1. Introduction
Nitrogen (N) is a limiting nutrient in many soils, particularly in those suitable for intensive agricultural production. Indeed, within agricultural management, N addition is a widespread practice to increase crop production. Land application of livestock effluents as untreated slurries and digested slurries may represent an alternative to the use of mineral fertilizers and contribute to the natural closure of nutrient cycles in soils, with economic and environmental benefits. However, the application of these by-products may lead to nitrate (NO
3−-N) leaching and runoff, ammonia (NH
3) volatilization, and nitrous and nitric oxide (N
2O, NO) emissions [
1,
2]. This occurs particularly with low N uptake by crops, and where the soil moisture is higher than the water-holding capacity [
3,
4]. However, it is widely recognized that nitrification inhibitors are able to maintain the N applied to the soil in the ammonium form (NH
4+-N) [
5] through the biological immobilization of the N fertilizer [
6]. Therefore, nitrification inhibitors can reduce the NH
4+ to NO
3− oxidation rate, thus increasing N uptake [
4].
A wide range of nitrification inhibitors are available, and one of the most common is nitrapyrin (2-chloro-6-(trichloromethyl) pyridine), which is able to inhibit nitrification through copper chelation, thereby inactivating the ammonia monooxygenase enzyme that catalyzes NH
4+ oxidation [
5,
7,
8]. Moreover, by hampering the oxidation of NH
4+-N by chemoautotrophic nitrifiers, nitrapyrin reduces NO
3−-N supply to the leaching process, helping to maintain N in the root zone [
9].
The activity of nitrapyrin as a nitrification inhibitor was first described in 1962 by Goring [
10]. Subsequent studies have been carried out in combination with mineral fertilizers, both under laboratory and field conditions [
11] (Wolt, 2000), particularly concerning the effects of nitrapyrin on plant growth and crop yield [
12]. Previous studies on nitrapyrin efficacy as a nitrification inhibitor have demonstrated its ability to inhibit the nitrification process and reduce NO
3−-N leaching by 7 and 27%. Moreover, Abbasi et al. [
5] demonstrated that nitrapyrin addition could increase the soil NH
4+-N concentration, decrease the NO
3−-N content, and reduce the N
2O emissions, with a 30% increase in N recovery.
The majority of the studies in the literature dealing with nitrapyrin efficiency have been carried out using mineral fertilizers as the N source, such as urea, and few have taken into consideration the use of cattle or pig slurry, or manure [
3,
4,
7,
13]. Indeed, no studies were found in which nitrapyrin had been tested in co-application with different N sources derived from animal slurries and digestates, and when these N sources were considered in the same work, the nitrification inhibitor was not nitrapyrin.
Therefore, the current study tested nitrapyrin efficacy in a laboratory experiment under controlled conditions, characterized by a soil treated with two animal slurries (pig and cattle slurry) and one digestate. The aim was to test the influence of nitrapyrin applied jointly with livestock effluents on nitrification inhibition and leaching under aerobic conditions. Specifically, the hypotheses were that nitrapyrin application together with animal slurry and digestate would be able to reduce N leaching and increase N retention, also affecting soil content of microbial N positively.
2. Materials and Methods
An Aquic Xeropsamment soil [
14] was taken from an agricultural area located in the south-east of the Po valley (Rimini, Italy). Samples were collected from the top 20 cm layer, freshly sieved to 2 mm, air-dried, and characterized according to Sparks et al. [
15]. The soil physico-chemical characteristics are reported in
Table 1.
Untreated pig slurry (PS) and cattle slurry (CS) were collected directly from the stables of two farms, while digestate (D) was taken from an anaerobic digestion plant processing cattle slurry together with other organic wastes from the agro-processing and food industry (
Table 2). The nitrification inhibitor used was a commercial formulation (N-Lock
TM, Corteva Agriscience, Wilmington, DE, USA) containing 200 g L
−1 of the active ingredient, nitrapyrin (2-chloro-6-(trichloromethyl)-pyridine).
