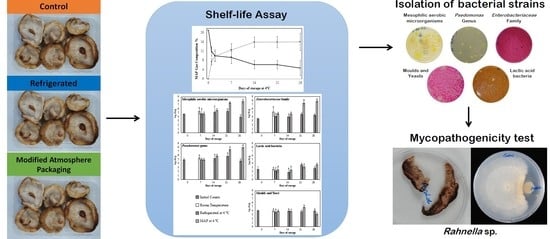
Effect of Bacterial Strains Isolated from Stored Shiitake (Lentinula edodes) on Mushroom Biodeterioration and Mycelial Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Storage Conditions
2.2. Shelf-Life Assay
2.2.1. Modified Atmosphere Gas Measurement
2.2.2. Determination of Weight Loss
2.2.3. Microbial Analysis
2.2.4. Sensory Analysis
2.3. Strains Isolation and Identification
2.4. Pathogenicity Test
2.5. Antibiogram Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Modification of Package Atmosphere Composition
3.2. Time Trend of Weight
3.3. Time Trend of Microbial Populations
3.4. Sensory Evaluation and Shelf Life
3.5. Identification of Bacterial Isolates
3.6. Biodeterioration Capacity of the Isolated Strains
3.7. Inhibition of Mycelium Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gil-Ramírez, A.; Soler-Rivas, C. The use of edible mushroom extracts as bioactive ingredients to design novel functional foods with hypocholesterolemic activities. In Mushrooms: Cultivation, Antioxidant Properties and Health Benefits.; Pesti, G., Ed.; Nova Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 44–73. ISBN 978-1-63117-521-3. [Google Scholar]
- Ares, G.; Parentelli, C.; Gámbaro, A.; Lareo, C.; Lema, P. Sensory shelf life of shiitake mushrooms stored under passive modified atmosphere. Postharvest Biol. Technol. 2006, 41, 191–197. [Google Scholar] [CrossRef]
- Han Lyn, F.; Maryam Adilah, Z.A.; Nor-Khaizura, M.A.R.; Jamilah, B.; Nur Hanani, Z.A. Application of modified atmosphere and active packaging for oyster mushroom (Pleurotus ostreatus). Food Packag. Shelf Life 2020, 23, 100451. [Google Scholar] [CrossRef]
- Rivera, C.S.; Blanco, D.; Marco, P.; Oria, R.; Venturini, M.E. Effects of electron-beam irradiation on the shelf life, microbial populations and sensory characteristics of summer truffles (Tuber aestivum) packaged under modified atmospheres. Food Microbiol. 2011, 28, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wei, Q.; Huang, L.; Fang, T.; Chen, B.; Jiang, Y. Mathematical modeling Pseudomonas spp. growth and microflora composition variation in Agaricus bisporus fruiting bodies during chilled storage. Postharvest Biol. Technol. 2020, 163, 111144. [Google Scholar] [CrossRef]
- Rivera, C.S.; Venturini, M.E.; Marco, P.; Oria, R.; Blanco, D. Effects of electron-beam and gamma irradiation treatments on the microbial populations, respiratory activity and sensory characteristics of Tuber melanosporum truffles packaged under modified atmospheres. Food Microbiol. 2011, 28, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.E.; Venturini, M.E.; Oria, R.; Blanco, D. Prevalence of Ewingella americana in retail fresh cultivated mushrooms (Agaricus bisporus, Lentinula edodes and Pleurotus ostreatus) in Zaragoza (Spain). FEMS Microbiol. Ecol. 2004, 47, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, P.R.; Heinemann, J.A. The general secretory pathway of Burkholderia gladioli pv. agaricicola BG164R is necessary for cavity disease in white button mushrooms. Appl. Environ. Microbiol. 2006, 60, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barghouthi, S.A. A Universal Method for the Identification of Bacteria Based on General PCR Primers. Indian J. Microbiol. 2011, 51, 430–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Combet, E.; Henderson, J.; Henderson, J.; Combet, E. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Venturini, M.E.; Rivera, C.S.; Gonzalez, C.; Blanco, D. Antimicrobial activity of extracts of edible wild and cultivated mushrooms against foodborne bacterial strains. J. Food Prot. 2008, 71, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Luo, S.; Chen, Q.; Shen, L.; Ying, T. Effect of integrated application of gamma irradiation and modified atmosphere packaging on physicochemical and microbiological properties of shiitake mushroom (Lentinus edodes). Food Chem. 2010, 122, 761–767. