsons, J.; Sahi, S.V. Chemical speciation and cellular deposition of lead in Sesbania drummondii. Environ. Toxicol. Chem. 2004, 23, 2068–2073. [Google Scholar] [CrossRef] [Green Version]
- Islam, E.; Yang, X.; Li, T.; Liu, D.; Jin, X.; Meng, F. Effect of Pb toxicity on root morphology, physiology and ultrastructure in the two ecotypes of Elsholtzia argyi. J. Hazard. Mater. 2007, 147, 806–816. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Asher, C.J.; Kopittke, R.A.; Menzies, N.W. Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environ. Pollut. 2007, 150, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Meyers, D.E.R.; Auchterlonie, G.J.; Webb, R.I.; Wood, B. Uptake and localisation of lead in the root system of Brassica juncea. Environ. Pollut. 2008, 153, 323–332. [Google Scholar] [CrossRef]
- Arias, J.A.; Peralta-Videa, J.R.; Ellzey, J.T.; Ren, M.; Viveros, M.N.; Gardea-Torresdey, J.L. Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environ. Exp. Bot. 2010, 68, 139–148. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Shahid, M.; Khalid, S.; Abbas, G.; Shahid, N.; Nadeem, M.; Sabir, M.; Aslam, M.; Dumat, C. Heavy metal stress and crop productivity. In Crop Production and Global Environmental Issues; Hakeem, K.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–26. [Google Scholar]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef]
- Bi, X.; Ren, L.; Gong, M.; He, Y.; Wang, L.; Ma, Z. Transfer of cadmium and lead from soil to mangoes in an uncontaminated area, Hainan Island, China. Geoderma 2010, 155, 115–120. [Google Scholar] [CrossRef]
- Mirecki, N.; Agič, R.; Šunić, L.; Milenković, L.; Ilić, Z.S. Transfer factor as indicator of heavy metals content in plants. Fresenius Environ. Bull. 2015, 24, 4212–4218. [Google Scholar]
- Abdelhameed, R.E.; Metwally, R.A. Alleviation of cadmium stress by arbuscular mycorrhizal symbiosis. Int. J. Phytoremediation 2019, 21, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.; Silvestre, J.; Pinelli, E.; Kallerhoff, J.; Kaemmerer, M.; Tarigo, A.; Shahid, M.; Guiresse, M.; Pradere, P.; Dumat, C. A field study of lead phytoextraction by various scented Pelargonium cultivars. Chemosphere 2008, 71, 2187–2192. [Google Scholar] [CrossRef] [Green Version]
- Brunet, J.; Varrault, G.; Zuily-Fodil, Y.; Repellin, A. Accumulation of lead in the roots of grass pea (Lathyrus sativus L.) plants triggers systemic variation in gene expression in the shoots. Chemosphere 2009, 77, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Bini, C.; Wahsha, M.; Fontana, S.; Maleci, L. Effects of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J. Geochem. Explor. 2012, 123, 101–108. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Küpper, H.; Šetlik, I.; Spiller, M.; Kupper, F.C.; Prášil, O. Heavy metal-induced inhibition of photosynthesis: Targets of in vivo heavy metal chlorophyll formation. J. Phycol. 2002, 38, 429–441. [Google Scholar] [CrossRef]
- Küpper, H.; Šetlík, I.; Šetliková, E.; Ferimazova, N.; Spiller, M.; Küpper, F.C. Copper-induced inhibition of photosynthesis: Limiting steps of in vivo copper chlorophyll formation in Scenedesmus quadricauda. Funct. Plant Biol. 2003, 30, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Chen, T.; Chen, Y.; Lei, M. Role of trichome of Pteris vittata L. in arsenic hyper accumulation. Sci. China C Life Sci. 2005, 48, 148–154. [Google Scholar] [PubMed]
