Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Field Experimental Sites
2.2. Soil Sampling and Analyses
2.3. Source of Strains and Seeds
2.4. Treatments and Experimental Design
2.5. Nodulation Assessment
2.6. Determination of Nitrogen and Phosphorus Uptake
2.7. Determination of Nitrogen Fixation
2.8. Determination of Soil Nitrogen Balance
2.9. Statistical Analysis
3. Results
3.1. Rhizobium Strains Inoculation Effect on Nodulation
3.2. Effect of Inoculation on Nutrient Concentration and Uptake
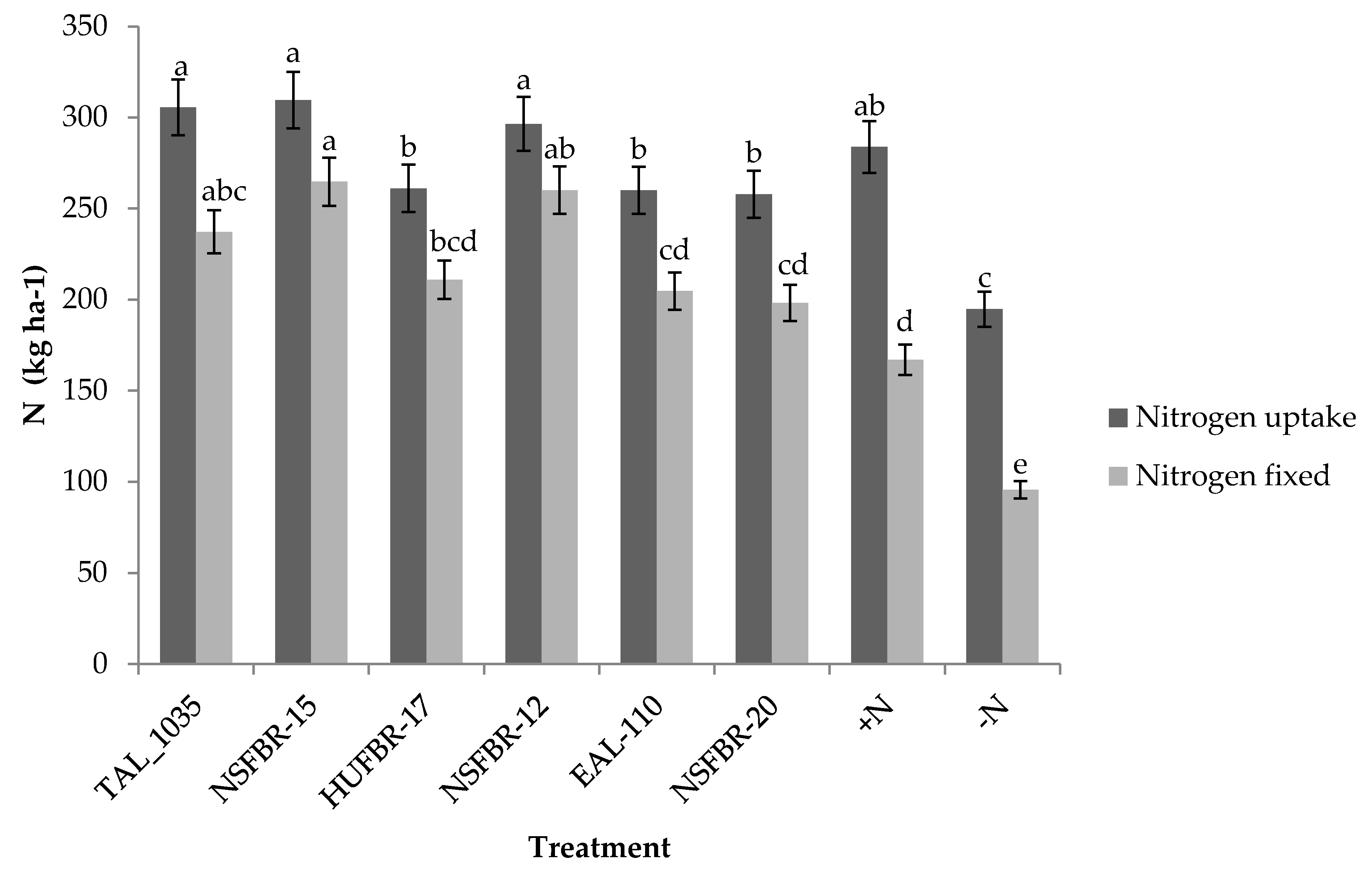
3.3. Inoculation Effects on Nitrogen Fixation
3.4. Effects of Inoculation on Soil Nitrogen Balance
3.5. Correlation among Nodulation, Nitrogen Fixation and Nutrient Uptake
4. Discussion
4.1. Inoculation Effect on Nodulation
4.2. Inoculation Effects on Nutrient Uptake
4.3. Inoculation Effect on Nitrogen Fixation
4.4. Effect of Inoculation on Soil Nitrogen Balance
4.5. Correlation among Nodulation, Nitrogen Fixation and Nutrient Uptake
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smaling, E.M.A.; Stoorvogel, J.J.; Windmeijer, P.N. Calculating soil nutrient balances in Africa at different scales: II district scale. Fertil. Res. 1993, 35, 237–250. [Google Scholar] [CrossRef]
- Ladha, J.K.; Padre, A.T.; Punzalan, G.C.; Castillo, E.; Singh, U.; Reddy, C.K. Non destructive estimation of shoot nitrogen in different rice genotypes. Agron. J. 1998, 90, 33–40. [Google Scholar] [CrossRef]
- Siczek, A.; Lipiec, J. Impact of faba bean seed rhizobial inoculation on microbial activity in the rhizosphere soil during growing season. Int. J. Mol. Sci. 2016, 17, 784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, E.S.; Hauggard-Nielsen, H. How can increased use of biological N2 fixation in agriculture benefit the environment? Plant Soil 2003, 252, 177–186. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Soe, K.M.; Moe, K.; Yamakawa, T. Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant, growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of mung bean, cowpea and soybean. Agronomy 2019, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Laguerre, G.; Louvrier, P.; Allard, M.R.; Amarger, N. Compatibility of rhizobial genotypes within natural populations of Rhizobium leguminosarum biovar viciae for Nodulation of Host Legumes. Appl. Environ. Microbiol. 2003, 69, 2276–2283. [Google Scholar] [CrossRef] [Green Version]
- Mutch, L.A.; Young, J.P.W. Diversity and specificity of Rhizobium leguminosarum boivar viciae on wild and cultivated legumes. Mol. Ecol. 2004, 13, 2435–2444. [Google Scholar] [CrossRef] [Green Version]
- Laguerre, G.; Depret, G.; Bourion, V.; Duc, G. Rhizobium leguminosarum biovar viciae genotypes interact with pea plants in developmental responses of nodules, roots and shoots. New Phytol. 2007, 176, 680–690. [Google Scholar] [CrossRef]
