Nutrient Dynamics in Switchgrass as a Function of Time
Abstract
:1. Introduction
2. Materials and Methods
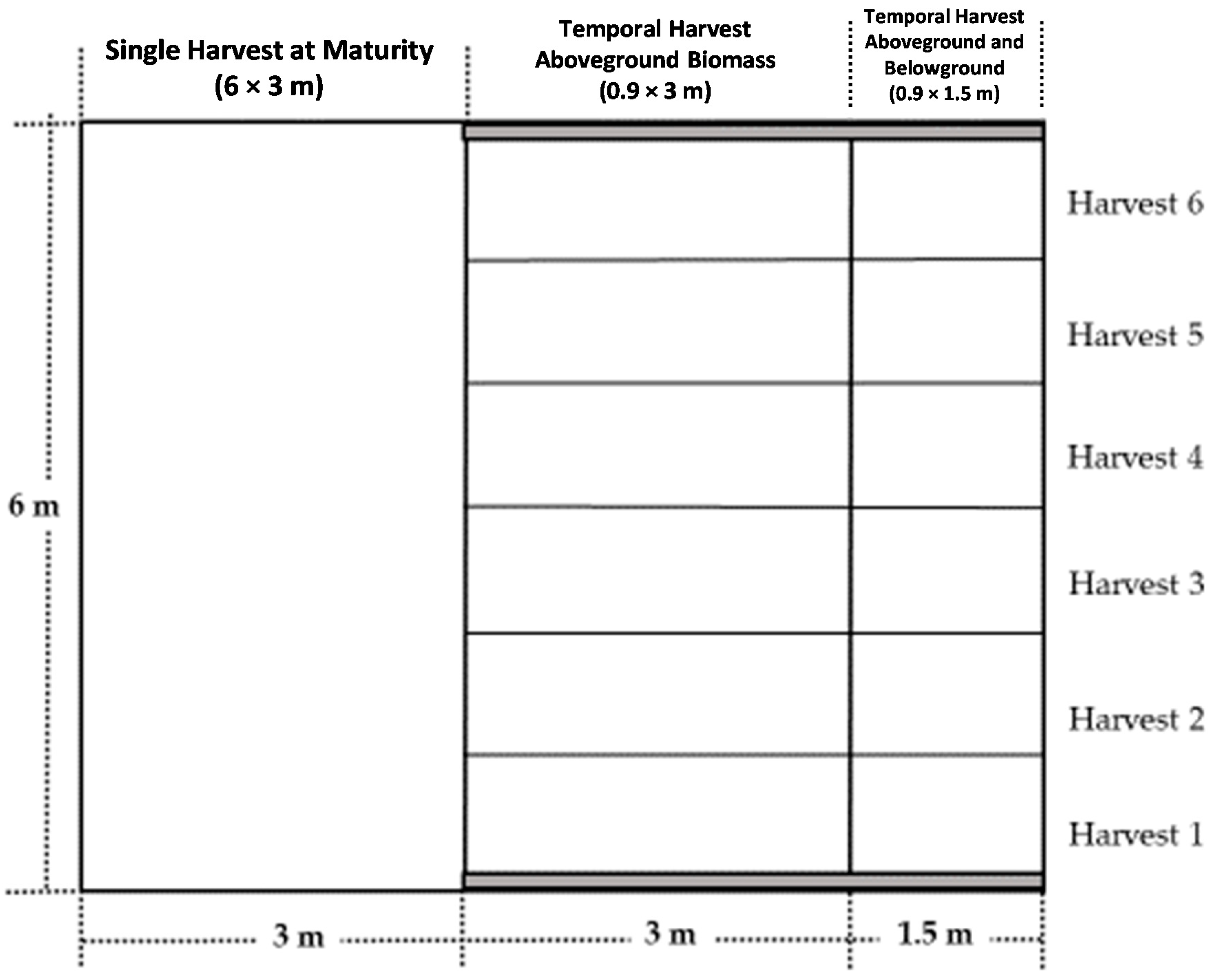
2.1. Experimental Area and Treatments Description
2.2. Soil Sampling and Analysis
2.3. Switchgrass Sampling and Analyses
2.4. Statistical Analysis
3. Results
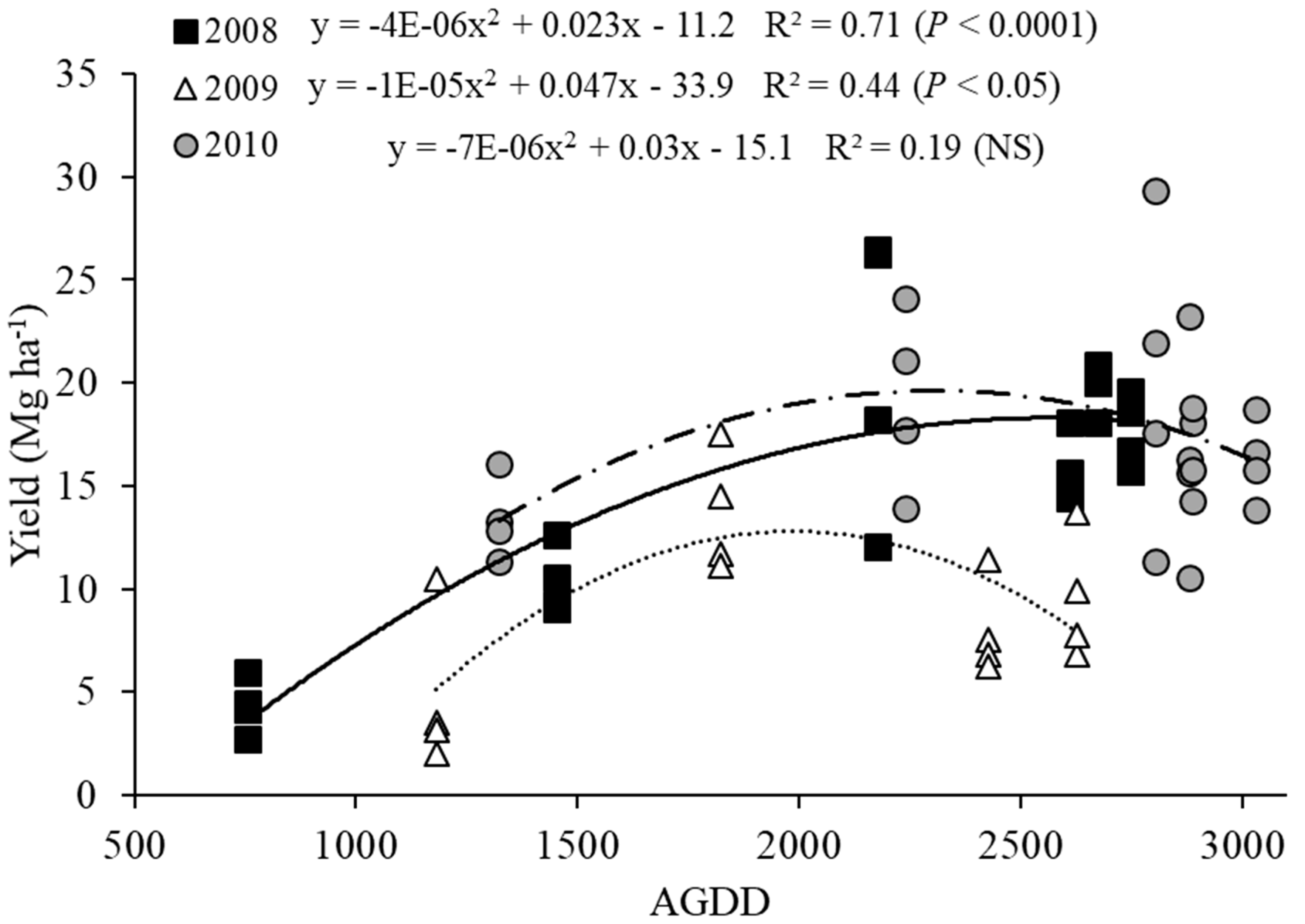
3.1. Switchgrass Yield
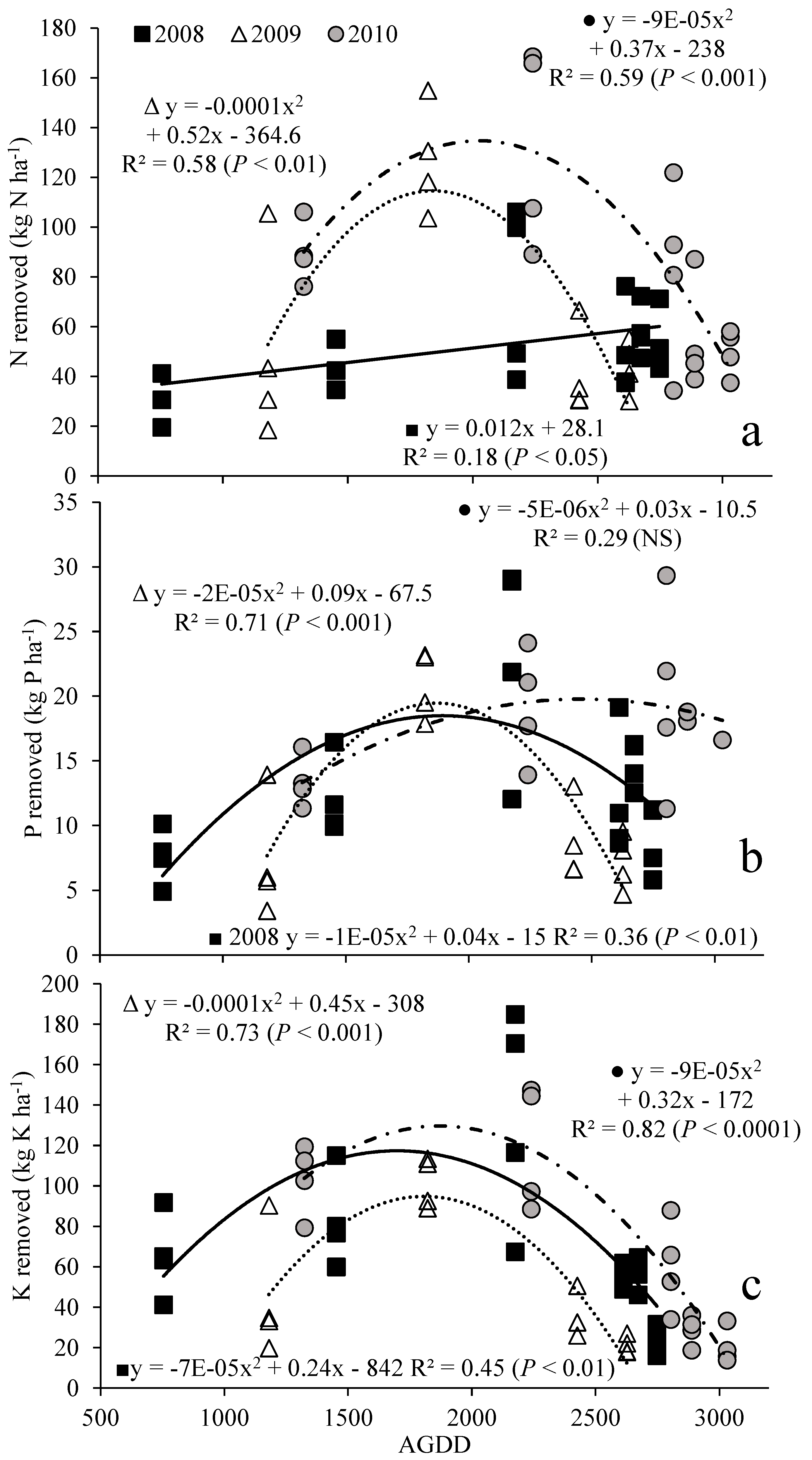
3.2. Nutrient Removal with Harvest
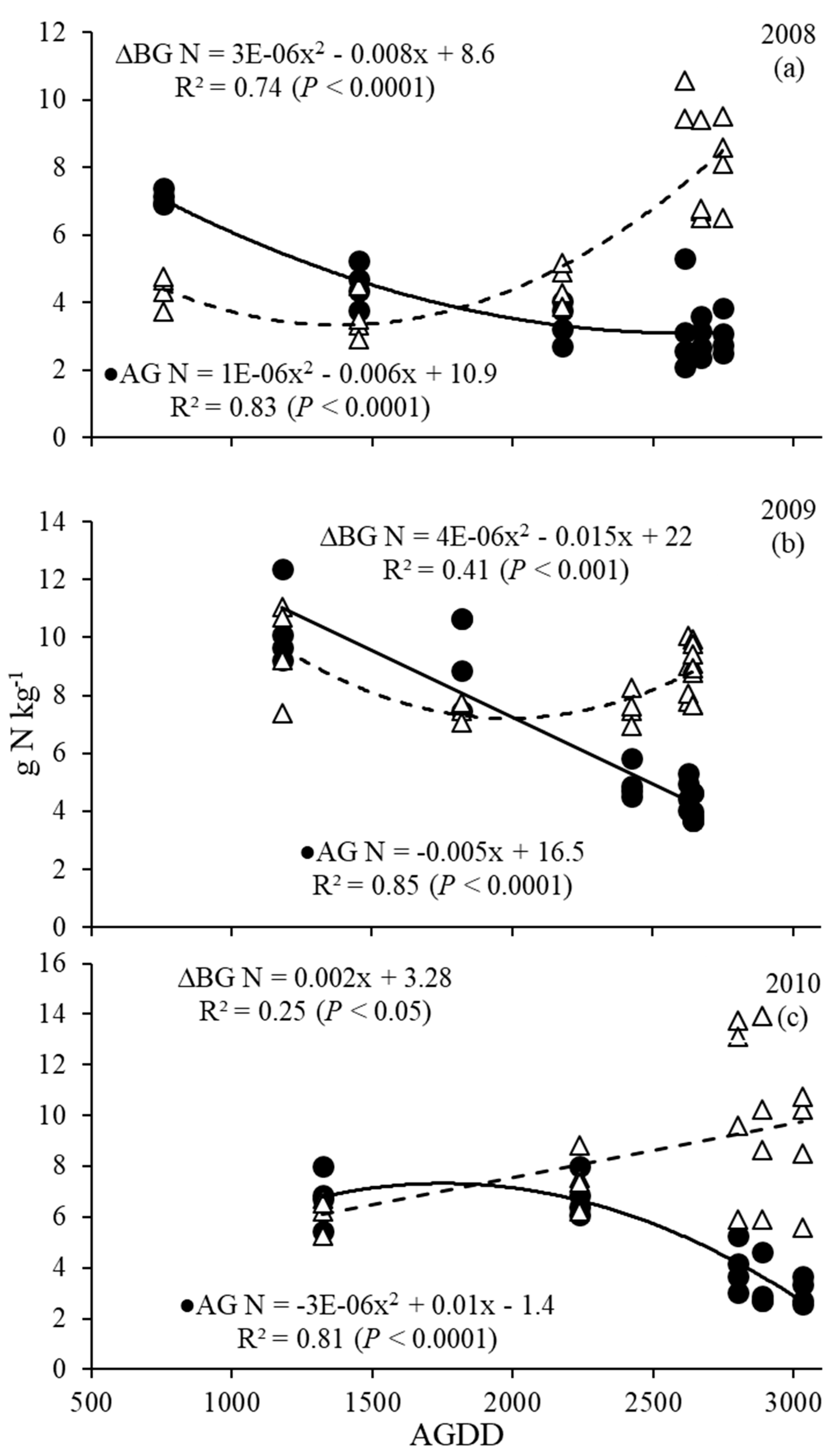
3.3. Nitrogen Dynamics
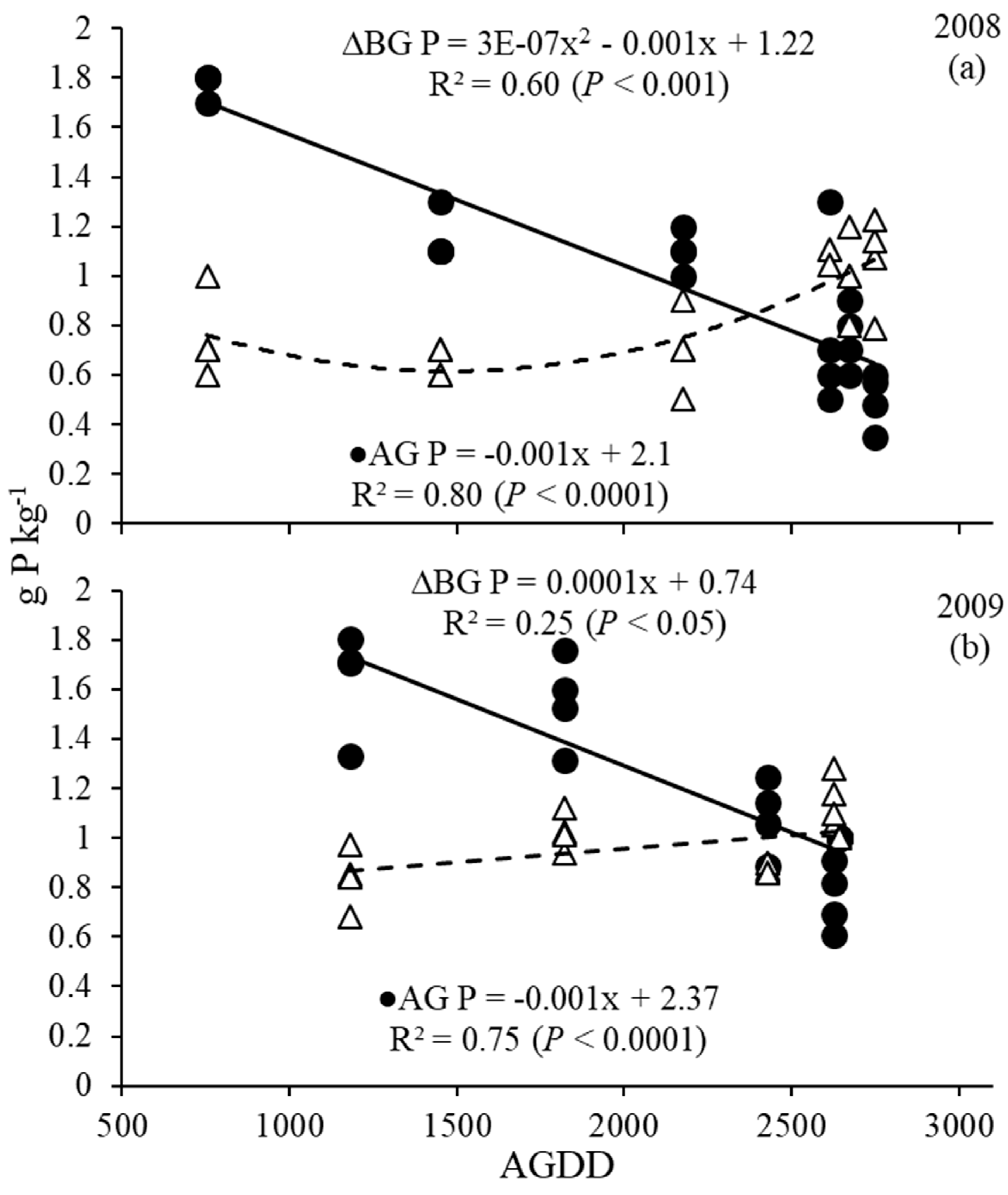
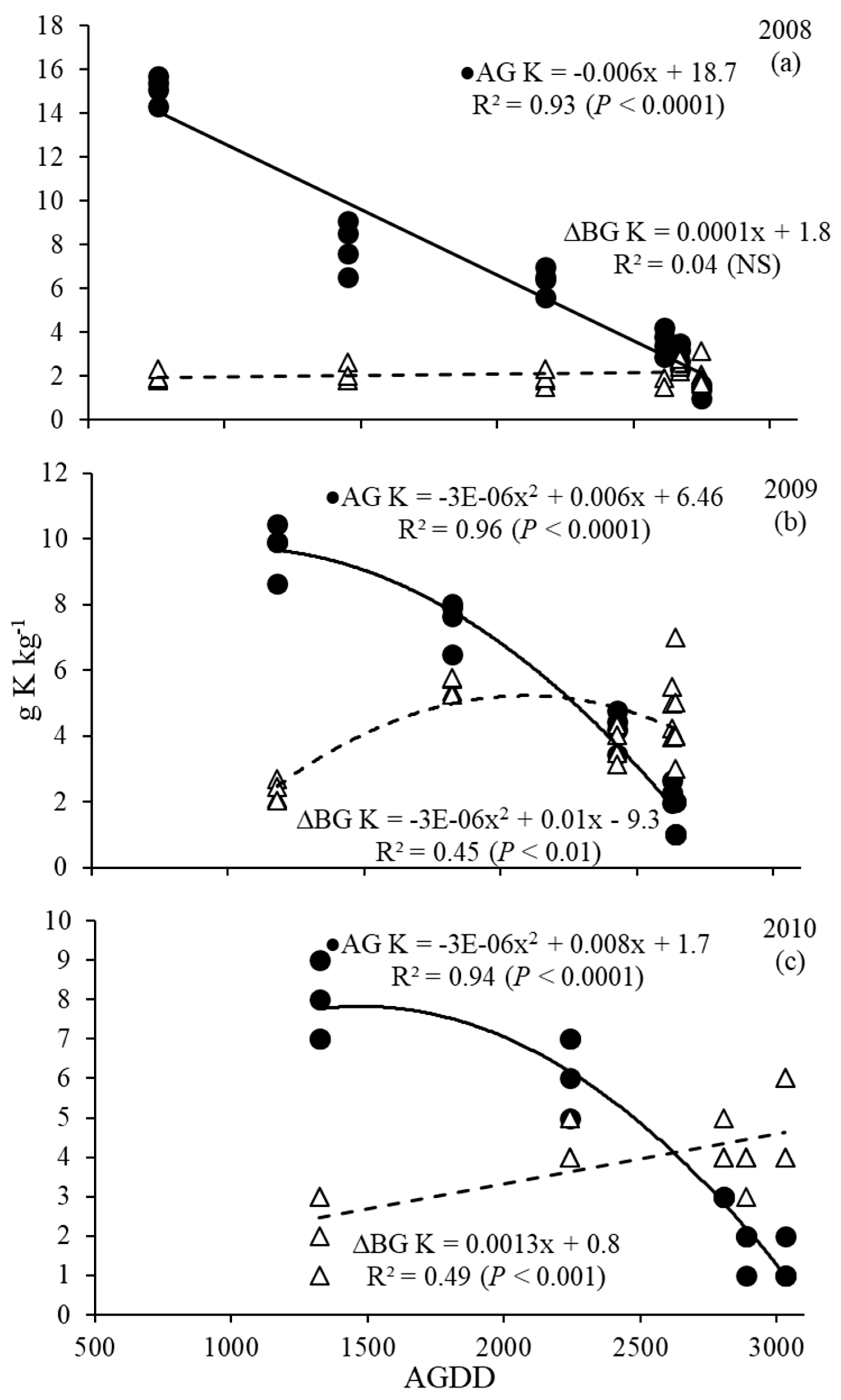
3.4. Phosphorus and Potassium Dynamics
3.5. Secondary and Micronutrient Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Von Haden, A.C.; Dornbush, M.E. Prairies thrive where row crops drown: A comparison of yields in upland and lowland topographies in the Upper Midwest US. Agronomy 2016, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Márquez, C.O.; García, V.J.; Schultz, R.C.; Isenhart, T.M. Assessment of soil aggradation through soil aggregation and particulate organic matter by riparian switchgrass buffers. Agronomy 