- Rasouli-Sadaghiani, M.H.; Barin, M.; Khodaverdiloo, H.; Moghaddam, S.S.; Damalas, C.A.; Kazemalilou, S. Arbuscular mycorrhizal fungi and rhizobacteria promote growth of Russian knapweed (Acroptilon repens L.) in a Cd-contaminated soil. J. Plant Growth Regul. 2019, 38, 113–121. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Liang, Y.; Ghosh, A.; Chen, J.; Tang, M. The Combined effectsof arbuscular mycorrhizal fungi (AMF) and lead (Pb) stresson Pb accumulation, plant growth parameters, photosynthesis, and antioxidant enzymesin Robinia pseudoacacia L. PLoS ONE 2015, 10, e0145726. [Google Scholar] [CrossRef] [PubMed]
- Sengar, R.S.; Gautam, M.; Sengar, R.S.; Garg, S.K.; Sengar, K.; Chaudhary, R. Lead stress effects on physiobiochemical activities of higher plants. Rev. Environ. Contam. Toxicol. 2008, 196, 73–93. [Google Scholar]
- Bonifacio, R.S.; Montaño, M.N. Inhibitory effects of mercury and cadmium on seed germination of Enhalus acoroides (L.f.) Royle. Bull. Environ. Contam. Toxicol 1998, 60, 45–51. [Google Scholar] [PubMed]
- Tomulescu, I.M.; Radoviciu, E.M.; Merca, V.V.; Tuduce, A.D. Effect of copper, zinc and lead and their combinations on the germination capacity of two cereals. J. Agric. Sci. 2004, 15, 39–42. [Google Scholar] [CrossRef]
- Muhammad, Z.I.; Maria, K.S.; Mohammad, A.; Muhammad, S.; Zia-Ur-Rehman, F.; Muhammad, K. Effect of mercury on seed germination and seedling growth of Mungbean (Vigna radiata (L.) Wilczek). J. Appl. Sci. Environ. Manag. 2015, 19, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.; Santos, C.; Mann, R.M.; Soares, A.M.V.M.; Lopes, T. Evaluation of cadmium genotoxicity in Lactuca sativa L. using nuclear microsatellites. Environ. Exp. Bot. 2007, 60, 421–427. [Google Scholar] [CrossRef]
- Roth, J.S.; Inglis, L.; Bachmurski, D. Ribonuclease: VIII studies on the inactive ribonuclease in rat liver the supernatant fraction of rat liver. J. Biol. Chem. 1958, 231, 1097–1106. [Google Scholar]
- Jana, S.; Choudhuri, M.A. Senescence in submerged aquatic angiosperms: Effects of heavy metals. New Phytol. 1982, 90, 477–484. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Titov, V.; Hohn, B.; Kovalchuk, O. Transcriptome profiling reveals similarities and differences in plant responses to cadmium and lead. Mutat. Res. 2005, 570, 149–161. [Google Scholar] [CrossRef]
- Gopal, R.; Rizvi, A.H. Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere 2008, 70, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Al Huqail, A.A.; Egamberdieva, D.; Wirth, S. Alleviation of cadmium stress in Solanum lycopersicum L. by arbuscular mycorrhizal fungi via induction of acquired systemic tolerance. Saudi J. Biol. Sci. 2016, 23, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Abd-Allah, E.F.; Abeer, H.; Alqarawi, A.A.; Hend, A. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015, 47, 785–795. [Google Scholar]
- Deng, H.; Li, M.S.; Chen, Y.X.; Luo, Y.P.; Yu, F.M. A new discovered manganese hyperaccumulator-Polygonum pubescens Blume. Fresenisu Environ Bull. 2010, 19, 94–99. [Google Scholar]
- Iannone, M.F.; Rosales, E.P.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf disks exposed to cadmium. Protoplasma 2010, 245, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Roelfsema, M.R.G.; Hedrich, R. In the light of stomatal opening: New insights into “the Watergate”. New Phytol. 2005, 167, 665–691. [Google Scholar] [CrossRef]