- Sachs, J.L.; Kembel, S.W.; Lau, A.M.; Simms, E.L. In situphylogenetic structure and diversity of wild Bradyrhizobium communities. Appl. Environ. Microbiol. 2009, 75, 4727–4735. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Cañizares, C.; Jorrín, B.; Durán, D.; Nadendla, S.; Albareda, M.; Rubio-Sanz, L.; Lanza, M.; González-Guerrero, M.; Prieto, R.I.; Brito, B.; et al. Genomic diversity in the endosymbiotic bacterium Rhizobium leguminosarum. Genes 2018, 9, 60. [Google Scholar] [CrossRef] [Green Version]
- McKenzie, R.H.; Middleton, A.B.; Solberg, E.D.; De-Mulder, J.; Flore, N.; Clayton, G.W. Response of peat or rhizobia inoculation and start nitrogen in Alberta. Can. J. Plant Sci. 2001, 81, 637–643. [Google Scholar] [CrossRef]
- Downie, J.A. The roles of extracellular proteins, polysaccharides and signals in the interactions of rhizobia with legume roots. FEMS Microbiol. Rev. 2010, 34, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.L.; Ehinger, M.O.; Simms, E.L. Origins of cheating and loss of symbiosis in wild Bradyrhizobium. J. Evol. Biol. 2010, 23, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Aoki, S.; Kawaguchi, M. Evolutionary dynamics of nitrogen fixation in the legume-rhizobia symbiosis. PLoS ONE 2014, 9, e3670. [Google Scholar] [CrossRef] [PubMed]
- Beltayef, H.; Melki, M.; Saidi, W.; Samaali, S.; Muscolo, A.; Cruz, C.; Garoui, T. Statement of biological nitrogen fixation in snap bean under mediterranean semi-arid conditions. Bulg. J. Agric. Sci. 2018, 24, 244–251. [Google Scholar]
- Stajković, O.; Delić, D.; Jošić, D.; Kuzmanović, D.; Rasulić, N.; Knežević-Vukčević, J. Improvement of common bean growth by co-innoculation with Rhizobium and plant growth-promoting bacteria. Rom. Biotechnol. Lett. 2011, 16, 5919–5926. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improvement for making particle size analysis of soils. Agron. J. 1962, 54, 179–186. [Google Scholar] [CrossRef]
- Black, G.R.; Hertge, K.H. Bulk density. In Methods of Soil Analysis; Klute, A., Ed.; SSSA: Madison, WI, USA, 1986; pp. 377–382. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Canadian Soil Science Society. In Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Mclean, E.O. Aluminum. In Methods of Soil Analysis; Part 2 chemical methods; Black, C.A., Ed.; America Sci. Agron.: Madison, WI, USA, 1965; pp. 978–998. [Google Scholar]
- Van Reeuwijk, L.P. Technical paper/international soil reference and information center. In Procedures for Soil Analysis, 6th ed.; Scientific Research: Wageningen, The Netherlands, 2002. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis, Part 2, Chemical And Microbiological Properties; Page, A.L., Ed.; SSSA: Madison, Wiscosin, 1982; pp. 595–622. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dea, L.A. Estimation of Available Phosphorous in Soils by Extraction with Sodium Bicarbonate; USDA: Washington, DC, USA, 1954; Volume 939, pp. 1–19.
