Betaine Hydrochloride Treatment Affects Growth and Phenylpropanoid Accumulation in Tartary Buckwheat (Fagopyrum tataricum) Seedlings under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Phenylpropanoid Extraction
2.3. HPLC Analysis of Phenylpropanoid Content
2.4. Statistical Analysis
3. Results
3.1. Effect of NaCl Treatment on the Growth of Tartary Buckwheat Sprouts
3.2. Effect of 50 mM NaCl Combined with Different Concentrations of Betaine Hydrochloride on the Growth of Tartary Buckwheat Sprouts
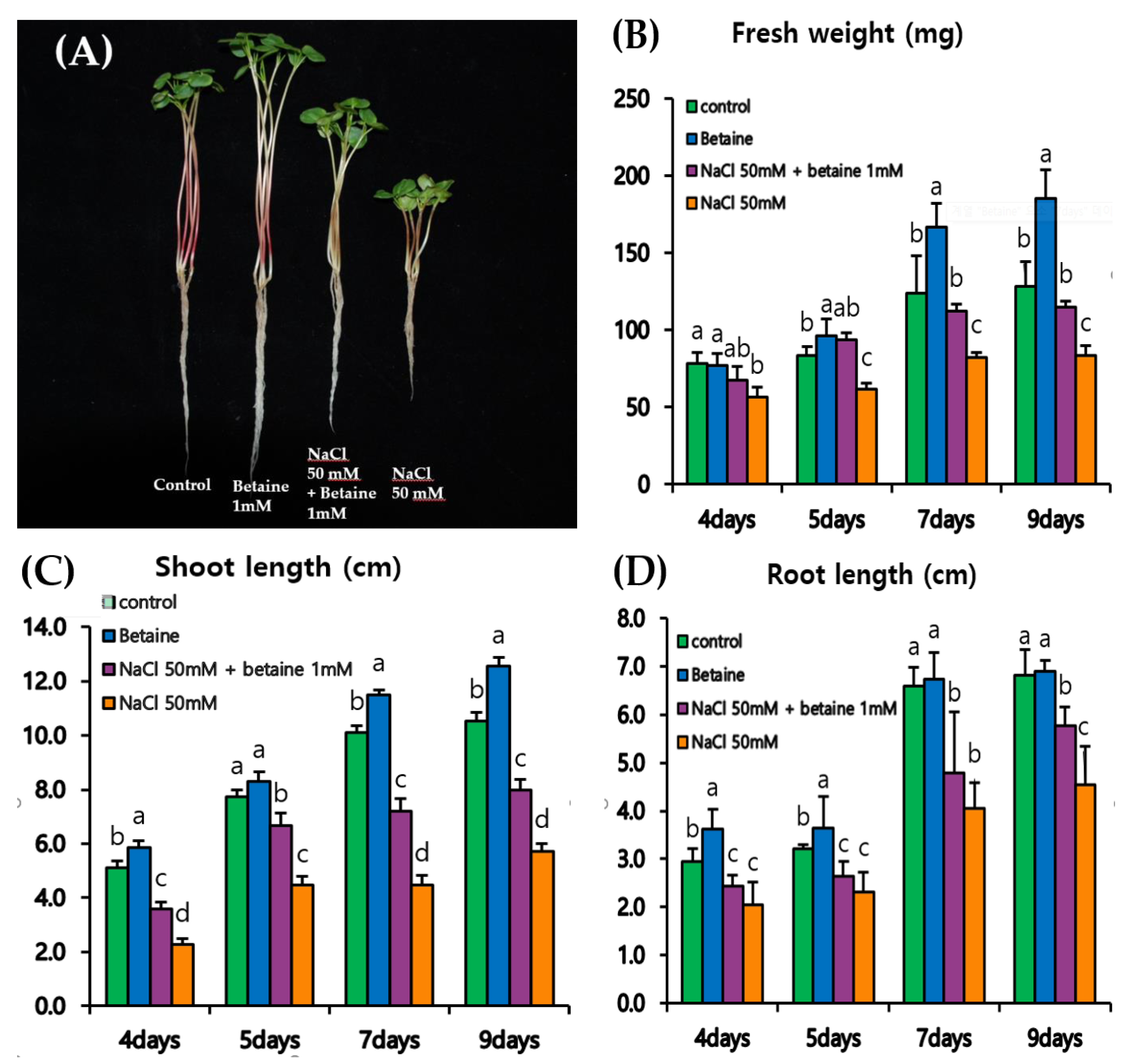
3.3. Growth of Tartary Buckwheat Sprouts under a Time-Course Experiment
3.4. Effect of 50 mM NaCl, 1 mM Betaine, and Their Combination on Phenylpropanoid Content (mg/g Dry wt.) of Buckwheat Sprouts at Different Harvest Times
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tsugane, K.; Kobayashi, K.; Niwa, Y.; Ohba, Y.; Wada, K.; Kobayashi, H. A recessive Arabidopsis mutant that grows photoautotrophically under salt stress shows enhanced active oxygen detoxification. Plant Cell 1999, 11, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2.−/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Isayenkov, S. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Passioura, J. Hydraulic resistance of plants. III. Effects of NaCl in barley and lupin. Funct. Plant Biol. 1984, 11, 351–359. [Google Scholar] [CrossRef]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Funct. Plant Biol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Rajendran, K.; Tester, M.; Roy, S.J. Quantifying the three main components of salinity tolerance in cereals. Plant Cell Environ. 2009, 32, 237–249. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.J.; Negrão, S.; Tester, M. Salt resistant crop plants. Curr. Opin. Biotechnol. 2014, 26, 115–124. [Google Scholar] [CrossRef]
- Petrusa, L.M.; Winicov, I. Proline status in salt-tolerant and salt-sensitive alfalfa cell lines and plants in response to NaCl. Plant Physiol. Biochem. 1997, 35, 303–310. [Google Scholar]
- Pedranzani, H.; Racagni, G.; Alemano, S.; Miersch, O.; Ramírez, I.; Peña-Cortés, H.; Taleisnik, E.; Machado-Domenech, E.; Abdala, G. Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul. 2003, 41, 149–158. [Google Scholar] [CrossRef]
- Oomah, B.D.; Mazza, G. Flavonoids and antioxidative activities in buckwheat. J. Agric. Food Chem. 1996, 44, 1746–1750. [Google Scholar] [CrossRef]