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Jolivet, S.; Arpin, N.; Olivier, J.M.; Wichers, H.J. Biochemical and physiological aspects of brown blotch disease of Agaricus bisporus. FEMS Microbiol. Rev. 1999, 23, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Aslani, M.A.; Harighi, B.; Abdollahzadeh, J. Screening of endofungal bacteria isolated from wild growing mushrooms as potential biological control agents against brown blotch and internal stipe necrosis diseases of Agaricus bisporus. Biol. Control 2018, 119, 20–26. [Google Scholar] [CrossRef]
- Hamilton-Miller, J.M.; Shah, S. Identity and antibiotic susceptibility of enterobacterial flora of salad vegetables. Int. J. Antimicrob. Agents 2001, 18, 81–83. [Google Scholar] [CrossRef]
- Helps, C.R.; Harbour, D.A.; Corry, J.E. PCR-based 16S ribosomal DNA detection technique for Clostridium estertheticum causing spoilage in vacuum-packed chill-stored beef. Int. J. Food Microbiol. 1999, 52, 57–65. [Google Scholar] [CrossRef]
- Rao, J.R.; Millar, B.C.; Moore, J.E. Antimicrobial properties of shiitake mushrooms (Lentinula edodes). Int. J. Antimicrob. Agents 2009, 33, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Lo Cantore, P.; Giorgio, A.; Iacobellis, N.S. Bioactivity of volatile organic compounds produced by Pseudomonas tolaasii. Front. Microbiol. 2015, 6, 1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 dashed line) and O2 (
dashed line) and O2 (  continuous line) of shiitake mushrooms packaged under modified atmosphere stored at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples.
continuous line) of shiitake mushrooms packaged under modified atmosphere stored at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples.
 dashed line) and O2 (
dashed line) and O2 (  continuous line) of shiitake mushrooms packaged under modified atmosphere stored at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples.
continuous line) of shiitake mushrooms packaged under modified atmosphere stored at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples.
 initial counts,
initial counts,  room temperature,
room temperature,  refrigerated at 4 °C, and
refrigerated at 4 °C, and  modified atmosphere packaging at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples. a, b Different superscript letters within the same day of storage indicate statistically significant differences at p < 0.05.
modified atmosphere packaging at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples. a, b Different superscript letters within the same day of storage indicate statistically significant differences at p < 0.05.
 initial counts,
initial counts,  room temperature,
room temperature,  refrigerated at 4 °C, and
refrigerated at 4 °C, and  modified atmosphere packaging at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples. a, b Different superscript letters within the same day of storage indicate statistically significant differences at p < 0.05.
modified atmosphere packaging at 4 °C. Data expressed as mean ± standard deviation (SD) of three samples. a, b Different superscript letters within the same day of storage indicate statistically significant differences at p < 0.05.
 0: no appreciable damage,
0: no appreciable damage,  1: slight damage in the inoculation area,
1: slight damage in the inoculation area,  2: extended damage in the inoculation area,
2: extended damage in the inoculation area,  3: severe damage throughout the carpophore sample.
3: severe damage throughout the carpophore sample.
 0: no appreciable damage,
0: no appreciable damage,  1: slight damage in the inoculation area,
1: slight damage in the inoculation area,  2: extended damage in the inoculation area,
2: extended damage in the inoculation area,  3: severe damage throughout the carpophore sample.
3: severe damage throughout the carpophore sample.