The leaching experiment was carried out following the Organization for Economic Cooperation and Development [
16] method, with some modifications. Briefly, leaching columns were constructed using a plexiglass pipe (35 cm long and 3.4 cm inner diameter). A plastic mesh (1 mm) was glued to the bottom of the column and a glass fiber filter was then put on the mesh and 30 g of sand (white quartz, Aldrich, St. Louis, MO, USA) was added to ensure water drainage. A second glass fiber filter was added on top of the sand, over which 300 g of air-dried, sieved soil was packed at a bulk density of 1.1 g cm
−3, up to a height of 30 cm. Soil columns were pre-incubated for 14 days in the dark at 18 °C, with the moisture corresponding to 70% of the water holding capacity (WHC). At the end of the pre-incubation, three replicates of the top layer (0–5 cm) of the soil columns were treated with an amount of CS, PS, and D at 100 mg N kg
−1 [
17], plus an amount of N-Lock
TM of 2.5 mg kg
−1. There were six treatments in total: (1) pig slurry (PS); (2) pig slurry plus N-Lock
TM (PSN); (3) cattle slurry CS); (4) cattle slurry plus N-Lock
TM (CSN); (5) digestate (D); and (6) digestate plus N-Lock
TM (DN). The animal sludges (PS and CS) and digestate (D) were mixed with N-lock before application and surface-applied using a pipette. An untreated control (C) and a control receiving 2.5 mg kg
−1 of N-Lock
TM (CN) were included as well. Water was added to the treatments and controls to achieve a uniform soil moisture.
Destructive sampling was carried out after 0, 7, and 28 days of incubation [
17] in the dark at 18 °C. At each sampling date, a glass fiber filter was placed on the surface of the columns and a 0.01 M CaCl
2 solution was applied drop-wise to simulate a rainfall of 200 mm over 4 h. The leachate was then collected, brought up to a final volume of 200 mL, filtrated through Wathman 2V filter paper, and stored at −20 °C. After leaching and allowing the columns to drain, the soil was removed from the columns and sectioned into 5 cm segments. For both leachate and soil, the NH
4+-N, nitrite-N (NO
2−-N), and NO
3−-N were measured by an automatic analyzer (AACE 5.46, Bran Luebbe GmbH, Norderstedt, Germany) following, for soil samples, the ISO 14256-2 method [
18]. Microbial biomass N was determined by the fumigation-extraction method [
19]. Finally, at each sampling time, the net concentrations of the measured parameters in the soil segments and leachates were calculated as the difference between the concentration in the treated and control columns.
The data obtained for each destructive sampling were analyzed independently using a split-split-plot design, with two independent factors (T = type of organic fertilizers and N = nitrapyrin presence) and one related factor (D = soil depth). Parametric ANOVA assumptions were verified through Bartlett’s and Shapiro–Wilk’s tests. The presence of sphericity was tested using Mauchly’s test and the Greenhouse–Geisser correction was used for adjusting the degrees of freedom. The significance of all statistical tests was settled at α = 0.05. An HSD Tukey test was performed to investigate the differences between the means when the ANOVA returned a significant global test. All data are expressed on an oven-dried basis. All statistical analyses were performed using the statistical ambient R [
20].
3. Results
Concentrations of NO2−-N are not reported because they were under the detection limit in both the leachates and soil extracts of all the treatments.
In
Table 3, the main results of the ANOVA statistical analysis are reported. Results concerning the sphericity and the Greenhouse–Geisser correction are reported in the
Supplementary Materials (
Table S1).
Considering the significant interaction of the three factors considered for each of the parameters measured, it was decided to graphically report only the interaction (T × N × D).
Only in the columns treated with PS did the addition of the inhibitor show the highest NH
4+-N concentration in all the measurement times, while the other treatments displayed trends of change with layer depth and days of incubation (
Figure 1). At the beginning of the incubation period (Day 0), no difference in the net concentrations of the soil NH
4+-N were observed among the columns treated with nitrapyrin and the untreated columns. After 7 and 28 days of incubation, the PSN soil columns showed higher net concentrations of NH
4+-N compared to the PS columns, particularly within the first 15 cm. Similar differences were observed between CS and CSN, but in the case of the latter treatment, after 7 days of incubation, differences also occurred within the 20–30 cm soil depth. Differences between the treatments with and without the inhibitor at Days 7 and 28 were also observed in the D application, while at Day 7 differences only occurred in the first 5 cm; at Day 28, differences were also detected in the deeper soil layers, with a higher NH
4+-N concentration in the columns not treated with nitrapyrin.