- Barros, J.; Serrani-Yarce, J.C.; Chen, F.; Baxter, D.; Venables, B.J.; Dixon, R.A. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nature Plants 2016, 2, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.; Amenta, M.; Lamattina, L.; Cassia, R. Retracted: Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ. 2011, 34, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Compatible rhizosphere microbes mediated alleviation of biotic stress in chickpea through enhanced antioxidant and phenylpropanoid activities. Microbiol. Res. 2013, 168, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Routaboul, J.-M.; Dubos, C.; Beck, G.; Marquis, C.; Bidzinski, P.; Loudet, O.; Lepiniec, L. Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. J. Exp. Bot. 2012, 63, 3749–3764. [Google Scholar] [CrossRef] [Green Version]
- Saito, K.; Yonekura-Sakakibara, K.; Nakabayashi, R.; Higashi, Y.; Yamazaki, M.; Tohge, T.; Fernie, A.R. The flavonoid biosynthetic pathway in Arabidopsis: Structural and genetic diversity. Plant Physiol. Biochem. 2013, 72, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, D.; Hanson, A. Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant Biol. 1993, 44, 357–384. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Protection of plasma membrane of onion epidermal cells by glycinebetaine and proline against NaCl stress. Plant Physiol. Biochem. 1998, 36, 767–772. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.; Ashraf, M.; Foolad, M. Improving plant abiotic-stress resistance by exogenous application of osmoprotectants glycine, betaine and proline. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Cuong, D.M.; Kwon, S.-J.; Nguyen, B.V.; Chun, S.W.; Kim, J.K.; Park, S.U. Effect of Salinity Stress on Phenylpropanoid Genes Expression and Related Gene Expression in Wheat Sprout. Agronomy 2020, 10, 390. [Google Scholar] [CrossRef] [Green Version]
- Koca, H.; Bor, M.; Özdemir, F.; Türkan, İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007, 60, 344–351. [Google Scholar] [CrossRef]
- Saha, P.; Chatterjee, P.; Biswas, A.K. NaCl pretreatment alleviates salt stress by enhancement of antioxidant defense system and osmolyte accumulation in mungbean (Vigna radiata L. Wilczek). Indian J. Exp. Biol. 2010, 48, 593–600. [Google Scholar] [PubMed]
- Agarwal, S.; Pandey, V. Antioxidant enzyme responses to NaCl stress in Cassia angustifolia. Biol. Plant. 2004, 48, 555–560. [Google Scholar] [CrossRef]
- Jamil, M.; Bae, D.; Yong, K.; Ashraf, M.; Chun, L.; Shik, R. Effect of salt (NaCl) stress on germination and early seedling growth of four vegetables species. J. Cent. Eur. Agric. 2006, 7, 273–282. [Google Scholar]