| Day | C | R | MAP |
|---|---|---|---|
| 0 | 0.0 ± 0.0 b | 0.0 ± 0.0 d | 0.0 ± 0.0 c |
| 7 | 78.1 ± 5.9 a | 24.7 ± 4.6 c | 2.3 ± 0.6 b |
| 14 | - | 36.4 ± 4.0 bc | 2.5 ± 0.4 b |
| 21 | - | 50.5 ± 5.1 b | 3.0 ± 0.1 b |
| 28 | - | 84.2 ± 5.7 a | 4.9 ± 0.7 a |
| Day | Storage Condition | External Appearance | Hymenium Appearance | Texture | Aroma | Taste | General Acceptability |
|---|---|---|---|---|---|---|---|
| 0 | C | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a |
| R | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | |
| MAP | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 9.0 ± 0.0 a | |
| 7 | C | 5.8 ± 1.5 b | 4.7 ± 0.9 b | 6.5 ± 0.8 b | 7.1 ± 0.5 b | 6.8 ± 0.8 b | 5.8 ± 1.2 b |
| R | 8.4 ± 0.5 a | 8.2 ± 0.3 a | 8.6 ± 0.2 a | 8.5 ± 0.1 a | 8.5 ± 0.2 a | 8.3 ± 0.5 a | |
| MAP | 8.3 ± 0.7 a | 8.6 ± 0.3 a | 8.8 ± 0.1 a | 8.7 ± 0.2 a | 8.6 ± 0.3 a | 8.5 ± 0.4 a | |
| 14 | C | 1.7 ± 0.9 b | 1.1 ± 0.3 b | 3.0 ± 0.5 b | 4.5 ± 0.5 b | - | 2.1 ± 0.5 b |
| R | 7.5 ± 0.7 a | 7.9 ± 0.5 a | 7.2 ± 0.5 a | 7.3 ± 0.4 a | 7.7 ± 0.6 a | 7.5 ± 0.4 a | |
| MAP | 7.1 ± 0.9 a | 7.7 ± 0.6 a | 8.1 ± 0.4 a | 7.9 ± 0.6 a | 7.5 ± 0.7 a | 7.0 ± 0.6 a | |
| 21 | C | - | - | - | - | - | - |
| R | 7.0 ± 1.1 a | 7.1 ± 0.8 a | 5.5 ± 0.4 b | 6.6 ± 0.8 a | 6.6 ± 0.8 a | 6.5 ± 0.8 a | |
| MAP | 5.5 ± 0.7 a | 5.8 ± 0.9 a | 7.2 ± 0.8 a | 5.7 ± 1.1 a | 4.9 ± 0.3 b | 5.0 ± 1.1 b | |
| 28 | C | - | - | - | - | - | - |
| R | 5.4 ± 0.8 a | 5.1 ± 0.3 a | 3.8 ± 0.7 a | 5.9 ± 0.4 a | 6.0 ± 1.2 | 5.1 ± 1.0 a | |
| MAP | 1.5 ± 1.1 b | 2.1 ± 0.3 b | 5.1 ± 0.4 a | 3.1 ± 0.7 b | - | 1.3 ± 0.4 b |
| Day-Storage | Accession Number | Genus | L. edodes | A. bisporus | |
|---|---|---|---|---|---|
| Slices | Cap | Slices | |||
| D0 | MT335659 | Microbacterium | 3 | 3 | 0 |
| D0 | MT335657 | Paenibacillus | 0 | 2 | 2 |
| D0 | MT335639 | Ewingella | 1 | 1 | 0 |
| D0 | MT335646 | Pseudomonas | 0 | 1 | 1 |
| D0 | MT335652 | Pseudomonas | 0 | 1 | 0 |
| D0 | MT335647 | Pseudomonas | 0 | 1 | 1 |
| D7-C | MT335641 | Ewingella | 0 | 2 | 2 |
| D7-C | MT335650 | Pseudomonas | 1 | 1 | 1 |
| D7-C | MT335667 | Pseudomonas | 3 | 1 | 0 |
| D7-C | MT335653 | Pseudomonas | 3 | 1 | 2 |
| D7-C | MT335673 | Pseudomonas | 0 | 1 | 2 |
| D7-C | MT335654 | Pseudomonas | 3 | 1 | 3 |
| D7-R | MT335642 | Ewingella | 1 | 2 | 3 |
| D7-R | MT335644 | Ewingella | 2 | 1 | 1 |
| D7-R | MT335648 | Ewingella | 3 | 1 | 1 |
| D7-R | MT335656 | Ewingella | 3 | 1 | 2 |
| D7-R | MT335683 | Pseudomonas | 3 | 1 | 1 |
| D7-R | MT335651 | Pseudomonas | 1 | 0 | 0 |