- Yang, H.M.; Zhang, X.Y.; Wang, G.X. Effects of heavy metals on stomatal movements in broad bean leaves. Russ. J. Plant Physiol. 2004, 51, 464–468. [Google Scholar] [CrossRef]
- Chandra, R.; Kang, H. Mixed heavy metal stress on photosynthesis, transpiration rate, and chlorophyll content in poplar hybrids. For. Sci. Technol. 2015, 12, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, U.; Kanu, A.S.; Mo, Z.; Hussain, S.; Anjum, S.A.; Khan, I.; Abbas, R.N.; Tang, X. Lead toxicity in rice: Effects, mechanisms, and mitigation strategies—A mini review. Environ. Sci. Pollut. Res. 2015, 22, 18318–18332. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouziad, F.; Hildebrandt, U.; Schmelzer, E.; Bothe, H. Differential gene expressions in arbuscular mycorrhizal-colonized tomato grown under heavy metal stress. J. Plant Physiol. 2005, 162, 634–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Punamiya, P.; Datta, R.; Sarkar, D.; Barber, S.; Patel, M.; Das, P. Symbiotic role of Glomusmosseae in phytoextraction of lead in vetiver grass Chrysopogon zizanioides L. J. Hazard. Mater. 2010, 177, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. The symbionts forming arbuscular mycorrhizas. In Mycorrhizal Symbiosis; Smith, E.S., Read, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 13–41. [Google Scholar]
- Vogel Mikuš, K.; Pongrac, P.; Kump, P.; Nečemer, M.; Regvar, M. Colonisation of a Zn, Cd and Pb hyper accumulator Thlaspi praecoxWulfen with indigenous arbuscular mycorrhizal fungal mixture induces changes in heavy metal and nutrient uptake. Environ. Pollut. 2006, 139, 362–371. [Google Scholar] [CrossRef]
- Göhre, Y.; Paszkowski, U. Contribution of the arbuscular mycorrhizal symbiosis to heavy metal phytoremediation. Planta 2006, 223, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Zhipeng, W.U.; Weidong, W.U.; Shenglu, Z.H.O.U.; Shaohua, W.U. Mycorrhizal inoculation affects Pb and Cd accumulation and translocation in Pakchoi (Brassica chinensis L.). Pedosphere 2016, 26, 13–26. [Google Scholar]
- Carvalho, L.M.; Cacador, I.; Martins-Loucao, M.A. Arbuscular mycorrhizal fungi enhance root cadmium and copper accumulation in the roots of the salt marsh plant Aster tripolium L. Plant Soil 2006, 285, 161–169. [Google Scholar] [CrossRef]
- Jankong, P.; Visoottiviseth, P. Effects of arbuscular mycorrhizal inoculation on plants growing on arsenic contaminated soil. Chemosphere 2008, 72, 1092–1097. [Google Scholar] [CrossRef]
- Janeeshma, E.; Puthur, J.T. Direct and indirect influence of arbuscular mycorrhizae on enhancing metal tolerance of plants. Arch. Microbiol. 2020, 202, 1–16. [Google Scholar] [CrossRef]
- Bi, Y.; Xiao, L.; Liu, R. Response of arbuscular mycorrhizal fungi and phosphorus solubilizing bacteria to remediation abandoned solid waste of coal mine. Int. J. Coal Sci. Technol. 2019, 6, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Johri, A.K.; Oelmüller, R.; Dua, M.; Yadav, V.; Kumar, M.; Tuteja, N.; Varma, A.; Bonfante, P.; Persson, B.L.; Stroud, R.M. Fungal association and utilization of phosphate by plants: Success, limitations, and future prospects. Front. Microbiol. 2015, 16, 984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benedetto, A.; Magurno, F.; Bonfante, P.; Lanfranco, L. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 2005, 15, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Mendoza, I.E.; Dewbre, G.R.; Harrison, M.J. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol. Plant Microbe Interact. 