2017, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Fike, J.H.; Parrish, D.J.; Wolf, D.D.; Balasko, J.A.; Green, J.T.; Rasnake, M.; Reynolds, J.H. Long-Term yield potential of switchgrass-for-biofuel systems. Biomass Bioenergy 2006, 30, 198–206. [Google Scholar] [CrossRef]
- Guretzky, J.A.; Biermacher, J.T.; Cook, B.J.; Kering, M.K.; Mosali, J. Switchgrass for forage and bioenergy: Harvest and nitrogen rate effects on biomass yields and nutrient composition. Plant Soil 2011, 339, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Kering, M.K.; Butler, T.J.; Biermacher, J.T.; Guretzky, J.A. Biomass yield and nutrient removal rates of perennial grasses under nitrogen fertilization. Bioenergy Res. 2011, 5, 61–70. [Google Scholar] [CrossRef]
- Muir, J.P.; Sanderson, M.A.; Ocumpaugh, W.R.; Jones, R.M.; Reed, R.L. Biomass production of ‘Alamo’ switchgrass in response to nitrogen, phosphorus, and row spacing. Agron. J. 2001, 93, 896–901. [Google Scholar] [CrossRef]
- Jach-Smith, L.C.; Jackson, R.D. Inorganic N addition replaces N supplied to switchgrass (Panicum virgatum by arbuscular mycorrhizal fungi. Ecol. Appl. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Parrish, S.D.J.; Wolfe, D.D.; Fike, J.H.; Daniels, W.L. Switchgrass as a Biofuels Crop for the Upper Southeast: Cultivar Trials and Cultural Environments; Final Report for 1997–2001. ORNL/SUB-03-19XSY163/01; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2003. [Google Scholar]
- Ashworth, A.J.; Allen, F.L.; Bacon, J.L.; Sams, C.E.; Hart, W.E.; Grant, J.F.; Moore, P.A., Jr.; Pote, D.H. Switchgrass cultivar, yield, and nutrient removal responses to harvest timing. Agron. J. 2017, 109, 2598–2605. [Google Scholar] [CrossRef] [Green Version]
- Ashworth, A.J.; Rocateli, A.C.; West, C.P.; Brye, K.R.; Popp, M.P. Switchgrass growth and effects on biomass accumulation, moisture content, and nutrient removal. Agron. J. 2017, 109, 1359–1367. [Google Scholar] [CrossRef] [Green Version]
- Clark, F.E. Internal cycling of 15Nitrogen in shortgrass prairie. Ecology 1977, 58, 1322–1333. [Google Scholar] [CrossRef]
- Chapin, F.S., III. The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Parrish, D.J.; Fike, J.H. The biology and agronomy of switchgrass for biofuels. Crit. Rev. Plant Sci. 2005, 24, 423–459. [Google Scholar] [CrossRef]