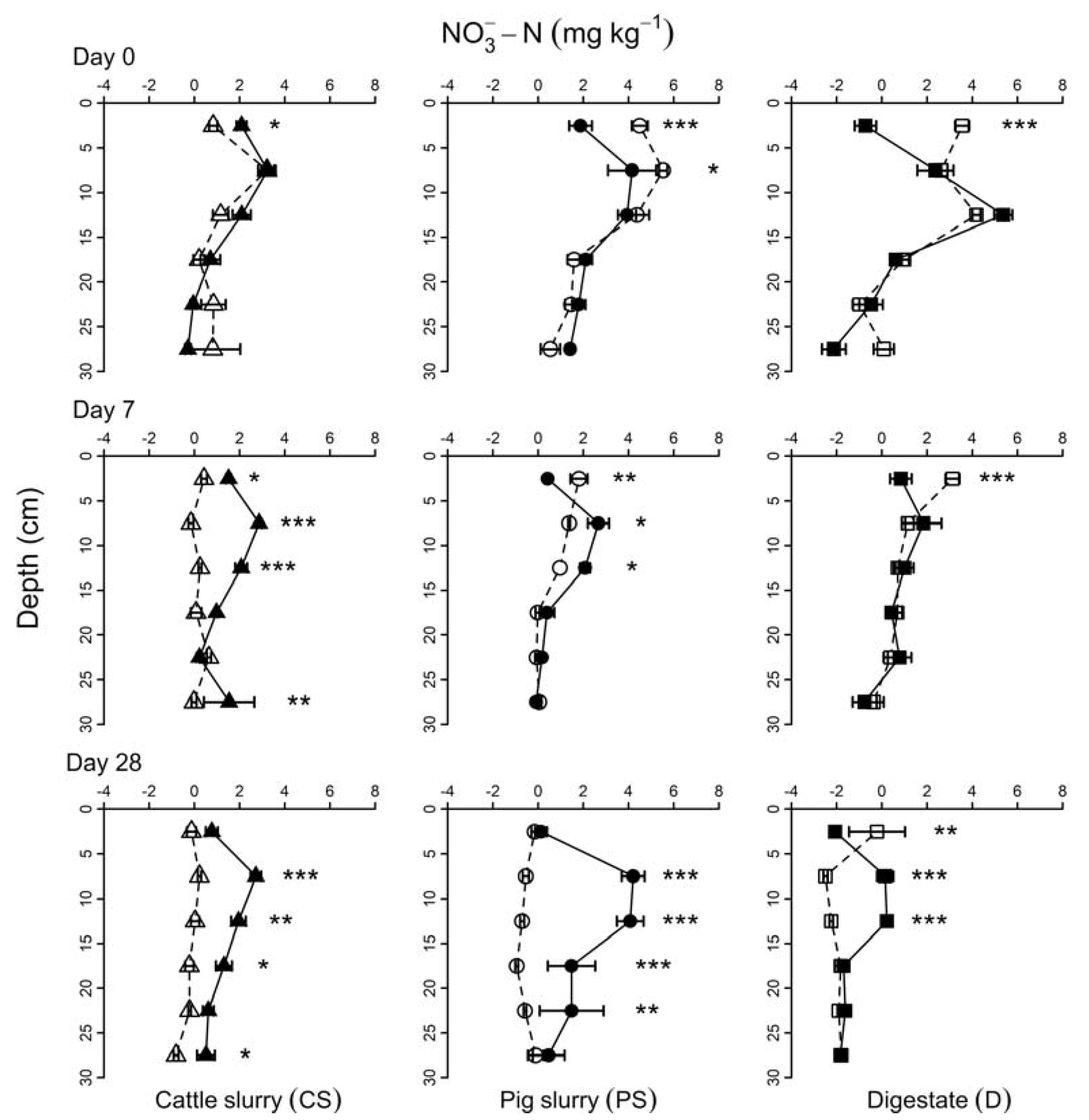
In
Figure 2, it could be observed that, particularly after 0 and 7 days, the nitrate concentrations in the soil profile are similar, except for the superficial layer (first 5 cm). Specifically, after 0 and 7 days of incubation, the NO
3−-N net concentration was significantly higher in the upper layer (0–5 cm) of the PS and D treatments, in comparison with PSN and DN (
Figure 2). On the contrary, nitrapyrin addition to CS (CSN) enhanced the net concentration of the NO
3−-N after Day 0. After seven days of incubation, the CSN treatment showed a higher NO
3−-N net concentration up to a depth of 20 cm. After 28 days of incubation, the CSN sample showed the same trend for the entire column up to a depth of 30 cm. The PSN treatment on Day 28 showed a higher net concentration of NO
3−-N compared with the PS treatment, although, in this case, the differences were only statistically significant within a depth of 5–15 cm. The same result was observed for both the D and DN treatments where the NO
3−-N concentrations overlapped below a depth of 15 cm.
The net concentrations of NO
3−-N and NH
4+-N measured in the leachates are reported in
Table 4. At Day 0, the concentration of NO
3−-N measured in the PSN and DN leachates was significantly higher than in PS and D, while no significant differences between CS and CSN were observed. After 7 and 28 days of incubation, nitrapyrin significantly reduced the concentration of leachable NO
3−-N compared to the PS and D applications. On the contrary, inhibitor addition with CS as a N source resulted in a decrease in NO
3−-N concentration only on Day 7. Nitrapyrin treatment produced differences in the NH
4+-N content in the leachates only with the CS and PS substrates. At Days 7 and 28, nitrapyrin was able to reduce the NH
4+-N content in the CSN- and PSN-treated columns, respectively.
A comparison of the net microbial biomass N measured in the PS and PSN showed differences only in the lower layers of the columns, without a clear trend, while the CS and CSN were the same (
Figure 3). In columns treated with D, DN showed higher values than the D in some of the soil layers, without a discernable relationship to soil depth.
4. Discussion
As expected, the results obtained on Day 0 of incubation indicate that the amount of NH