2001, 14, 1140–1148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, M.J.; van Buuren, M.L. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 1995, 378, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Porras-Soriano, L.Z.; Soriano-Martín, M.L.; Porras-Piedra, A.; Azcón, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 2009, 166, 1350–1359. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Al-Mana, F.A.; El-Hendawy, S.; Al-Selwey, W.A.; Elgorban, A.M. Arbuscular mycorrhizal fungi mitigates heavy metal toxicity adverse effects in sewage water contaminated soil on Tagetes erecta L. Soil Sci. Plant Nutr. 2018, 64, 662–668. [Google Scholar] [CrossRef] [Green Version]
- Garg, N.; Singla, P.; Bhandari, P. Metal uptake, oxidative metabolism, and mycorrhization in pigeon pea and pea under arsenic and cadmium stress. Turk. J. Agric. For. 2015, 39, 234–250. [Google Scholar] [CrossRef]
- Garg, N.; Singla, P. The role of Glomus mosseae on key physiological and biochemical parameters of pea plants grown in arsenic contaminated soil. Sci. Hortic. 2012, 143, 92–101. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Chen, S.; Polle, A.; Rennenberg, H.; Luo, Z.B. Physiological and molecular mechanisms of heavy metal accumulation in non mycorrhizal versus mycorrhizal plants. Plant Cell Environ. 2019, 42, 1087–1103. [Google Scholar] [CrossRef]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Evolution of metal tolerance in higher plants. For. Snow Landsc. Res. 2006, 80, 251–274. [Google Scholar]
- Lux, A.; Martinka, M.; Vaculik, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2010, 62, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Chu, C. Towards understanding plant response to heavy metal stress. In Abiotic Stress in Plants-Mechanisms and Adaptations; Shanker, A., Venkateswarlu, B., Eds.; InTech Open: London, UK, 2011; pp. 59–78. [Google Scholar]
- Soudek, P.; Petrova, S.; Vankova, R.; Song, J.; Vanek, T. Accumulation of heavy metals using Sorghum sp. Chemosphere 2014, 104, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Vogeli-Lange, R.; Wagner, G.J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: Implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990, 92, 1086–1093. [Google Scholar] [CrossRef] [Green Version]
- Joiner, E.J.; Briones, R.; Leyval, C. Metal binding capacity of arbuscular mycorrhizal mycelium. Plant Soil 2000, 226, 227–234. [Google Scholar] [CrossRef]
- Cornejo, P.; Pérez-Tienda, J.; Meier, S.; Valderas, A.; Borie, F.; Azcón-Aguilar, C.; Ferrol, N. Copper compartmentalization in spores as a survival strategy of arbuscular mycorrhizal fungi in Cu-polluted environments. Soil Biol. Biochem. 2013, 57, 925–928. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, M.; Melville, L.H.; Ferrol, N.; Lott, J.N.; Azcon-Aguilar, C.; Peterson, R.L. Ultrastructural localization of heavy metals in the extraradical mycelium and spores of the arbuscular mycorrhizal fungus Glomus intraradices. Can. J. Microbiol. 2008, 54, 103–110. [Google Scholar] [CrossRef]
- Zhang, X.F.; Hu, Z.H.; Yan, T.X.; Lu, R.R.; Peng, C.L.; Li, S.S.; Jing, Y.X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.; Venkatramanan, V.; Senthilkumar, M.; Anandham, R.; Kumutha, K.; Sa, T. Management of Heavy Metal Polluted Soils: Perspective of Arbuscular Mycorrhizal Fungi. In Sustainable Green Technologies for Environmental Management; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer: Singapore, 2019; pp. 67–85. [Google Scholar]