- Heggenstaller, A.H.; Moore, K.J.; Liebman, M.; Anex, R.P. Nitrogen influences biomass and nutrient partitioning by perennial, warm-season grasses. Agron. J. 2009, 101, 1363–1371. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; DeLucia, E.H. Drought-Induced nitrogen translocation in perennial C4 grasses of tallgrass prairie. Ecology 1994, 75, 1877–1886. [Google Scholar] [CrossRef]
- Heckathorn, S.A.; DeLucia, E.H. Retranslocation of shoot nitrogen to rhizomes and roots in prairie grasses may limit loss of N to grazing and fire during drought. Funct. Ecol. 1996, 10, 396–400. [Google Scholar] [CrossRef]
- Clark, R.B. Differences among mycorrhizal fungi for mineral uptake per root length of switchgrass grown in acidic soil. J. Plant Nutr. 2002, 25, 1753–1772. [Google Scholar] [CrossRef]
- Petipas, R.H.; Bowsher, A.W.; Bekkering, C.S.; Jack, C.N.; Mclachlan, E.E.; White, R.A.; Younginger, B.S.; Tiemann, L.K.; Evans, S.E.; Friesen, M.L. Interactive effects of microbes and nitrogen on Panicum virgatum root functional traits and patterns of phenotypic selection. Int. J. Plant Sci. 2020, 181, 20–32. [Google Scholar] [CrossRef]