- Noreen, Z.; Ashraf, M. Assessment of variation in antioxidative defense system in salt-treated pea (Pisum sativum) cultivars and its putative use as salinity tolerance markers. J. Plant Physiol. 2009, 166, 1764–1774. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Demiral, T.; Türkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Harinasut, P.; Tsutsui, K.; Takabe, T.; Nomura, M.; Takabe, T.; Kishitani, S. Exogenous glycinebetaine accumulation and increased salt-tolerance in rice seedlings. Biosci. Biotechnol. Biochem. 1996, 60, 366–368. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, P.; Peltonen-Sainio, P.; Jokinen, K.; Pehu, E.; Setälä, H.; Hinkkanen, R.; Somersalo, S. Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Sci. 1996, 121, 221–230. [Google Scholar] [CrossRef]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, C. Photosynthesis is improved by exogenous glycinebetaine in salt-stressed maize plants. Physiol. Plant. 2005, 124, 343–352. [Google Scholar] [CrossRef]
- Nawaz, K.; Ashraf, M. Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci. 2010, 196, 28–37. [Google Scholar] [CrossRef]
- Raza, S.H.; Athar, H.R.; Ashraf, M.; Hameed, A. Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ. Exp. Bot. 2007, 60, 368–376. [Google Scholar] [CrossRef]
- Hossain, M.A.; Fujita, M. Evidence for a role of exogenous glycinebetaine and proline in antioxidant defense and methylglyoxal detoxification systems in mung bean seedlings under salt stress. Physiol. Mol. Biol. Plants 2010, 16, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Heuer, B. Influence of exogenous application of proline and glycinebetaine on growth of salt-stressed tomato plants. Plant Sci. 2003, 165, 693–699. [Google Scholar] [CrossRef]
- Grieve, C.; Francois, L.; Poss, J. Effect of salt stress during early seedling growth on phenology and yield of spring wheat. Cereal Res. Commun. 2001, 29, 167–174. [Google Scholar] [CrossRef]
- Pandey, M.; Penna, S. Time course of physiological, biochemical, and gene expression changes under short-term salt stress in Brassica juncea L. Crop J. 2017, 5, 219–230. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.M.; Flores, P.; Garrido, C.; Martinez, V. Changes in the contents of antioxidant compounds inpepper fruits at different ripening stages, as affected by salinity. Food Chem. 2006, 96, 66–73. [Google Scholar] [CrossRef]
- Ben Abdallah, S.; Aung, B.; Amyot, L.; Lalin, I.; Lachaal, M.; Karray-Bouraoui, N.; annoufa, A. Salt stress (NaCl) a_ects plant growth and branch pathways of carotenoid and flavonoid biosyntheses in Solanum nigrum. Acta Physiol. Plant. 2016, 38, 72. [Google Scholar] [CrossRef]
