Synthesis of Dacus Pheromone, 1,7-Dioxaspiro[5.5]Undecane and Its Encapsulation in PLLA Microspheres for Their Potential Use as Controlled Release Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of Dacus Pheromone
NMR Spectroscopy (1H and 13C)
2.3. PLLA Microparticles
2.3.1. Preparation of PLLA Microparticles
2.3.2. Characterization of Prepared Microspheres
Thermogravimetric Analysis (TGA)
Fourier Transform-Infrared Spectroscopy (FT-IR)
X-ray Diffraction (XRD)
Scanning Electron Microscopy (SEM)
Differential Scanning Calorimetry (DSC)
2.4. DSU Release from Microparticles
2.4.1. Determination of Amounts of Residual DSU in PLLA Microparticles
2.4.2. Determination of DSU Amount Released from PLLA Microspheres
2.4.3. GC-MS
3. Results and Discussion
3.1. Synthesis and Characterization of Dacus Pheromone
3.2. Characterization of Prepared Microparticles
3.3. DSU Release from PLLA Microparticles
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gömez-Caravaca, A.M.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; delCarlo, M.; Compagnone, D.; Cichelli, A. Effects of fly attack (Bactrocera oleae) on the phenolic profile and selected chemical parameters of olive oil. J. Agric. Food Chem. 2008, 56, 4577–4583. [Google Scholar] [CrossRef]
- Bayega, A.; Djambazian, H.; Tsoumani, K.T.; Gregoriou, M.E.; Sagri, E.; Drosopoulou, E.; Mavragani-Tsipidou, P.; Giorda, K.; Tsiamis, G.; Bourtzis, K.; et al. De novo assembly of the olive fruit fly (Bactrocera oleae) genome with linked-reads and long-read technologies minimizes gaps and provides exceptional Y chromosome assembly. BMC Genom. 2020, 21, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canale, A.; Benelli, G.; Germinara, G.S.; Fusini, G.; Romano, D.; Rapalini, F.; Desneux, N.; Rotundo, G.; Raspi, A.; Carpita, A. Behavioural and electrophysiological responses to overlooked female pheromone components in the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Chemoecology 2015, 25, 147–157. [Google Scholar] [CrossRef]
- Varikou, K.; Garantonakis, N.; Birouraki, A. Exposure of bombus terrestris l. To three different active ingredients and two application methods for olive pest control. Entomol. Gen. 2019, 39, 53–60. [Google Scholar] [CrossRef]
- Rebora, M.; Salerno, G.; Piersanti, S.; Gorb, E.; Gorb, S. Role of Fruit Epicuticular Waxes in Preventing. Insects 2020, 11, 1–17. [Google Scholar]
- Witzgall, P.; Kirsch, P.; Cork, A. Sex pheromones and their impact on pest management. J. Chem. Ecol. 2010, 36, 80–100. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Herbert, R.; Howse, P.E.; Jones, O.T.; Francke, W.; Reith, W. Identification and synthesis of the major sex pheromone of the olive fly (Dacus oleae). J. Chem. Soc. Chem. Commun. 1980, 2, 52–53. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. A multicomponent female sex pheromone of Dacus oleae Gmelin: Isolation and bioassay. J. Chem. Ecol. 1981, 7, 437–444. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E. Male olive fruit fly attraction to synthetic sex pheromone components in laboratory and field tests. J. Chem. Ecol. 1985, 11, 397–405. [Google Scholar] [CrossRef]
- Mazomenos, B.E.; Haniotakis, G.E.; Ioannou, A.; Spanakis, I.; Kozirakis, A. Field evaluation of the olive fruit fly pheromone traps with various dispensers and concentrations. In Fruit Flies of Economic Importance, Proceedings of the CEC/IOBC International Symposium, Athens, Greece, 16–19 November 1982; pp. 506–512.