4+-N added to the different substrates (PS, CS, and D) was not affected by the presence of nitrapyrin in the mixture. Results obtained after 7 and 28 days of incubation, within the first 10 cm of soil, indicated that nitrapyrin effectively inhibited the nitrification process and caused an accumulation of NH
4+-N in the soil. Similar results were observed by Calderón et al. [
3] in a laboratory experiment on soils treated with cattle manure and nitrapyrin. However, on the 28th day, in the deeper layers of the soil columns, the presence of the inhibitor did not affect the NH
4+-N content. Indeed, it has been demonstrated that at 20 °C of incubation, the half-life of nitrapyrin is only 16 days [
21].
Results of the CS and D treatments at 0 and 7 days of incubation suggest that a nitrification inhibitor based on nitrapyrin will reduce the concentration of NO
3−-N in the soil columns before the rainfall simulation, and therefore less NO
3−-N remains in the soil. The differences observed between the treatments may be ascribed to small differences in the efficiency of the leaching process. However, the contrasting effects of nitrapyrin on the net concentration of NO
3−-N detected at different times of incubation (especially after 28 days) in the soil columns may be also due to a reduction of its inhibitory efficacy as incubation proceeds. On Day 28, a reduction in nitrapyrin efficiency was observed in all the soil columns. Higher net concentrations of NO
3−-N were measured for the CS treatment at a depth of 30 cm. The rate of degradation, and hence the efficacy of nitrapyrin, is strongly influenced by many factors. Bundy and Bremner [
21] reported that the half-life of nitrapyrin in soil ranged from 43 to 77 days at 10 °C, but only from 9 to 16 days at 20 °C. Wolt [
11] reported that when the soil temperature, pH, moisture content, and soil organic matter increase, nitrapyrin effectiveness decreases. This may be due to the transformation of nitrapyrin into degradation products, such as 6-chloropicolinic acid, which is less effective as a nitrification inhibitor [
22]. However, adsorption on soil colloids and volatilization can also play an important role in determining the persistence and efficacy of nitrapyrin [
11]. Therefore, in this study, even if the soil had a low clay content and a low content of organic carbon (1.3%), the incubation temperature (18 °C) and soil pH (8.1) may have acted to decrease the nitrapyrin efficacy [
16] in terms of nitrification inhibition and leaching.