- Wu, Q.S.; Zou, Y.N.; AbduAllah, E.F. Mycorrhizal association and ros in plants. In Oxidative Damage to Plants; Ahmad, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 453–475. [Google Scholar]
- Garg, N.; Kaur, H. Response of Antioxidant Enzymes, Phytochelatins and Glutathione Production Towards Cd and Zn Stresses in Cajanus cajan (L.) Mill sp. Genotypes Colonized by Arbuscular Mycorrhizal Fungi. J. Agron. Crop Sci. 2013, 199, 118–133. [Google Scholar] [CrossRef]
- Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of metal/metalloid chelation trade in plants-an overview. Front. Plant Sci. 2015, 6, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Millsp. under NaCl and Cd stresses. J. Plant Growth Regul. 2012, 31, 292–308. [Google Scholar] [CrossRef]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- Grill, E.; Loffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Feng, Y.; He, Z.; Stoffella, P. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Y.; Zhuo, F.; Long, S.H.; Zhao, H.D.; Yang, D.J.; Ye, Z.H.; Li, S.S.; Jing, Y.X. Can arbuscular mycorrhizal fungi reduce Cd uptake and alleviate Cd toxicity of Lonicera japonica grown in Cd-added soils? Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Degola, F.; Fattorini, L.; Bona, E.; Sprimuto, C.T.; Argese, E.; Berta, G.; di Toppi, L.S. The symbiosis between Nicotiana tabacum and the endomycorrhizal fungus Funneliformis mosseae increases the plant glutathione level and decreases leaf cadmium and root arsenic contents. Plant Physiol. Biochem. 2015, 92, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Jia, D.U.; Jing-Li, Y.; Cheng-Hao, L.I. Advances in metallothionein studies in forest trees. Plant Omics 2012, 5, 46–51. [Google Scholar]
- Hasegawa, I.; Terada, E.; Sunairi, M.; Wakita, H.; Shinmachi, F.; Noguchi, A.; Nakajima, M.; Yazaki, J. Genetic improvement of heavy metal tolerance in plants by transfer of the yeast metallothionein gene (CUP1). Plant Soil 1997, 196, 277–281. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, G.; Zhou, L.; Li, Y.; Liu, J. Expression patterns of the rice class I metallothionein gene family in response to lead stress in rice seedlings and functional complementation of its members in lead-sensitive yeast cells. Chin. Sci. Bull. 2007, 52, 2203–2209. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Guan, H.; Ma, L.; Chen, Z.; Gu, H.; Qu, L.J. Transcriptional profiling of Arabidopsis seedlings in response to heavy metal lead (Pb). Environ. Exp. Bot. 2009, 67, 377–386. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Driver, J.D.; Holben, W.E.; Rillig, M.C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2005, 37, 101–106. [Google Scholar] [CrossRef]
- Singh, P.K.; Singh, M.; Tripathi, B.N. Glomalin: An arbuscular mycorrhizal fungal soil protein. Protoplasma 2012, 250, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R. Use of fungi in mitigating cadmium toxicity in plants. In Cadmium Toxicity and Tolerance in Plants; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 397–426. [Google Scholar]