- Rice, W.A.; Clyton, G.W.; Lupwayi, N.Z.; Olsen, P.E. Evaluation of coated seeds as a rhizobium delivery system for field pea. Can. J. Plant Sci. 2001, 81, 248–249. [Google Scholar] [CrossRef]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 6th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- NSL. Manual for Plant Analysis and Interpretation; Food and Agriculture Organization of the United Nations: Addis Ababa, Ethiopia, 1994. [Google Scholar]
- Unkovich, M.J.; Herridge, D.; Peoples, M.; Cadisch, G.; Boddey, R.; Giller, K.; Alves, B.; Chalk, P. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR Monography: Canberra, Australia, 2008; Volume 136, p. 258.
- Kermaha, M.; Frankeb, A.C.; Adjei-Nsiahc, S.; Adjei-Nsiahc, S.; Ahiabord, B.D.K.; Abaidoo, R.C.; Giller, K.E. N2-fixation and N contribution by grain legumes under different soil fertility status and cropping systems in the Guinea savanna of northern Ghana. Agric. Ecosyst. Environ. 2018, 261, 201–210. [Google Scholar] [CrossRef]
- SAS. SAS/STAT Software Syntax; Version 9.0; SAS Institute: Cary, NC, USA, 2010. [Google Scholar]
- Solomon, T.; Pant, L.M.; Angaw, T. Effects of inoculation by Bradyrhizobium japonicum strains on nodulation, nitrogen fixation, and yield of soybean (Glycine max L. Merill) varieties on Nitosols of Bako, western Ethiopia. Int. Sch. Res. Netw. ISRN Agron. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Farid, M.; Navabi, A. N2 fixation ability of different dry bean genotypes. Can. J. Plant Sci. 2015, 95, 1243–1257. [Google Scholar] [CrossRef]
- Voisin, A.S.; Salon, C.; Warembourg, F.R. Seasonal patterns of 13C partitioning between shoot and nodulated roots of N2- or nitrate-fed Pisum sativum (L.). Ann. Bot. 2003, 91, 539–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, P.H.; Hungria, M.; Tlusty, B. Breeding for better nitrogen fixation in grain legumes: Where do the rhizobia fit in? Crop Manag. 2004. [Google Scholar] [CrossRef]
- Tairo, E.V.; Ndakidemi, P.A. Micronutrients’ uptake in soybean (Glycine max L.) as affected by Bradyrhizobium japonicum inoculation and phosphorus (P.) supplements. World J. Soil Crop Sci. Res. 2014, 1, 1–9. [Google Scholar]
- Zoundji, C.C.; Houngnandan, P.; Amidou, M.H.; Kouelo, F.A.; Toukourou, F. Inoculation and phosphorus application effects on soybean (Glycine max L. Merrill) productivity grown in farmers’ fields of Benin. J. Anim. Plant Sci. 2015, 25, 1384–1392. [Google Scholar]
- Aliyu, I.M.; Yusuf, A.A.; Abaidoo, R.C. Response of grain legumes to rhizobial inoculation in two savanna soils of Nigeria. Afr. J. Microbiol. Res. 2013, 7, 1332–1342. [Google Scholar]
- Aziz, A.L.; Ahiabor, B.D.K.; Opoku, A.; Abaidoo, R.C. Contribution of rhizobium inoculants and phosphorus fertilizer to biological nitrogen fixation, growth and grain yield of three soybean varieties on a Fluvic Luvisol. Am. J. Exp. Agric. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Talaat, N.B.; Abdallah, A.M. Response of faba bean (Vicia faba L.) to dual inoculation with Rhizobium and VA mycorrhiza under different levels of N and P fertilization. J. Appl. Sci. Res. 2008, 4, 1092–1102. [Google Scholar]