- Brejda, J.J. Fertilization of Native Warm-Season Grasses. In Native Warm Season Grasses: Research Trends and Issues; Moore, K.J., Anderson, B.E., Eds.; CSSA Special Publication; CSSA: Madison, WI, USA, 2000; Volume 30. [Google Scholar] [CrossRef]
- Makaju, S.O.; Wu, Y.Q.; Zhang, H.; Kakani, V.G.; Taliaferro, C.M.; Anderson, M.P. Switchgrass winter yield, year-Round elemental concentrations, and associated soil nutrients in a zero input environment. Agron. J. 2013, 105, 463–470. [Google Scholar] [CrossRef] [Green Version]
- Vogel, K.P.; Brejda, J.J.; Walters, D.T.; Buxton, D.R. Switchgrass biomass production in the Midwest USA: Harvest and nitrogen management. Agron. J. 2002, 94, 413–420. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Seasonal nitrogen dynamics of Miscanthus x giganteus and Panicum virgatum. Glob. Chang. Biol. Bioenergy 2009, 1, 297–307. [Google Scholar] [CrossRef]
- Propheter, J.L.; Staggenborg, S.A.; Wu, X.; Wang, D. Performance of annual and perennial biofuel crops: Yield during the first two years. Agron. J. 2010, 102, 806–814. [Google Scholar] [CrossRef]