- Chern, E.C.; Tsai, D.W.; Ogunseitan, O.A. Deposition of glomalin-related soil protein and sequestered toxic metals into water sheds. Environ. Sci. Technol. 2007, 41, 3566–3572. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Chavez, M.C.; Carrillo-Gonzalez, R.; Gutierrez-Castorena, M.C. Natural attenuation in a slag heap contaminated with cadmium: The role of plants and arbuscular mycorrhizal fungi. J. Hazard. Mater. 2009, 161, 1288–1298. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. Erratum: A fern that hyperaccumulates arsenic. Nature 2001, 411, 438. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.; Biswas, A.K. Role of higher plants in remediation of metal contaminated sites. Sci. Rev. Chem. Commun. 2012, 2, 141–146. [Google Scholar]
- Meier, S.; Borie, F.; Bolan, N.; Cornejo, P. Phytoremediation of metal-polluted soils by arbuscular mycorrhizal fungi. Crit. Rev. Environ. Sci. Technol. 2012, 42, 741–775. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Bai, J.; Wang, J.; Yin, R.; Wang, J.; Lin, X. Arbuscular mycorrhizal fungi alleviate the heavy metal toxicity on sunflower (Helianthus annuus L.) plants cultivated on a heavily contaminated field soil at a WEEE-recycling site. Sci. Total Environ. 2018, 628–629, 282–290. [Google Scholar] [CrossRef]
- Pivetz, B.E. Phytoremediation of Contaminated Soil and Ground Water at Hazardous Waste Sites; US Environmental Protection Agency, Office of Research and Development, Office of Solid Waste and Emergency Response: Washington, DC, USA, 2001. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/epa_540_s01_500.pdf (accessed on 10 July 2019).
- Ma, Y.; Prasad, M.N.V.; Rajkumar, M.; Freitas, H. Plant growth promoting rhizobacteria and endophytes accelerate phytoremediation of metalliferous soils. Biotechnol. Adv. 2011, 29, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Hashem, A.; Rasool, S.; Abd_Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D.; Jan, S.; Anjum, N.A.; Ahmad, P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016, 59, 407–426. [Google Scholar] [CrossRef]
- Lanfranco, L.; Bolchi, A.; Ros, E.C.; Ottonello, S.; Bonfante, P. Differential expression of a metallothionein gene during the presymbiotic versus the symbiotic phase of an arbuscular mycorrhizal fungus. Plant Physiol. 2002, 130, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Guerrero, M.; Benabdellah, K.; Valderas, A.; Azcón-Aguilar, C.; Ferrol, N. GintABC1 encodes a putative ABC transporter of the MRP subfamily induced by Cu, Cd, and oxidative stress in Glomus intraradices. Mycorrhiza 2010, 20, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Azcón, R.; Medina, A.; Aroca, R.; Ruiz-Lozano, J.M. Abiotic stress remediation by the arbuscular mycorrhizal symbiosis and rhizosphere bacteria/yeast interactions. Mol. Microb. Ecol. Rhizosphere 2013, 1, 991–1002. [Google Scholar]
- González-Guerrero, M.; Cano, C.; Azcón-Aguilar, C.; Ferrol, N. GintMT1 encodes a functional metallothionein in Glomus intraradices that responds to oxidative stress. Mycorrhiza 2007, 17, 327–335. [Google Scholar] [CrossRef]
- González-Guerrero, M.; Azcón-Aguilar, C.; Mooney, M.; Valderas, A.; MacDiarmid, C.W.; Eide, D.J.; Ferrol, N. Characterization of a Glomus intraradices gene encoding a putative Zn transporter of the cation diffusion facilitator family. Fungal Genet. Biol. 2005, 42, 130–140. [Google Scholar] [CrossRef]
- Stommel, M.; Mann, P.; Franken, P. EST-library construction using spore RNA of the arbuscular mycorrhizal fungus Gigaspora rosea. Mycorrhiza 2001, 10, 281–285. [Google Scholar] [CrossRef]
- Benabdellah, K.; Merlos, M.Á.; Azcón-Aguilar, C.; Ferrol, N. GintGRX1, the first characterized glomeromycotan glutaredoxin, is a multifunctional enzyme that responds to oxidative stress. Fungal Genet. Biol. 2009, 46, 94–103. [Google Scholar] [CrossRef]