- Lugtenberg, B.; Kamilova, F. Plant-growth-proming rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Regar, M.K.; Meena, R.H.; Jat, G.; Mundra, S.L. Effect of different rhizobial strains on growth and yield of soybean (Glycine max L. Merrill). Int. J. Curr. Microbiol. App. Sci. 2017, 6, 3653–3659. [Google Scholar] [CrossRef]
- Gasim, S.; Hamad, S.A.A.; Abdelmula, A.; Ahmed, I.A.M. Yield and quality attributes of faba bean inbred lines grown under marginal environmental condition of Sudan. Food Sci. Nutr. 2015, 3, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Khogali, M.E.; Dagash, Y.M.I.; EL-Hag, M.G. Nitrogen fertilizer effects on quality of fodder beet (Beta vulgaris var. Crassa). Agric. Biol. J. N. Am. 2011, 292, 270–278. [Google Scholar] [CrossRef]
- Araujo, A.P.; Teixeira, M.G. Relationship between grain yield and accumulation of biomass, nitrogen and phosphorus in common bean cultivars. Revista Brasileira de Ciência do Solo 2008, 32, 1977–1986. [Google Scholar] [CrossRef] [Green Version]
- Neugschwandtner, R.; Ziegler, K.; Kriegner, S.; Wagentristl, H.; Kaul, H.P. Nitrogen yield and nitrogen fixation of winter faba Beans. Acta Agric. Scand. Sect. B Soil Plant Sci. 2015, 65, 658–666. Available online: http://www.tandfonline.com/loi/sagb20 (accessed on 25 January 2018). [CrossRef]
- Pleijel, H.; Mortensen, L.; Fuhrer, J.; Ojanpera, K.; Danielsson, H. Grain protein accumulation in relation to grain yield of spring wheat (Triticum eastivum L.) grown in open-top chambers with different concentrations of ozone, carbon dioxide and water availability. Agric. Ecosyst. Environ. 1999, 72, 265–270. [Google Scholar] [CrossRef]
- Cox, M.C.; Qualset, C.O.; Rains, D.W. Genetic variation for nitrogen assimilation and translocation in wheat. III. Nitrogen translocation in relation to grain yield protein. Crop Sci. 1986, 26, 737–740. [Google Scholar] [CrossRef]
- Nyoki, D.; Ndakidemi, P.A. Effects of Bradyrhizobium japonicum inoculation and supplementation with phosphorus on macronutrients uptake in cowpea (Vigna unguiculata L. Walp). Am. J. Plant Sci. 2014, 5, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Korir, H.; Mungal, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation effect of rhizobia and plant growth promoting rhizobacteria on common bean in a low phosphorus soil. Front. Plant Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Israel, D.W. Symbiotic dinitrogen fixation and host-plant growth during development of and recovery from phosphorus deficiency. Physiol. Plant. 1993, 88, 294–300. [Google Scholar] [CrossRef]
- Ziadi, N.; Be’langer, G.; Cambouris, A.N.; Tremblay, N.; Nolin, M.C. Relationship between P and N concentrations in corn. Agron. J. 2007, 199, 833–841. [Google Scholar] [CrossRef]
- Agren, G.I.; Wetterstedt, M.J.Å.; Billberger, M.F.K. Nutrient limitation on terrestrial plant growth-modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 153–960. [Google Scholar] [CrossRef]
- Ball, B.C.; Crichton, I.; Horgan, G.W. Dynamics of upward and downward N2O and CO2 fluxes in ploughed and no-tilled soils in relation to water-filled pore space, compaction and crop presence. Soil Tillage Res. 2008, 101, 20–30. [Google Scholar] [CrossRef]
- Tahir, M.M.; Abbasi, M.K.; Rahim, N.; Khaliq, A.; Kazmi, M.H. Effect of Rhizobium inoculation and NP fertilization on growth, yield and nodulation of soybean (Glycine max L.) in sub-humid hilly region of Rawa Lakot, Azud Jammu and Kashmir, Pakistan. Afr. J. Biotechnol. 2009, 8, 6191–6200. [Google Scholar]