- Liu, B.; Syu, K.J.; Zhang, Y.X.; Gupta, S.; Shen, Y.J.; Chien, W.J.; Tseng, J.C.; Lou, D.W. Practical Synthesis and Field Application of the Synthetic Sex Pheromone of Rice Stem Borer, Chilo suppressalis (Lepidoptera: Pyralidae). J. Chem. 2020. [Google Scholar] [CrossRef] [Green Version]
- Chamberlain, D.J.; Brown, N.J.; Jones, O.T.; Casagrande, E. Field evaluation of a slow release pheromone formulation to control the American bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) in Pakistan. Bull. Entomol. Res. 2000, 90, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Mazomenos, B.; Moustakali-Mavridis, I. Inclusion Complexes of Cyclodextrin and their Use in Slow Release Formulations for Attracting Insects. PCT/EP93/01536, 17 June 1993. [Google Scholar]
- Kikionis, S.; Ioannou, E.; Konstantopoulou, M.; Roussis, V. Electrospun Micro/Nanofibers as Controlled Release Systems for Pheromones of Bactrocera oleae and Prays oleae. J. Chem. Ecol. 2017, 43, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization study and comparative in vitro-in vivo hydrolysis of PLA reinforcement ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanaki, S.; Barmpalexis, P.; Iatrou, A.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D.N. Risperidone controlled release microspheres based on poly(lactic acid)-poly(propylene adipate) novel polymer blends appropriate for long acting injectable formulations. Pharmaceutics 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Nanaki, S.; Barmpalexis, P.; Papakonstantinou, Z.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D.N. Preparation of new risperidone depot microspheres based on novel biocompatible poly(alkylene adipate) polyesters as long acting formulations. J. Pharm. Sci. 2018, 107, 2891. [Google Scholar] [CrossRef]
- Nanaki, S.; Tseklima, M.; Terzopoulou, Z.; Nerantzaki, M.; Giliopoulos, D.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Use of mesoporous cellular foam (mcf) in preparation of polymeric microspheres for long acting injectable release formulations of paliperidone antipsychotic drug. Eur. J. Pharm. Biopharm. 2017, 117, 77–90. [Google Scholar] [CrossRef]
- Nanaki, S.; Viziridou, A.; Zamboulis, A.; Kostoglou, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New Biodegradable Poly (l -lactide) -Block- for Long-Acting Injectables of Naltrexone Drug. Polymers 2020, 12, 852. [Google Scholar] [CrossRef] [Green Version]
- Bragagni, M.; Beneitez, C.; Martín, C.; De La Ossa, D.H.P.; Mura, P.A.; Gil-Alegre, M.E. Selection of PLA polymers for the development of injectable prilocaine controlled release microparticles: Usefulness of thermal analysis. Int. J. Pharm. 2013, 441, 468–475. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Bera, S.; Mondal, D. Nascent-HBr-Catalyzed Removal of Orthogonal Protecting Groups in Aqueous Surfactants. J. Org. Chem. 2020, 85, 2635–2645. [Google Scholar] [CrossRef]
- Bernat, V.; Brox, R.; Heinrich, M.R.; Yves, P.; Auberson, Y.P.; Tschammer, N. Ligand-Biased and Probe-Dependent Modulation of Chemokine Receptor CXCR3 Signaling by Negative Allosteric Modulators. Chem. Med. Chem. 2015, 10, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Kranidiotis, N.S.; Grammatoglou, C.E.; Gallos, J.K. A new short synthesis of (±)-olean through cross metathesis. Heterocycles 2018, 96, 1441–1444. [Google Scholar]