- Benabdellah, K.; Azcón-Aguilar, C.; Valderas, A.; Speziga, D.; Fitzpatrick, T.B.; Ferrol, N. GintPDX1 encodes a protein involved in vitamin B6 biosynthesis that is up-regulated by oxidative stress in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2009, 184, 682–693. [Google Scholar] [CrossRef]
- Hildebrandt, U.; Regvar, M.; Bothe, H. Arbuscular mycorrhiza and heavy metal tolerance. Phytochemistry 2007, 38, 139–146. [Google Scholar] [CrossRef] [PubMed]
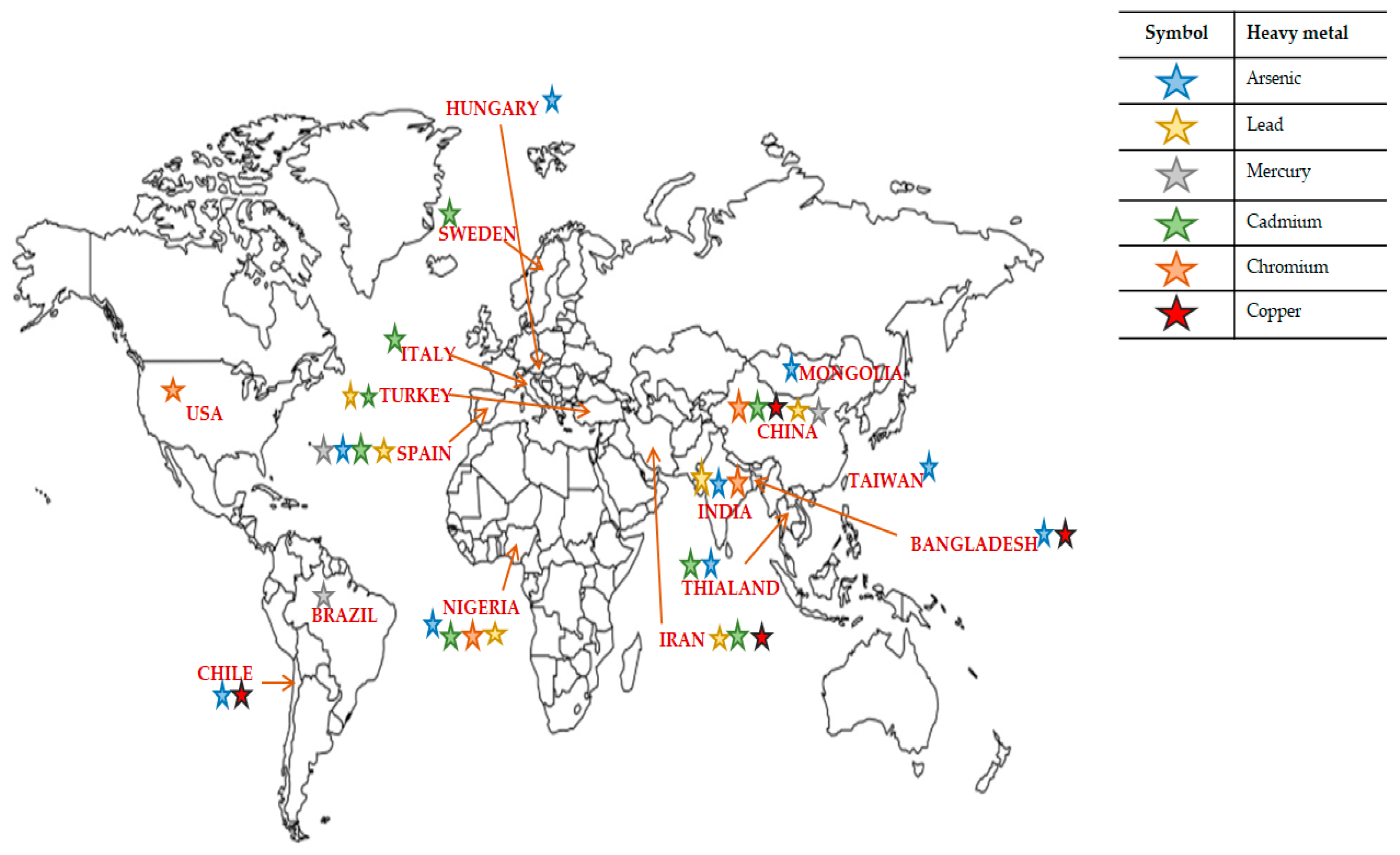

| Heavy Metal | Source | Country | Biological Effects | Ref |
|---|---|---|---|---|
| Arsenic (As) | Drinking water | Hungary | Increase in stillbirths and spontaneous abortion | [22] |
| Drinking water | Chile | Increase in stillbirths and infant mortality | [23] | |
| Drinking water | Bangladesh | Increase in spontaneous abortions, stillbirths and preterm births | [24] | |
| Drinking water | Taiwan | Reduced birth rate | [25] | |
| Drinking water | India | Increase in stillbirths | [26] | |
| Drinking water | Chile | Increased liver cancer mortality for ages 10–19 | [27] | |
| Drinking water | Chile | Increased lung cancer and chronic obstructive pulmonary disease (COPD) deaths for ages 30–39 | [28] | |
| Drinking water | Bangladesh | Chronic cough, breathing problems or blood in the sputum of 39 aged people | [29] | |
| Drinking water | Mongolia | Skin hyperkeratosis, hyperpigmentation and depigmentation and other skin lesions among men | [30] | |
| Soil and vegetables | Nigeria | Liver damage, gastro-intestinal effects, lung cancer and skin lesions | [31] | |
| Rice and edible mushrooms | China | NS | [32] | |
| Food | Spain | NS | [33] | |
| Cadmium (Cd) | Soil | Thailand | High prevalence of renal dysfunction, bone mineral loss, hypertension and urinary stones | [34] |
| NS | Sweden | Tubular and glomerular kidney effects in women | [35] | |
| Industrial plants | Italy | Delayed onset of puberty in male adolescents and impaired testicular growth | [36] | |
| Soil and vegetables | Turkey | Gastro-intestinal, renal prostate and ovarian cancer | [37] | |
| Soil | Nigeria | Damage to central nervous system | [31] | |
| Rice and edible mushrooms | China | NS | [32] | |
| Vegetables and Fish | China | NS | [38] | |
| Food | Spain | NS | [33] | |
| Smelting | China | Renal dysfunction | [39] | |
| Rice | Thailand | Kidney, lung and liver problems | [40] | |
| Food | South west china | Bone damage, kidney injury and cancer | [41] | |
| Mining areas | South china | Renal effects, particularly in children | [42] | |
| Drinking water | Iran | NS | [43] | |
| Chromium (Cr) | Ground water | India | Gastrointestinal and dermatological complaints and abnormal hematological function | [44] |
| Chromate production plant | USA | Nasal irritation and nasal ulceration | [45] | |
| Vegetables | Nigeria | Respiratory problems, lung cancer and skin rashes | [31] | |
| Vegetables | China | NS | [38] | |
| Copper (Cu) | Drinking water | Chile | Nausea, abdominal pain or vomiting | [46] |
| Vegetables | Bangladesh | Kidney damage or tumors | [47] | |
| Vegetables | China | NS | [38] | |
| Drinking water | Iran | NS | [43] | |
| Mercury (Hg) | Mercury mining sites | China | Postural sway, as well as hand tremor, may be affected by elemental mercury vapor exposure | [48] |
| Mercury mines | China | Finger and eyelid tremor, gingivitis, and typical darkline on gums | [49] | |