- Sardans, J.; Rivas-Ubach, A.; Uelas, J.P. The C:N:P stoichiometry of organisms and ecosystems in a changing world: A review and perspectives. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 33–47. [Google Scholar] [CrossRef]
- Lu, X.T.; Kong, D.L.; Pan, Q.M.; Simmons, M.E.; Han, X.G. Nitrogen and water availability interact to affect leaf stoichiometry in semi-arid grassland. Oecologia 2012, 168, 301–310. [Google Scholar] [CrossRef]
- Belanger, G.; Claessens, A.; Ziadi, N. Grain nitrogen and phosphorus relationships in maize. Field Crops Res. 2012, 126, 1–7. [Google Scholar] [CrossRef]
- Taylor, B.N.; Menge, D.N.L. Light regulates tropical symbiotic nitrogen fixation more strongly than soil nitrogen. Nat. Plants 2018, 4, 655–661. [Google Scholar] [CrossRef]
- Reem, M.H. The effect of phosphorus in nitrogen fixation in legumes. Agric. Res. Technol. Access J. 2017, 5, 555652. [Google Scholar] [CrossRef]
- Raven, J.A. Protein turnover and plant RNA and phosphorus requirement in relation to nitrogen fixation. Plant Sci. 2012, 189, 25–35. [Google Scholar] [CrossRef]
- Bello, S.K.; Yusuf, A.A.; Cargele, M. Performance of cowpea as influenced by native strain of rhizobia, lime and phosphorus in Samaru, Nigeria. Symbiosis 2018, 75, 167–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keneni, A.; Feten, A.; Prabu, P.C. Characterization of acid and salt tolerant rhizobial strains isolated from faba bean fields of Wollo, northern Ethiopia. J. Agric. Sci. Technol. 2010, 2, 365–376. [Google Scholar]
- Tena, W.; Wolde-Meskel, E.; Walley, F. Symbiotic efficiency of native and exotic Rhizobium strains nodulating lentil (Lens culinaris Medik.) in soils of southern Ethiopia. Agronomy 2016, 6, 11. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Li, X.; Ai, W.; Liu, D.; Qi, W.; Zhang, M.; Yang, C.; Liao, H. Characterization of genetic basis on synergistic interactions between root architecture and biological nitrogen fixation in soybean. Front. Plant Sci. 2017, 8, 1466. [Google Scholar] [CrossRef] [Green Version]
- Smil, V. Nitrogen in crop production: An account of global flows. Glob. Biogeochem. Cycles 1999, 13, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Diouf, A.; Diop, T.A.; Gueye, M. Nodulation in situ of common bean (Phaseolus vulgaris L.) and field outcome of an elite symbiotic association in Senegal. Res. J. Agric. Biol. Sci. 2008, 4, 810–818. [Google Scholar]
- Youseif, S.H.; Fayrouz, H.; El-Megeed, F.H.A.; Sahel, S.A. Improvement of faba bean yield using Rhizobium/Agrobacterium inoculant in low fertility sandy soil. Agronomy 2017, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Jensen, E.S.; Peoples, M.B.; Hauggaard-Nielsen, H. Faba bean in cropping systems. Field Crops Res. 2010, 115, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Herridge, D.F.; Peoples, M.B.; Boddey, R.M. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 2008, 311, 1–18. [Google Scholar] [CrossRef]
- Lindemann, W.C.; Glover, C.R. Nitrogen Fixation by Legumes; Cooperative Extension Service, College of Agriculture and Home Economics New Mexico State University: New Mexico, NM, USA, 2003; pp. 1–4. [Google Scholar]
