3.1. Experiment I
The viability of the four seed lots was very low, ranging from 5 to 12.5% (without significant differences (at
p ≤ 0.05;
Table 1)) when healthy seeds (category H) were considered, and between 10% and 35% (corresponding with the lowest value to 2-year old seeds (
p ≤ 0.05)) in seeds with viable tissues (including weak ones; category H + category WV). These results are in line with those obtained by [
33], who reported, for a commercialized seed lot of standard category, a viability of 19.5% considering H, and 29.3% considering H + WV.
Seeds cannot retain their viability indefinitely and, after a period of time, they deteriorate. In addition to the species characteristics, seed longevity also depends on the individual seed characteristics and on the storage conditions. The researchers in [
9] reported that caper seed germination declined when the seeds were kept at room temperature for more than 12 months. However, the authors in [
34] found that the seed viability (harvested, cleaned, dried, and stored by themselves) was maintained over 84% after 3-years of storage, with germination percentages (without scarification or GA addition) similar to those of the recently-harvested seeds (around 30%). These authors also determined that the caper seed longevity (considered as the time taken for 50% of the seeds to die [
33,
34,
35]) stored at 7 °C was 3.85 years [
34]. Therefore, the low viability of the caper seeds used in this study (particularly the 0.5 and 2-year old seeds) may not be due to a natural deterioration during storage, but rather to intrinsic seed characteristics.
Germination data was fitted to the logistic function (
p ≤ 0.01), presenting coefficients of determination (R
2) for the 32 curves (four replicates from eight combinations of variation sources) greater than 0.91. This allows the utilization of the variable
A (instead of G), as well as other variables, such as
β and
k, and then
β/k and
k/2, as done in previous studies of caper seed germination [
18,
34].
Figure 1 presents the cumulative germination curves fitted to the logistic model obtained for the average values of each seed age and saturation solution combination. In all cases, germination was very low, and the GA increased the
A value in relation to those saturated with water. The highest
A value (
p ≤ 0.05;
Table 2) was obtained for 5-year old seeds using GA (6%), not differing from that for 0.5-year old seeds. The lowest
A value was obtained for 2-year old seeds, not differing from that for 4-year old seeds. GA increased
A (
p ≤ 0.01). The seed age x saturation solution (water or GA) interaction was significant (
p ≤ 0.05) given that GA significantly (
p ≤ 0.05) increased the value of
A in the 5-year old seeds and did not significantly change the other lots.
No differences (at
p ≤ 0.05) were detected for
Gt50 or
k/2. Although
Gt50 can seem long, it is short in relation to that obtained in similar studies using the owned seeds, but in which much higher germination percentages were obtained (
A = 90.5%,
Gt50 = 50 days; [
13]). The
k/2 values of the seeds with greater germination percentages (0.5- and 5-year old) were comparable to those of the aforementioned study (0.13 d
−1). These results were in agreement with those obtained by [
33], who reported germination percentages lower than 0.5% in the standard seeds moistened both with water and GA. They were also consistent with the low viability of these seeds, particularly considering only the healthy ones.
The germination percentage values were lower than those of viability, as expected, taking into account that, on one hand, germination loss precedes the viability loss, as stated by [
36], and, on the other hand, that if seeds had imbibed the substrate solution, the main reason preventing germination in many H, and probably all WV seeds, was the low embryo growth potential (“push power”), i.e., these seeds presented PD, according to [
22]. This theory is also supported by the fact that the GA application improved germination. The GA application to saturate the two filter papers or to soak the seeds, are treatments, alone or in combination with scarification or NO
3K utilization, that succeeded in increasing the germination of caper seeds, probably due to breaking their PD [
13,
16], increasing the embryo vigor, or weakening the seed coat.