- Hichem, H.; Mounir, D.; Naceur, E. Di_erential responses of two maize (Zea mays L.) varieties to salt stress: Changes on polyphenols composition of foliage and oxidative damages. Ind. Crop. Prod. 2009, 30, 144–151. [Google Scholar] [CrossRef]
- Kim, N.S.; Kwon, S.J.; Cuong, D.M.; Jeon, J.; Park, J.S.; Park, S.U. Accumulation of phenylpropanoids in tartary buckwheat (Fagopyrum tataricum) under salt stress. Agronomy 2019, 9, 739. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.; Jung, H.J.; Rahim, M.A.; Park, N.K.; Kuk, Y. Molecular analysis of genes related to phenylpropanoid and ascorbate biosynthesis in salt and UV-B treated pak choi grown under LEDs. Botany 2019, 97, 513–519. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kim, J.K.; Li, X.; Kim, Y.B.; Uddin, M.R.; Kim, S.J.; Suzuki, T.; Park, N.I.; Park, S.U. Metabolomic analysis and phenylpropanoid biosynthesis in hairy root culture of tartary buckwheat cultivars. PLoS ONE 2013, 8, 6. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Kwon, D.Y. Salt in irrigation water a_ects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Lopez-Berenguer, C.; Martinez-Ballesta, M.D.; Moreno, D.A.; Carvajal, M.; Garcia-Viguera, C. Growing hardier crops for better health: Salinity tolerance and the nutritional value of Broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef]
| Treatment | Fresh Weight (mg) | Shoot Length (cm) | Root Length (cm) |
|---|---|---|---|
| Control | 123.90 ± 11.70 a 1 | 8.86 ± 0.25 a | 6.66 ± 0.51 a |
| NaCl 30 mM | 96.0 ± 3.54 b | 5.81 ± 1.27 b | 5.81 ± 0.44 b |
| NaCl 50 mM | 82.20 ± 3.19 c | 4.48 ± 0.31 c | 4.06 ± 0.47 c |
| NaCl 70 mM | 40.80 ± 1.02 d | 1.08 ± 0.18 d | 3.59 ± 0.60 c |
| NaCl 100 mM | 28.05 ± 3.44 e | 0.90 ± 0.21 d | 2.05 ± 2.08 d |
| Treatment | Fresh Weight (mg) | Shoot Length (cm) | Root Length (cm) |
|---|---|---|---|
| Control | 118.80 ± 15.68 c 1 | 8.72 ± 0.34 d | 6.59 ± 0.25 a |
| Betaine 0.1 mM | 167.00 ± 6.10 a | 10.40 ± 0.80 b | 6.28 ± 1.00 a |
| Betaine 0.5 mM | 179.60 ± 3.61 a | 10.86 ± 0.51 a b | 6.67 ± 0.50 a |
| Betaine 1 mM | 181.60 ± 6.68 a | 11.48 ± 0.29 a | 6.23 ± 0.82 a |
| Betaine 5 mM | 141.40 ± 5.00 b | 10.00 ± 0.55 b c | 5.59 ± 0.73 b |
| Betaine 10 mM | 146.80 ± 13.64 b | 9.40 ± 0.73 c d | 3.51 ± 0.37 c |
| Betaine 20 mM | 152.20 ± 9.37 b | 6.32 ± 0.36 e | 3.21 ± 0.39 c |
| Betaine 30 mM | 121.80 ± 14.19 c | 6.68 ± 0.93 e | 2.99 ± 0.66 c |
| Treatment | Fresh Weight (mg) | Shoot Length (cm) | Root Length(cm) |
|---|---|---|---|
| Control | 134.40 ± 6.50 a 1 | 11.62 ± 0.19 a | 7.90 ± 0.97 a |
| NaCl 50 mM | 69.20 ± 5.11 c | 4.48 ± 0.34 e | 4.06 ± 0.52 d |