- Asad, S.; Malik, K.A.; Hafeez, F.Y. Competition between inoculated and indigenous Rhizobium/Bradyrhizobium spp. strains for nodulation of grain and fodder legumes in Pakistan. Biol. Fertil. Soils 1991, 12, 107–111. [Google Scholar] [CrossRef]
- Habtemichial, K.H.; Singh, B.R.; Aune, J.B. Wheat response to N2 fixed by faba bean (Vicia faba L.) as affected by sulfur fertilization and rhizobial inoculation in semi-arid northern Ethiopia. J. Plant Nutr. Soil Sci. 2007, 170, 412–418. [Google Scholar] [CrossRef]
- Whalen, J.K. Managing soil biota-mediated decomposition and nutrient mineralization in sustainable agroecosystems. Adv. Agric. 2014, 2014, 384604. [Google Scholar] [CrossRef]
- Frank, A.C.; Laberge, G.; Oyewole, B.D.; Schulz, S. A comparison between legume technologies and fallow, and their effects on maize and soil traits, in two distinct environments of the West Africa savannah. Nutr. Cycl. Agroecosys 2008, 82, 117–135. [Google Scholar] [CrossRef] [Green Version]
- Martin, G.; Moraine, M.; Ryschawy, J.; Magne, M.A.; Asai, M.; Sarthou, J.P.; Duru, M.; Therond, O. Crop-livestock integration beyond the farm level: A review. Agron. Sustain. Dev. 2016, 36, 53. [Google Scholar] [CrossRef]
- Argaw, A. Characterization of symbiotic effectiveness of rhizobia Nodulating faba bean (Vicia faba L.) isolated from central Ethiopia. Res. J. Microbiol. 2012, 7, 280–296. [Google Scholar] [CrossRef]
- Minalku, A.; Gebrekidan, H.; Assefa, F. Symbiotic effectiveness and characterization of Rhizobium strains of faba bean (Vicia faba L.) Collected from eastern and western Hararghe highlands of Ethiopia. Ethiop. J. Nat. Resour. 2009, 11, 223–244. [Google Scholar]
- Ferreira, P.A.A.; Bomfeti, C.A.; Soares, B.L.; Moreira, F.M.S. Efficient nitrogen-fixing rhizobium strains isolated from Amazonian soils are highly tolerant to acidity and aluminum. World J. Microbiol. Biotechnol. 2012, 28, 1947–1959. [Google Scholar] [CrossRef]
- Delić, D.; Stajković, O.; Rasulić, N.; Kuzmanović, D.; Jošić, D.; Milićić, B. Nodulation and N2 fixation effectiveness of Bradyrhizobium strains in symbiosis with Adzuki bean. Vigna angularis. Braz. Arch. Biol. Technol. 2010, 53, 293–299. [Google Scholar]
- Patra, R.K.; Pant, L.M.; Pardhan, K. Response of soybean to inoculation with rhizobial strains: Effect on growth, yield, N uptake and soil status. World J. Agric. Sci. 2012, 8, 51–54. [Google Scholar]
- Ruiz-Diez, B.; Fajardo, S.; Fernández-Pascual, M. Selection of rhizobia from agronomic legumes grown in semi-arid soils to be employed as bio-inoculants. Agron. J. 2012, 104, 550–559. [Google Scholar] [CrossRef]
- Kantar, F.; Elkoca, E.; Oğutcu, H.; Algur, Ö.F. Chickpea yields in relation to Rhizobium inoculation from wild chickpea at high altitudes. J. Agron. Crop Sci. 2003, 189, 291–297. [Google Scholar] [CrossRef]
- Oğutcu, H.; Algur, O.F.; Elkoca, E.; Kantar, F. The determination of symbiotic effectiveness of Rhizobium strains isolated from wild chickpeas collected from high altitudes in Erzurum. Turk. J. Agric. 2008, 32, 241–248. [Google Scholar]
- Boughribil, S.; Abumsimir, B.; Montassir, L.; Tarek, F.; Ennaji, M.M.; Bessi, H. Effect of competitiveness on nodulation and nitrogen fixation in common bean (Phaseolus vulgaris L.). J. Mater. Environ. Sci. 2018, 9, 828–833. [Google Scholar]