| D7-MAP | MT335643 | Micrococcus | 0 | 2 | 2 |
| D7-MAP | MT335658 | Ewingella | 3 | 1 | 1 |
| D7-MAP | MT335660 | Ewingella | 0 | 2 | 3 |
| D7-MAP | MT335679 | Pseudomonas | 1 | 1 | 1 |
| D7-MAP | MT335645 | Pseudomonas | 3 | 2 | 1 |
| D7-MAP | MT335661 | Serratia | 3 | 2 | 1 |
| D14-R | MT335662 | Ewingella | 3 | 2 | 1 |
| D14-R | MT335666 | Ewingella | 3 | 3 | 1 |
| D14-R | MT335665 | Ewingella | 3 | 2 | 0 |
| D14-R | MT335684 | Pseudomonas | 1 | 2 | 2 |
| D14-R | MT335664 | Pseudomonas | 3 | 1 | 2 |
| D14-R | MT335669 | Serratia | 3 | 0 | 2 |
| D14-MAP | MT335649 | Burkholderia | 3 | 2 | 3 |
| D14-MAP | MT335663 | Ewingella | 3 | 2 | 2 |
| D14-MAP | MT335671 | Ewingella | 3 | 1 | 3 |
| D14-MAP | MT335675 | Pseudomonas | 3 | 2 | 2 |
| D14-MAP | MT335640 | Pseudomonas | 3 | 2 | 2 |
| D14-MAP | MT335676 | Pseudomonas | 1 | 2 | 1 |
| D28-R | MT335686 | Ewingella | 3 | 1 | 3 |
| D28-R | MT335674 | Ewingella | 3 | 3 | 2 |
| D28-R | MT335680 | Ewingella | 3 | 2 | 3 |
| D28-R | MT335685 | Ewingella | 3 | 2 | 2 |
| D28-R | MT335670 | Pseudomonas | 3 | 2 | 1 |
| D28-R | MT335678 | Pseudomonas | 3 | 1 | 1 |
| D28-MAP | MT335682 | Paenarthrobacter | 1 | 1 | 3 |
| D28-MAP | MT335672 | Ewingella | 3 | 1 | 3 |
| D28-MAP | MT335668 | Ewingella | 3 | 1 | 3 |
| D28-MAP | MT335677 | Pseudomonas | 3 | 1 | 3 |
| D28-MAP | MT335681 | Pseudomonas | 0 | 1 | 2 |
| D28-MAP | MT335655 | Rahnella | 3 | 2 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejedor-Calvo, E.; García-Barreda, S.; Sánchez, S.; Marco, P. Effect of Bacterial Strains Isolated from Stored Shiitake (Lentinula edodes) on Mushroom Biodeterioration and Mycelial Growth. Agronomy 2020, 10, 898. https://doi.org/10.3390/agronomy10060898
Tejedor-Calvo E, García-Barreda S, Sánchez S, Marco P. Effect of Bacterial Strains Isolated from Stored Shiitake (Lentinula edodes) on Mushroom Biodeterioration and Mycelial Growth. Agronomy. 2020; 10(6):898. https://doi.org/10.3390/agronomy10060898
Chicago/Turabian StyleTejedor-Calvo, Eva, Sergi García-Barreda, Sergio Sánchez, and Pedro Marco. 2020. "Effect of Bacterial Strains Isolated from Stored Shiitake (Lentinula edodes) on Mushroom Biodeterioration and Mycelial Growth" Agronomy 10, no. 6: 898. https://doi.org/10.3390/agronomy10060898
APA StyleTejedor-Calvo, E., García-Barreda, S., Sánchez, S., & Marco, P. (2020). Effect of Bacterial Strains Isolated from Stored Shiitake (Lentinula edodes) on Mushroom Biodeterioration and Mycelial Growth. Agronomy, 10(6), 898. https://doi.org/10.3390/agronomy10060898