| NaCl 50 mM + Betaine 0.1 mM | 127.00 ± 15.06 a | 8.10 ± 0.66 c | 7.70 ± 0.24 a |
| NaCl 50 mM + Betaine 0.5 mM | 137.40 ± 3.83 a | 8.24 ± 0.56 c | 7.64 ± 0.59 a |
| NaCl 50 mM + Betaine 1 mM | 134.60 ± 5.12 a | 9.96 ± 0.22 b | 6.98 ± 0.90 a |
| NaCl 50 mM + Betaine 5 mM | 109.60 ± 11.88 b | 8.32 ± 0.19 c | 5.64 ± 0.64 b |
| NaCl 50 mM + Betaine 10 mM | 98.40 ± 4.76 b | 7.92 ± 0.17 c | 5.40 ± 0.49 b c |
| NaCl 50 mM + Betaine 20 mM | 97.80 ± 7.33 b | 7.28 ± 0.23 d | 4.64 ± 0.32 c d |
| NaCl 50 mM + Betaine 30 mM | 44.60 ± 4.76 d | 3.12 ± 0.07 f | 2.76 ± 0.25 e |
| Trivial Names | 4 Days | 5 Days | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Betaine 1 | NaCl 50 | Betaine 1 NaCl 50 | Control | Betaine 1 | NaCl 50 | Betaine 1 NaCl 50 | |
| Gallic acid | 0.01 ± 0.00 a 1 | 0.01 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a |
| Chlorogenic acid | 1.64 ± 0.10 a | 1.63 ± 0.06 a | 0.72 ± 0.00 c | 0.94 ± 0.02 b | 2.58 ± 0.06 a | 2.36 ± 0.00 b | 1.22 ± 0.04 d | 1.39 ± 0.01 c |
| Epicatechin | 2.74 ± 0.65 b | 3.47 ± 0.05 a | 2.26 ± 0.01 b | 2.42 ± 0.07 b | 3.28 ± 0.11 a | 3.25 ± 0.03 a | 3.18 ± 0.06 a | 2.77 ± 0.00 b |
| p-Coumaric acid | 0.25 ± 0.01 a b | 0.28 ± 0.02 a | 0.21 ± 0.00 b | 0.23 ± 0.02 b | 0.14 ± 0.00 | 0.15 ± 0.00 | 0.20 ± 0.01 | 0.18 ± 0.00 |
| Ferulic acid | 0.13 ± 0.07 a | 0.11 ± 0.00 a | 0.10 ± 0.01 a | 0.11 ± 0.00 a | 0.10 ± 0.01 | 0.08 ± 0.00 | 0.15 ± 0.00 | 0.14 ± 0.00 |
| Benzoic acid | 1.83 ± 0.48 a | 1.44 ± 0.01 a | 1.04 ± 0.01 c | 1.27 ± 0.06 b | 1.91 ± 0.03 | 1.91 ± 0.03 | 1.61 ± 0.04 | 1.70 ± 0.03 |
| Rutin | 26.26 ± 1.06 a | 26.34 ± 0.79 a | 24.71 ± 0.56 a | 27.56 ± 1.78 a | 38.81 ± 1.30 | 38.88 ± 0.29 | 31.72 ± 0.41 | 32.03 ± 0.06 |
| Trans-cinnamic acid | 0.02 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.02 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a | 0.03 ± 0.00 a |
| Quercetin | 0.43 ± 0.00 c | 0.42 ± 0.01 c | 0.66 ± 0.02 a | 0.52 ± 0.02 b | 0.47 ± 0.06 c | 0.60 ± 0.00 b | 0.78 ± 0.00 a | 0.65 ± 0.06 b |
| Kaempferol | 0.45 ± 0.06 b | 0.47 ± 0.02 b | 0.48 ± 0.07 a b | 0.58 ± 0.05 a | 0.67 ± 0.01 a | 0.63 ± 0.01 b | 0.61 ± 0.04 b | 0.63 ± 0.01 b |
| Total | 33.77 ± 1.10 a | 34.19 ± 0.80 a | 30.22 ± 0.52 b | 33.66 ± 1.85 a | 47.98 ± 1.56 a | 47.90 ± 0.37 a | 39.52 ± 0.59 b | 39.53 ± 0.06 b |
| Trivial Names | 7 Days | 9 Days | ||||||
| Gallic acid | 0.03 ± 0.00 b | 0.02 ± 0.00 c | 0.04 ± 0.00 a | 0.02 ± 0.00 c | 0.05 ± 0.02 a | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.02 ± 0.00 b |
| Chlorogenic acid | 3.69 ± 0.09 a | 3.19 ± 0.02 b | 1.65 ± 0.03 d | 1.87 ± 0.03 c | 4.49 ± 0.09 a | 4.43 ± 0.04 a | 1.38 ± 0.02 c | 2.62 ± 0.03 b |