- Mweetwa, M.; Chilombo, G.; Gondwe, B.M. Nodulation, nutrient uptake and yield of common bean inoculated with rhizobia and Trichoderma in an acid soil Alice. J. Agric. Sci. 2016, 8, 61–71. [Google Scholar]
- Ye, Y.; Liang, X.; Chen, Y.; Li, L.; Ji, Y.; Zhu, C. Carbon, nitrogen and phosphorus accumulation and partitioning, and C:N:P stoichiometry in late-season rice under different water and nitrogen managements. PLoS ONE 2014, 9, e101776. [Google Scholar] [CrossRef]


| Year | Cool Humid (Location: HK and AG) | Cool Sub–Humid (Location: HR and GA) | ||||||
|---|---|---|---|---|---|---|---|---|
| Rainfall | a Max. T | b Min. T | Rainfall | a Max. T | b Min. T | |||
| mm | °C | °C | mm | °C | °C | |||
| 2016 | June | 179.6 | 14.1 | 7.7 | 127.6 | 21.3 | 12.9 | |
| July | 133.5 | 16.4 | 5.6 | 96.8 | 24.3 | 12.5 | ||
| August | 182.2 | 16.3 | 6.1 | 191.5 | 22.8 | 11.6 | ||
| September | 159.6 | 16.5 | 7.2 | 104.0 | 23.7 | 13.4 | ||
| Annual | 1477.6 | 17.1 | 8.1 | 1303.0 | 25.2 | 15.1 | ||
| 2017 | June | 62.8 | 17.0 | 9.2 | 35.0 | 25.1 | 15.3 | |
| July | 218.8 | 15.6 | 5.2 | 160.7 | 23.3 | 11.9 | ||
| August | 218.8 | 14.1 | 6.5 | 165.7 | 20.9 | 11.3 | ||
| September | 206.0 | 14.0 | 7.8 | 204.1 | 19.1 | 10.9 | ||
| Annual | 1590.6 | 17.4 | 9.3 | 1199.2 | 24.5 | 14.4 | ||
| 10 years (2008–2017) | Annual Average | 1472.9 | 15.4 | 7.1 | 1092.5 | 22.4 | 11.7 | |
| Soil Paramete | Study Locations | ||||
|---|---|---|---|---|---|
| Hankomolicha | Abala Gase | Haramfama | Gike Atoye | ||
| pH (1:2; Soil:H2O) | 6.57 | 5.37 | 6.02 | 5.60 | |
| Available P (mg kg−1) | 12.60 | 5.70 | 8.40 | 6.03 | |
| Total nitrogen (%) | 0.17 | 0.17 | 0.16 | 0.22 | |
| Organic carbon (%) | 2.06 | 2.22 | 1.75 | 2.34 | |
| Organic matter (%) | 3.55 | 3.83 | 3.02 | 4.03 | |
| C:N ratio | 12.10 | 13.10 | 11.30 | 10.80 | |
| CEC (meq 100 g−1) | 29.40 | 27.56 | 22.60 | 32.81 | |
| Exchangeable bases (cmol(+) kg−1) | Na | 2.11 | 0.93 | 0.95 | 0.83 |
| K | 3.14 | 0.75 | 2.36 | 1.25 | |
| Ca | 13.40 | 15.09 | 12.60 | 17.73 | |
| Mg | 7.22 | 5.38 | 6.44 | 5.20 | |
| Exc. acidity (cmol(+) kg−1) | 0.40 | 0.48 | 0.12 | 0.52 | |
| Bulk density (g cm−3) | 1.24 | 1.21 | 1.35 | 1.25 | |
| Textural class | Clay | Clay loam | Loam | Clay | |
| Treatments | Hankomolicha | Haranfama | Abala Gase | Gike Atoye | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moti | Dosha | Gora | Moti | Dosha | Gora | Moti | Dosha | Gora | Moti | Dosha | Gora | |
| (mg Plant−1) | (mg Plant−1) | (mg Plant−1) | (mg Plant−1) | |||||||||
| TAL_1035 | 247.5 b | 260.3 a | 295.8 a | 211.3 a | 213.3 a | 261.3 a | 249.6 b | 271.8 bc | 414.3 a | 209.5 d | 219.2 b | 370.7 a |
| NSFBR-15 | 275.0 a | 258.0 a | 239.0 b | 212.5 a | 226.5 a | 240.3 b | 203.5 c | 463.9 a | 286.7 b | 258.0 ab | 174.0 c | 346.9 a |
| HUFBR-17 | 216.3 c | 235.8 b | 205.5 c | 198.5 ab | 163.3 cd | 167.8 d | 163.5 d | 155.7 e | 151.0 e | 232.4 bcd | 184.0 c | 118.2 e |
| NSFBR-12 | 228.8 bc | 224.5 b | 239.5 b | 207.3 ab | 192.0 b | 216.8 c | 346.7 a | 248.8 c | 234.3 cd | 278.9 a | 272.7 a | 284.3 b |
| EAL-110 | 236.0 bc | 174.8 c | 193.5 cd | 176.0 cd | 181.3 bc | 168.8 d | 228.3 bc | 201.3 d | 254.5 bc | 220.3 cd | 144.1 de | 252.2 c |