3.2. Experiment II
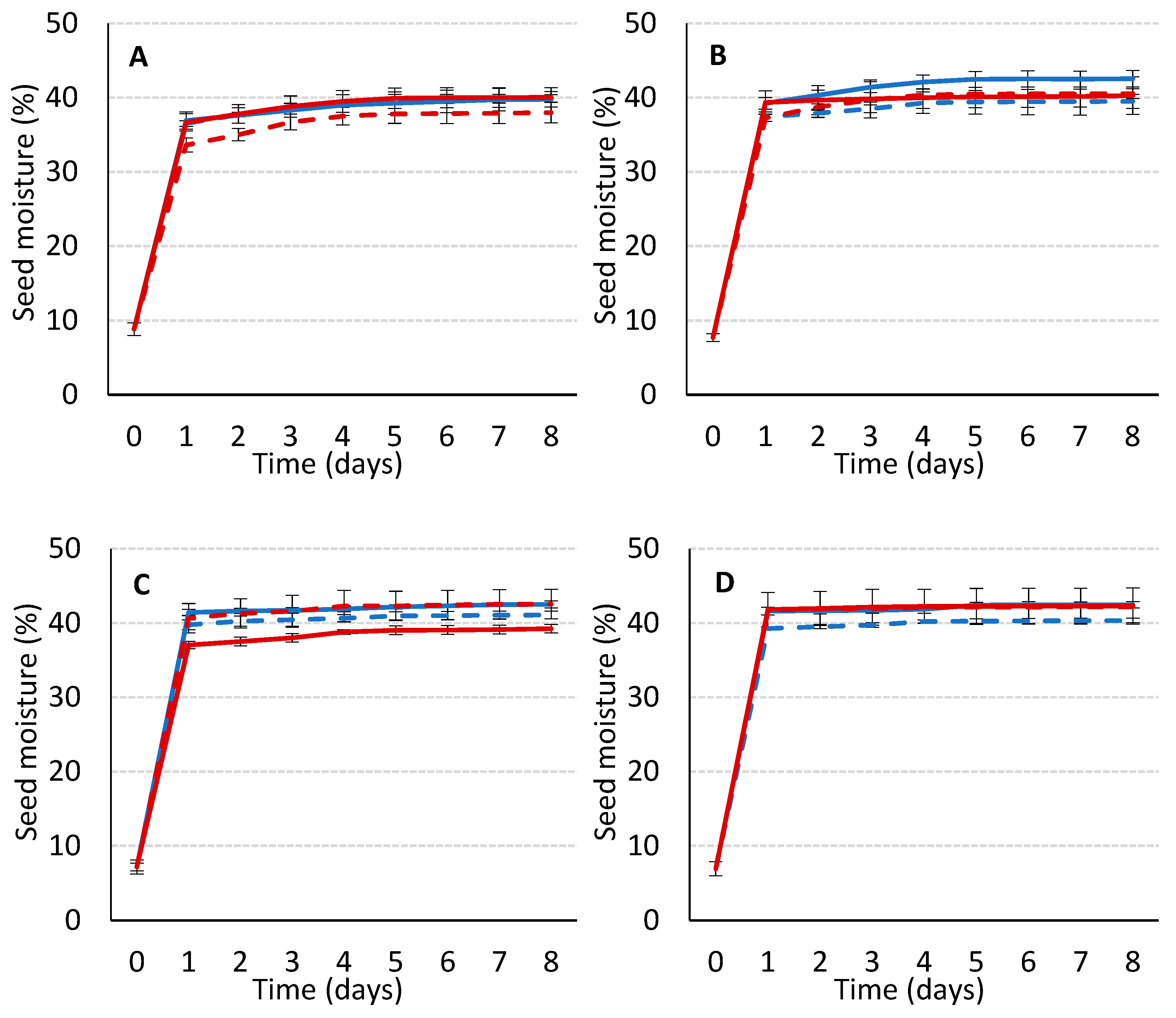
The distribution of the different categories established in the 0.5-year old seed lot was: IS, 40.1%; SS, 13.0%; CS, 30.7%; and BS, 16.2%. Initially, the seed moisture content of each category was: IS, 8.8%; SS, 7.7%; CS, 7.1%; and BS, 6.9%. Although it might be expected that an increase in seed coat damage results in a decrease of the initial moisture content of seeds, the difference in moisture contents were not significant (
p < 0.05; ANOVA not shown). The seed moisture content (
Figure 2) increased quickly during the first 24 h of soaking, stabilizing in the most deteriorated coat seeds (BS), while the water uptake continued slowly until the fourth day in intact seeds (IS). Although in previous studies our research team reported a linear water uptake during the first 24 h [
17], in view of the herein presented seed water content after one day of imbibition, and given that water uptake speed could influence the subsequent germination process, it would be interesting to determine the water uptake rate during the first 24 h, analyzing the seed water content every hour, which is intended to be addressed in a future study. As the researchers in [
37] stated, imbibition is a physical process and is a consequence of the matric forces that occur within dry seeds with water permeable seed coats, independently of whether they are alive or dead, or dormant or non-dormant.