Results confirm that nitrapyrin hampers the oxidation of NH
4+-N to NO
3−-N, and significantly reduces the supply of NO
3−-N to the leaching process [
9]. Compared to Day 7, differences observed at Day 28 were of a smaller magnitude. This concurs with the increase in NO
3−-N concentration observed in the soil columns and indicate that at the end of the incubation period, the effect of the nitrapyrin is partially lost. This phenomenon must not be regarded as a negative result. Nitrification inhibitors, such as nitrapyrin, are used with the intention of delaying the oxidation of ammonium to nitrate for a certain period of time, and not to hamper it completely. Ideally, the effective period of nitrification inhibitors should last long enough to bridge critical periods when the risk of NO
3−-N leaching is high, for example when crop N uptake is low and the soil is wet [
23,
24].
Contrary to what was observed by Calderón et al. [
3], the current study suggests that (under the conditions of this study) a nitrification inhibitor such as nitrapyrin does not have a negative effect on the soil microbial biomass, especially in co-application with the digestate. This microbial N is an organic N pool characterized by a short turnover and can, therefore, be available for plant uptake during the crop growing season through remineralization [
3].
Finally, the majority of the works on nitrapyrin in the literature compare different soils or one type of manure product with mineral fertilizers [
3,
4,
7,
23,
24]. The application of nitrapyrin together with different kinds of fertilizers demonstrates its higher efficacy with slurry, manure, or digestate than with mineral fertilizers. In this study, the three different organic by-products (PS, CS, and D) produced different responses in terms of nitrapyrin efficacy. Indeed, on Day 28 of incubation, only the PSN and DN increased the NH
4+-N soil content, and reduced the amount of NO
3−-N leached. CSN, even if able to slightly increase the NH
4+-N soil content, induced a reduction of NO
3−-N leaching only at Day 7. Therefore, the better efficacy of nitrapyrin over time, when added to PS and D, could be linked to their chemical characteristics, such as the C:N ratio [
25]. Moreover, the higher organic C content in the digestate (D) could be considered the driver for the higher microbial N measured under this treatment. On the other hand, this higher organic C content could also have reduced the nitrapyrin efficacy in the D compared to the PS treatment.
In this study, we have considered the effects of nitrapyrin and livestock effluent addition in relation to soil nitrification inhibition and leaching. Future studies should include also the influence of the rhizosphere and the possible N2O losses in order to have a complete balance of this process.
5. Conclusions
Under the conditions of this study, nitrapyrin effectively inhibited the nitrification process and kept most of the mineral N in the NH4+-N form, especially when applied jointly with pig slurries or digestate. As a result of the nitrification inhibition, less NO3−-N was available for leaching, and the concentration of NO3−-N in leachates from the soil columns treated with N-LockTM was negligible after 28 days of incubation. The data obtained after 7 and 28 days of incubation suggested that part of the efficacy of the nitrapyrin was lost as time passed by, particularly in the case of CS. No negative effects of nitrapyrin on the microbial biomass were observed. On the contrary, nitrapyrin applied with digestate favored the microbial biomass within the first 15 cm of the soil.
The results of this study show the potential of nitrapyrin used in conjunction with livestock effluents to reduce N losses through leaching. Considering the positive effects of pig slurry and digestate on nitrapyrin efficacy, future studies on nitrapyrin activity with different types of organic fertilizers should be carried out in both the laboratory and the field, including also crops in order to elucidate which kind of organic by-product effectively improves the nitrapyrin efficacy.