| Tapajos river basin | Brazil | Sensory disturbance (especially glove-and-stocking type, which is characteristic of Minamata disease), tremor, failure in two-point discrimination, and slight balancing failure | [50] | |
| Rice and edible mushrooms | China | NS | [32] | |
| Lead (Pb) | Glass work plant | China | Susceptible autonomic nervous function | [51] |
| Battery plant | Turkey | Increased erythrocyte malondialdehyde (MDA) levels, catalase and glucose-6-phosphate dehydrogenase (G6PD) activities, and decreased blood glutathione/glutathione disulfide ratio | [52] | |
| Automobile plant | India | High blood pressure, less heme biosynthesis | [53] | |
| Soil | China | Renal dysfunction | [54] | |
| Vegetables | Nigeria | Neurological, immunological effects | [31] | |
| Vegetables | China | NS | [38] | |
| Food | Spain | NS | [33] | |
| Battery plant | China | Neurological damage | [55] | |
| Drinking water | Iran | NS | [43] |
| Heavy Metals | Food Components | Maximum Levels (mg kg−1 wet weight) | Ref. |
|---|---|---|---|
| Lead (Pb) | Leafy vegetables, brassica vegetables and a few fungi like Pleurotus ostreatus (oyster mushroom), Agaricus bisporus (common mushroom) and Lentinula edodes (shiitake mushroom) | 0.3 | [57] with permission |
| Vegetables (excluding fresh herbs and fungi, leafy vegetables and brassica vegetables) | 0.1 | ||
| Berries and small fruits | 0.2 | ||
| Cereals, pulses and legumes | 0.2 | ||
| Fruits (excluding small fruits and berries) | 0.1 | ||
| Mercury (Hg) | Muscle meat of fish and fishery products | 0.50 | |
| Cadmium (Cd) | Fresh herbs, leafy vegetables, celeriac and some fungi like Pleurotus ostreatus (oyster mushroom) and Agaricus bisporus (common mushroom) | 0.2 | |
| Potatoes and root and stem vegetables (excluding celeriac plants) | 0.1 | ||
| Fruits and vegetables (excluding fresh herbs, root and stem vegetables, fungi, potatoes and leafy vegetables) | 0.05 | ||
| Soybeans | 0.2 | ||
| Cereals (excluding rice, wheat, germ and bran) | 0.1 | ||
| Rice, wheat, germ and bran | 0.2 | ||
| Tin (Sn) | Canned food (except beverages) | 200 | |
| Canned beverages (including vegetable and fruit juices) | 100 | ||
| Processed cereal based products (excluding powdered and dried products) | 50 | ||
| Chromium (Cr) | Fresh vegetables | 0.5 | [58] |
| Grains and its products | 1.0 | ||
| Beans | 1.0 | ||
| Meat and its products | 1.0 | ||
| Nickel (Ni) | Oil and its products (mainly hydrogenated vegetable oil) | 1.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhalaria, R.; Kumar, D.; Kumar, H.; Nepovimova, E.; Kuča, K.; Torequl Islam, M.; Verma, R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy 2020, 10, 815. https://doi.org/10.3390/agronomy10060815
Dhalaria R, Kumar D, Kumar H, Nepovimova E, Kuča K, Torequl Islam M, Verma R. Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy. 2020; 10(6):815. https://doi.org/10.3390/agronomy10060815
Chicago/Turabian StyleDhalaria, Rajni, Dinesh Kumar, Harsh Kumar, Eugenie Nepovimova, Kamil Kuča, Muhammad Torequl Islam, and Rachna Verma. 2020. "Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants" Agronomy 10, no. 6: 815. https://doi.org/10.3390/agronomy10060815
APA StyleDhalaria, R., Kumar, D., Kumar, H., Nepovimova, E., Kuča, K., Torequl Islam, M., & Verma, R. (2020). Arbuscular Mycorrhizal Fungi as Potential Agents in Ameliorating Heavy Metal Stress in Plants. Agronomy, 10(6), 815. https://doi.org/10.3390/agronomy10060815