- Nanaki, S.; Tseklima, M.; Christodoulou, E.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Thiolated chitosan masked microparticles with incorporated mesocellular silica foam (MCF) for intranasal delivery of paliperidone. Polymers 2017, 9, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koclenskl, P. Yeates, A new synthesis of 1,7-dioxasdipo[5,5] undecanes application to a rectal gland secretion of the olive fruit fly (Dacus Oleae). Tetrehedron Lett. 1983, 24, 3905–3906. [Google Scholar]
- Williams, B.A.; Barner, D.R. Synthetic Studies of 1,7-dioxaspir0[5.5]undecan-4-ones. Tetrahedron Lett. 1983, 24, 427–430. [Google Scholar] [CrossRef]
- Cala, L.; Fañanás, F.J.; Rodríguez, F. Enantioselective synthesis of spiroacetals: The conquest of a long-sought goal in asymmetric catalysis. Org. Biomol. Chem. 2014, 12, 5324–5330. [Google Scholar] [CrossRef] [Green Version]
- Řezanka, T.; Dvořáková, R.; Hanuš, L.O.; Dembitsky, V.M. Enteridinines A and B from slime mold Enteridium lycoperdon. Phytochemistry 2004, 65, 455–462. [Google Scholar] [CrossRef]
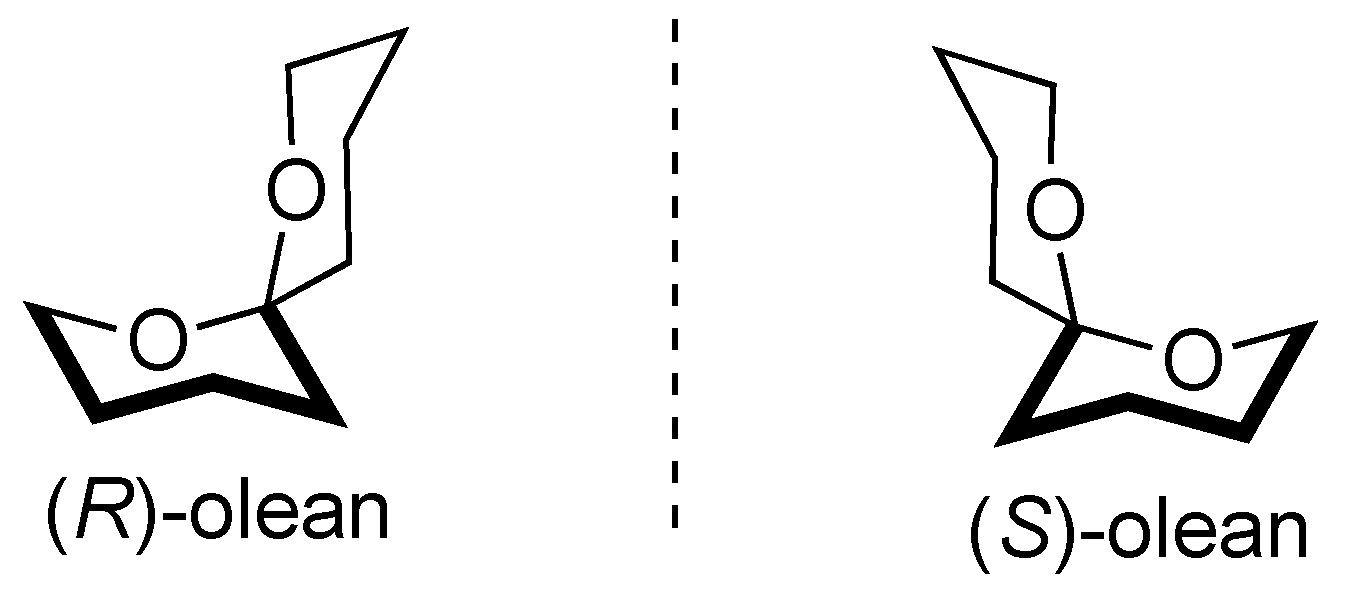








| Sample Name | Microparticles Yield (%) | Pheromone Loading (%) | Encapsulation Efficiency (%) | |
|---|---|---|---|---|
| PLLA-DSU | 5% w/w | 85.3 ± 0.2 | 4.8 ± 0.3 | 96.1 ± 0.2 |
| PLLA-DSU | 10% w/w | 91.5 ± 0.5 | 9.1 ± 0.2 | 91.8 ± 0.4 |
| PLLA-DSU | 20% w/w | 81.4 ± 0.3 | 16.8 ± 0.6 | 84.6 ± 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zisopoulou, S.A.; Chatzinikolaou, C.K.; Gallos, J.K.; Ofrydopoulou, A.; Lambropoulou, D.A.; Psochia, E.; Bikiaris, D.N.; Nanaki, S.G. Synthesis of Dacus Pheromone, 1,7-Dioxaspiro[5.5]Undecane and Its Encapsulation in PLLA Microspheres for Their Potential Use as Controlled Release Devices. Agronomy 2020, 10, 1053. https://doi.org/10.3390/agronomy10071053
Zisopoulou SA, Chatzinikolaou CK, Gallos JK, Ofrydopoulou A, Lambropoulou DA, Psochia E, Bikiaris DN, Nanaki SG. Synthesis of Dacus Pheromone, 1,7-Dioxaspiro[5.5]Undecane and Its Encapsulation in PLLA Microspheres for Their Potential Use as Controlled Release Devices. Agronomy. 2020; 10(7):1053. https://doi.org/10.3390/agronomy10071053
Chicago/Turabian StyleZisopoulou, Stavroula A., Christina K. Chatzinikolaou, John K. Gallos, Anna Ofrydopoulou, Dimitra A. Lambropoulou, Eleni Psochia, Dimitrios N. Bikiaris, and Stavroula G. Nanaki. 2020. "Synthesis of Dacus Pheromone, 1,7-Dioxaspiro[5.5]Undecane and Its Encapsulation in PLLA Microspheres for Their Potential Use as Controlled Release Devices" Agronomy 10, no. 7: 1053. https://doi.org/10.3390/agronomy10071053
APA StyleZisopoulou, S. A., Chatzinikolaou, C. K., Gallos, J. K., Ofrydopoulou, A., Lambropoulou, D. A., Psochia, E., Bikiaris, D. N., & Nanaki, S. G. (2020). Synthesis of Dacus Pheromone, 1,7-Dioxaspiro[5.5]Undecane and Its Encapsulation in PLLA Microspheres for Their Potential Use as Controlled Release Devices. Agronomy, 10(7), 1053. https://doi.org/10.3390/agronomy10071053