| NSFBR-20 | 155.5 d | 173.8 c | 177.8 d | 188.5 bc | 164.0 cd | 153.0 de | 209.1 c | 317.3 b | 201.5 d | 245.7 bc | 230.7 b | 188.5 d |
| +N | 112.3 e | 106.0 d | 111.5 e | 148.5 e | 156.8 d | 139.8 ef | 154.6 d | 135.1 e | 120.5 ef | 182.4 e | 121.9 e | 299.0 b |
| −N | 122.3 e | 154.5 c | 175.3 d | 162.8 de | 182.3 bc | 131.5 f | 100.1 e | 129.3 e | 105.3 f | 147.2 f | 169.5 cd | 113.9 e |
| CV (%) | 8.4 | 8.2 | 12.4 | 11.0 | ||||||||
| Treatments | N Concentration (%) | P Concentration (%) | ||||
|---|---|---|---|---|---|---|
| Root | Haulm | Grain | Root | Haulm | Grain | |
| TAL_1035 | 1.50 a | 1.68 a | 4.06 bc | 0.34 ab | 0.23 a | 1.32 a |
| NSFBR-15 | 1.37 a | 1.64 a | 4.02 bc | 0.35 ab | 0.25 a | 1.28 a |
| HUFBR-17 | 1.44 a | 2.01 a | 4.49 a | 0.31 b | 0.26 a | 1.24 ab |
| NSFBR-12 | 1.52 a | 1.75 a | 4.15 abc | 0.35 ab | 0.25 a | 1.31 a |
| EAL-110 | 1.38 a | 1.89 a | 4.36 ab | 0.34 ab | 0.26 a | 1.13 bcd |
| NSFBR-20 | 1.38 a | 1.94 a | 4.51 a | 0.33 ab | 0.28 a | 1.20 abc |
| +N | 1.43 a | 1.83 a | 3.82 c | 0.38 a | 0.30 a | 1.04 d |
| −N | 1.45 a | 1.81 a | 4.19 abc | 0.30 b | 0.27 a | 1.10 cd |
| CV (%) | 18.5 | 17.4 | 10.3 | 16.5 | 19.8 | 12.2 |
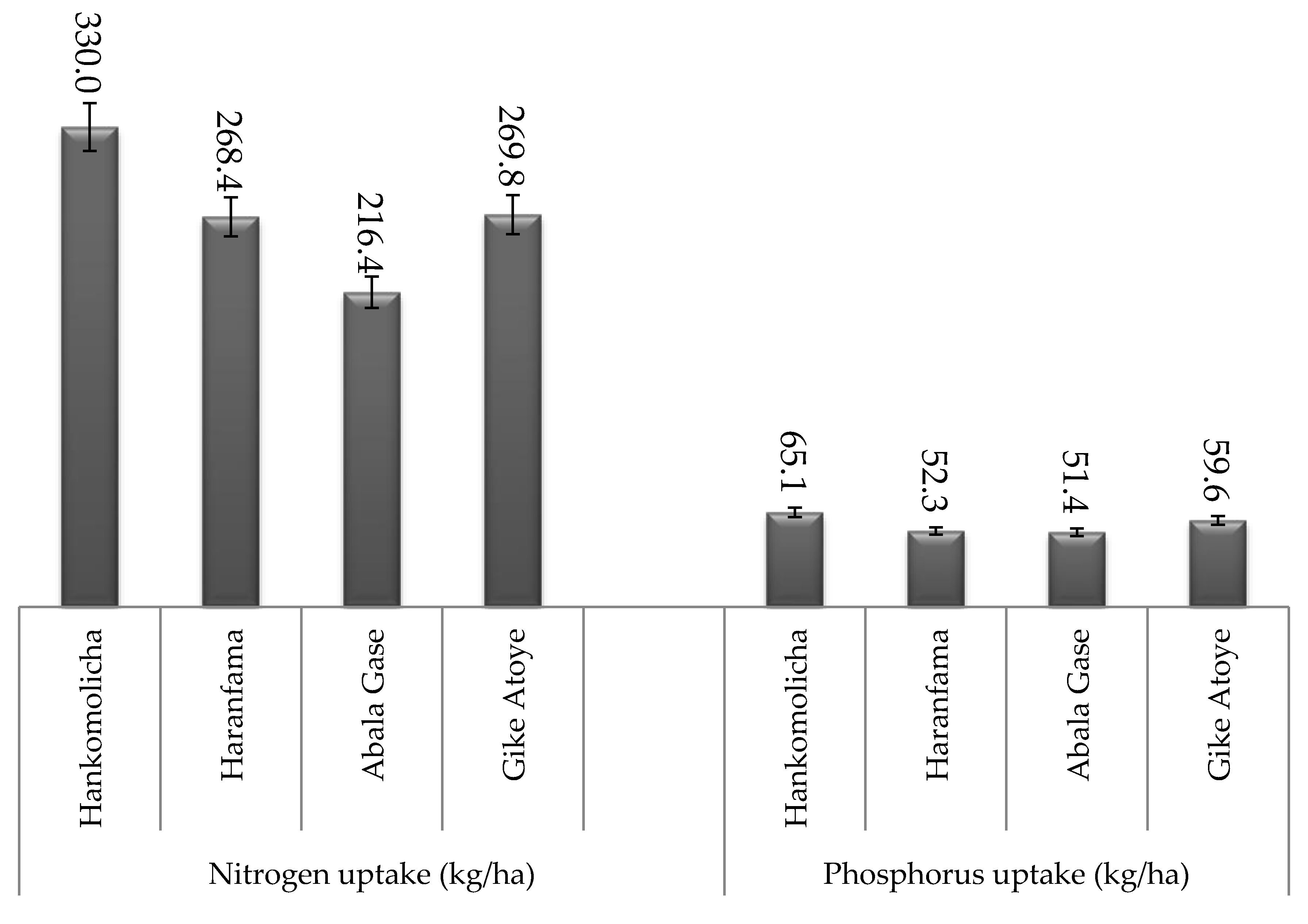
| Treatments | Nitrogen Uptake (kg N ha−1) | Phosphorus Uptake (kg P ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| Root | Haulm | Grain | Total | Root | Shoot | Grain | Total | |
| TAL_1035 | 28.6 a | 155.3 a | 121.7 a | 305.6 a | 6.4 abc | 21.7 ab | 39.9 a | 68.0 ab |
| NSFBR-15 | 28.5 a | 156.6 a | 124.5 a | 309.6 a | 7.4 a | 24.5 a | 39.9 a | 71.8 a |
| HUFBR-17 | 25.8 ab | 133.5 ab | 101.7 c | 261.1 b | 5.4 c | 16.5 cd | 28.0 b | 49.9 d |
| NSFBR-12 | 27.2 ab | 152.8 a | 116.5 ab | 296.5 a | 6.2 bc | 21.5 ab | 36.7 a | 64.4 bc |
| EAL-110 | 26.8 ab | 129.4 b | 103.8 c | 260.0 b | 6.7 ab | 18.0 bc | 27.1 b | 51.8 d |
| NSFBR-20 | 25.5 ab | 130.1 b | 102.3 c | 257.9 b | 6.0 bc | 18.4 bc | 27.3 b | 51.7 d |
| +N | 23.4 bc | 153.5 a | 106.8 bc | 283.8 ab | 6.4 abc | 24.8 a | 29.2 b | 60.4 c |
| −N | 20.3 c | 94.4 c | 80.1 d | 194.7 c | 4.0 d | 13.3 d | 21.2 c | 38.5 e |
| CV (%) | 18.2 | 18.8 | 13.3 | 14.1 | 19.5 | 22.2 | 16.1 | 14.7 |
| Variables | N-Uptake | P-Uptake | SNB | NF | ||||
|---|---|---|---|---|---|---|---|---|
| r | R2 | r | R2 | r | R2 | r | R2 | |
| NDW | 0.77 ** | 0.59 | 0.79 ** | 0.62 | 0.73 ** | 0.53 | 0.87 ** | 0.75 |
| NF | 0.86 ** | 0.74 | 0.81 ** | 0.65 | 0.95 ** | 0.90 | - | - |
| P-uptake | 0.96 ** | 0.92 | - | - | - | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allito, B.B.; Ewusi-Mensah, N.; Logah, V. Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.). Agronomy 2020, 10, 826. https://doi.org/10.3390/agronomy10060826
Allito BB, Ewusi-Mensah N, Logah V. Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.). Agronomy. 2020; 10(6):826. https://doi.org/10.3390/agronomy10060826
Chicago/Turabian StyleAllito, Bayou Bunkura, Nana Ewusi-Mensah, and Vincent Logah. 2020. "Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.)" Agronomy 10, no. 6: 826. https://doi.org/10.3390/agronomy10060826
APA StyleAllito, B. B., Ewusi-Mensah, N., & Logah, V. (2020). Legume-Rhizobium Strain Specificity Enhances Nutrition and Nitrogen Fixation in Faba Bean (Vicia faba L.). Agronomy, 10(6), 826. https://doi.org/10.3390/agronomy10060826