In
Figure 2, the seed moisture content along the soaking period followed the first two phases of the typical triphasic model of water uptake in seed germination. First (phase I of germination; imbibition itself), the water uptake was initially rapid, followed by a slower linear wetting step. At the end of phase I (from the first to the fourth day, depending on the category of the seed), the water uptake stopped as the seed entered the lag phase of germination (phase II) in which there was a limited water uptake, while, according to the literature [
37], the metabolism was supposedly active. Radicle protrusion would result from a second period of fresh weight gain driven by additional water uptake (phase III; not shown in
Figure 2).
Seeds do not hydrate uniformly during imbibition, since there is a “wetting front” that develops as the outer portions of the seed hydrates while the inner tissues are still dry; seed parts may hydrate differentially depending on their contents [
37]. As in other seeds [
38], caper seed coats contain the hilum (a scar formed when the funiculus detaches from the seed at maturity) and the micropyle (a scar that corresponds to the micropyle of the ovule), commonly known as the hilar region, and the rest is known as the extrahilar region. The hilar region can allow for water uptake, behaving like a water channel [
12,
37]. If these sites are large enough to admit dyes, the permeable areas can be visualized.
According to [
38], given that the methylene blue dye molecules are larger than those of water, dye entry into the seed coat indicates that this is a penetration point for water.
Figure 3b shows that seed hydration begins through the hilar region, while the extrahilar region is impermeable. Caper seeds do not have a water-impermeable coat sensu stricto and they imbibe water without the seed coat being disrupted. When this region is damaged, by scrapping (
Figure 3c) or by cracking (
Figure 3d,e), hydration begins through the damaged area, contrary to what was reported for
Opuntia tomentosa seeds [
22].
Table 3 presents the analysis of variance for the seed moisture content corresponding to the first, fourth, and eighth day of imbibition. After one day of soaking, BS reached a higher seed moisture content than CS (
p ≤ 0.05), which in turn, presented higher values than SS (
p ≤ 0.05), and those were greater than IS (
p ≤ 0.05). These differences decreased with the imbibition period. At the fourth and eighth day, only IS presented a lower seed moisture content (
p ≤ 0.05; on average 2.6% and 2.2%, respectively) than the other categories, which did not differ between them.
In seeds with a permeable seed coat, seed hydration is determined by the gradient of the water potential between the medium (in this case the solution column in which the seeds are soaked or the two filter papers) and the seed. Initially, the matric potential of dry seeds is very low, and water is absorbed on the dry coat surface, on the cell walls, and in the polymeric reserve compounds, principally proteins [
22], mainly due to the gradient between the osmotic potential of the seeds and the osmotic and pressure potential of the medium [
39]. When the seeds have a “water channel”, this process occurs through it. No differences at the
p ≤ 0.05 level in the seed moisture content were detected in relation to the imbibition medium (filter paper or liquid column) or the saturation solution (water or GA), which indicated that neither the saturated filter paper, nor the use of GA, restricted the solution uptake with respect to soaking in water; i.e., the osmotic potential of the seeds was much lower than the osmotic and pressure potential of the medium. In this experiment, the water column above the seeds had a height of approximately 10 cm, water Ψo = −0.13 MPa, and GA solution Ψo = −0.65 MPa.
The viability of the four seed categories was very low, ranging from 0 to 20% (
p ≤ 0.01;
Table 4) when healthy seeds (H) were considered, and between 2.5% and 32.5% in seeds with viable tissues (H + WV). Considering H, two groups (
p ˂ 0.05) were clearly detected, one consisting of IS and SS, which had greater viability than those of the other group, consisting of seeds with appreciable damage in their coat (CS and BS). The viability of BS seeds was nil. When considering all the seeds with viable tissues (H + WV categories), the viability of the BS seeds was lower (
p ≤ 0.05) than that of the other seeds.