| Epicatechin | 3.06 ± 0.07 a | 2.90 ± 0.01 b | 2.61 ± 0.03 c | 2.27 ± 0.05 d | 3.15 ± 0.03 a | 3.14 ± 0.08 a | 2.47 ± 0.03 b | 2.62 ± 0.04 b |
| p-Coumaric acid | 0.11 ± 0.00 c | 0.10 ± 0.00 d | 0.17 ± 0.00 a | 0.13 ± 0.00 b | 0.12 ± 0.02 b | 0.13 ± 0.00 b | 0.17 ± 0.02 a | 0.11 ± 0.00 c |
| Ferulic acid | 0.10 ± 0.00 c | 0.08 ± 0.00 d | 0.14 ± 0.00 b | 0.15 ± 0.00 a | 0.13 ± 0.06 a | 0.07 ± 0.00 b | 0.08 ± 0.00 b | 0.06 ± 0.01 b |
| Benzoic acid | 2.16 ± 0.05 a | 1.41 ± 0.01 b | 1.14 ± 0.01 c | 1.39 ± 0.13 b | 1.18 ± 0.86 a | 0.38 ± 0.01 b | 0.19 ± 0.01 c | 0.13 ± 0.06 c |
| Rutin | 38.43 ± 0.34 a | 38.45 ± 0.12 a | 30.08 ± 0.30 c | 32.40 ± 0.32 b | 32.31 ± 1.38 a | 28.66 ± 0.17 b | 24.78 ± 0.25 d | 26.72 ± 0.38 c |
| Trans-cinnamic acid | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.03 ± 0.00 b | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.00 a | 0.04 ± 0.01 a |
| Quercetin | 1.13 ± 0.02 a | 1.15 ± 0.02 a | 0.71 ± 0.00 b | 0.72 ± 0.03 b | 1.60 ± 0.03 a | 1.29 ± 0.01 b | 0.67 ± 0.02 c | 0.64 ± 0.03 c |
| Kaempferol | 0.69 ± 0.02 b | 0.74 ± 0.01 a | 0.69 ± 0.01 b | 0.70 ± 0.03 a b | 0.69 ± 0.02 c | 1.00 ± 0.00 a | 0.93 ± 0.07 b | 0.67 ± 0.02 c |
| Total | 49.44 ± 0.60 a | 48.09 ± 0.11 a | 37.27 ± 0.36 c | 39.69 ± 0.34 b | 43.77 ± 2.19 a | 39.16 ± 0.04 b | 30.74 ± 0.23 d | 33.63 ± 0.59 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.C.; Kim, N.S.; Kim, Y.B.; Kim, C.M.; Chung, Y.S.; Park, S.U. Betaine Hydrochloride Treatment Affects Growth and Phenylpropanoid Accumulation in Tartary Buckwheat (Fagopyrum tataricum) Seedlings under Salt Stress. Agronomy 2020, 10, 906. https://doi.org/10.3390/agronomy10060906
Kim MC, Kim NS, Kim YB, Kim CM, Chung YS, Park SU. Betaine Hydrochloride Treatment Affects Growth and Phenylpropanoid Accumulation in Tartary Buckwheat (Fagopyrum tataricum) Seedlings under Salt Stress. Agronomy. 2020; 10(6):906. https://doi.org/10.3390/agronomy10060906
Chicago/Turabian StyleKim, Min Cheol, Nam Su Kim, Yeon Bok Kim, Chul Min Kim, Yong Suk Chung, and Sang Un Park. 2020. "Betaine Hydrochloride Treatment Affects Growth and Phenylpropanoid Accumulation in Tartary Buckwheat (Fagopyrum tataricum) Seedlings under Salt Stress" Agronomy 10, no. 6: 906. https://doi.org/10.3390/agronomy10060906
APA StyleKim, M. C., Kim, N. S., Kim, Y. B., Kim, C. M., Chung, Y. S., & Park, S. U. (2020). Betaine Hydrochloride Treatment Affects Growth and Phenylpropanoid Accumulation in Tartary Buckwheat (Fagopyrum tataricum) Seedlings under Salt Stress. Agronomy, 10(6), 906. https://doi.org/10.3390/agronomy10060906