As in Experiment I, A was very low or nil; specifically it was null for BS, practically null (on average ≤ 0.3%) for CS and SS, and very low for IS; therefore, it was only possible for IS to adjust the cumulative germination to the logistic model. Coefficients of determination (R2) for the eight curves (four replicates from two combinations of variation sources) were greater than 0.94. The average parameters of the germination curves for IS were: A = 4.3, Gt50 = 23.4 days, and k/2 = 0.4 day−1. The use of GA increased the A value in relation to the use of water, as in previously cited studies, practically doubling its value (3.0% for water, 5.6% for GA). These results agreed with those obtained in Experiment I for the 0.5-year old seeds, which was the original seed lot. As stated above, the results were also consistent with the low viability of these seeds, particularly considering the healthy seeds.
As far as we know, this is the first time that data on caper seed imbibition has been reported. In this experiment, seeds imbibed the corresponding solution, and the embryo likely did not have enough growth potential to germinate [
22], as it was mentioned in the previous experiment. It remained to be confirmed whether the moisture content reached in these seeds was enough to allow their germination; thus, the third experiment was conducted.
3.3. Experiment III
The initial seed moisture content of the two types of seeds was rather similar (8.85% for standard seeds and 8.92% for the owned seeds), and evidently this difference was not significant (
p ≤ 0.05). As in Experiment II, (
Figure 4) the two first phases of germination were described by the water uptake, imbibition, and lag phase.
The analysis of variance for the seed moisture content corresponding to the first, fourth, and eighth day of imbibition are shown in
Table 5. Although dry seeds of the two types showed no differences in moisture content, differences (
p ≤ 0.01) appeared with imbibition, particularly after one day of soaking (9.3%), decreasing with the soaking period down to 4.6% at the eighth day. As in Experiment II, neither the saturated filter paper nor the use of GA restricted the solution uptake with respect to soaking in water. However, the seed lot × saturation method interaction was significant (
p ≤ 0.05) for all the analyzed dates, as the seed moisture reached in the BP method was greater than that in the 10 cm solution column, however, only in standard seeds.
The viability was greater in the owned seeds (
p ≤ 0.01;
Table 6) than in the standard seeds, considering both H and H + WV. The high viability of the owned seeds was expected since, on the one hand, they were manually extracted from the fruits, and mature dark-brown seeds were selected and, on the other hand, these seeds were only 0.5-years old. As mentioned above, the authors in [
9] reported that germination declined for caper seeds after 12 months of storage at room temperature, and [
34] demonstrated viability over 84% after three years of storage. Thus, a high viability was expected for 0.5-year old seeds.
Figure 5 presents the cumulative germination curves fitted to the logistic model obtained for the average values of each seed type and saturation solution combinations. The coefficients of determination (R
2) for the 16 curves (four replicates from four combinations of variation sources) were greater than 0.92.
Large differences (
p ≤ 0.01;
Table 7) were observed for
A between the owned seeds and the standard ones, in favor of the former. The use of GA also had a significant effect (
p ≤ 0.01), multiplying by two the germination in the standard seeds and by 16 in the owned seeds; in the latter, the viability and final germination values coincided totally. The seed lot x saturation solution interaction was significant (
p ≤ 0.01) due to the fact that GA increased
A more intensively in the owned seeds than in the standard ones, presenting greater viability. In addition to the higher germination,
Gt50 was longer for the owned seeds (
p ≤ 0.01) than for the standard seeds and, in both cases, was reduced using GA. With the use of GA all viable seeds germinated (including category WV), which implies that GA increased the “push power” of the weak embryo, so that it was able to expand, allowing the radicle protrusion, i.e., to germinate, and/or it also could reduce the mechanical resistance to expansion of the embryo. This should be studied in depth.
Given that the owned seeds reached a germination of 90% (all the viable ones), and taking into account that seed moisture was greater in the standard seeds than in the owned ones, we determined that caper seeds do not have a water-impermeable coat sensu stricto and that they imbibe water without the seed coat being disrupted.