- Wilson, D.M.; Dalluge, D.L.; Rover, M.; Heaton, E.A.; Brown, R.C. Crop management impacts biofuel quality: Influence of switchgrass harvest time on yield, nitrogen and ash of fast pyrolysis products. Bioenergy Res. 2013, 6, 103–113. [Google Scholar] [CrossRef]
- Reynolds, J.H.; Walker, C.L.; Kirchner, M.J. Nitrogen removal in switchgrass biomass under two harvest systems. Biomass Bioenergy 2000, 19, 281–286. [Google Scholar] [CrossRef]
- Kimura, E.; Collins, H.P.; Fransen, S. Biomass production and nutrient removal by switchgrass under irrigation. Agron. J. 2015, 107, 204–210. [Google Scholar] [CrossRef]
- Mislevy, P.; Martin, F.G. Biomass yield and forage nutritive value of Cynodon grasses harvested monthly. Soil Crop Sci. Soc. Fla. Proc. 2006, 65, 9–14. [Google Scholar]
- Soil Survey Staff. Natural Resources Conservation Service, United States Department of Agriculture. Web Soil Survey. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 2 October 2019).
- Anderson, E.K.; Parrish, A.S.; Voight, T.B.; Owens, V.N.; Hong, C.-H.; Lee, D.K. Nitrogen fertility and harvest management of switchgrass for sustainable bioenergy feedstock production in Illinois. Ind. Crops Prod. 2013, 48, 19–27. [Google Scholar] [CrossRef]
- Fike, J.H.; Parrish, D.J.; Wolf, D.D.; Balasko, J.A.; Green, J.T.; Rasnake, M.; Reynolds, J.H. Switchgrass production for the upper southeastern USA: Influence of cultivar and cutting frequency on biomass yields. Biomass Bioenergy 2006, 30, 207–213. [Google Scholar] [CrossRef]
- Thomas, J.S. Soil pH and Soil Acidity. In Methods of Soil Analysis; Sparks, D.L., Ed.; Part 3. SSSA Book Series 5; SSSA: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar] [CrossRef] [Green Version]
- Kachurina, O.M.; Zhang, H.; Raun, W.R.; Krenzer, E.G. Simultaneous determination of soil aluminum, ammonium- and nitrate-nitrogen using 1 M potassium chloride extraction. Commun. Soil Sci. Plant Anal. 2000, 31, 893–903. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Hanson, D.; Kotuby-Amacher, J.; Miller, R.O. Soil analysis: Western States Proficiency Testing Program for 1996. Anal. Bioanal. Chem. 1998, 360, 348–350. [Google Scholar] [CrossRef]
- Soltanpour, P.N.; Johnson, G.W.; Workman, S.M.; Jones, J.B., Jr.; Miller, R.O. Inductively Coupled Plasma Emission Spectrometry and Inductively Coupled Plasma-Mass Spectrometry. In Methods of Soil Analysis; Sparks, D.L., Ed.; Part 3. Chemical Methods. SSSA Book Series 5; SSSA: Madison, WI, USA; ASA: Madison, WI, USA, 1996; pp. 91–139. [Google Scholar] [CrossRef] [Green Version]
- Oklahoma Mesonet. Available online: www.mesonet.org (accessed on 10 October 2019).
- Sena, K.L.; Goff, B.; Davis, D.; Smith, S.R. Switchgrass growth and forage quality trends provide insight for management. Crop Forage Turfgrass Manag. 2018, 4, 170053. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Moore, K.J. Switchgrass morphological development predicted from day of the year or degree day models. Agron. J. 1999, 91, 732–734. [Google Scholar] [CrossRef]
- Dhillon, J.; Figueiredo, B.; Eickhoff, E.; Raun, W. Applied use of growing degree days to refine optimum times for nitrogen stress sensing in winter wheat (Triticum aestivum L.). Agron. J. 2019, 112, 537–549. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.B.; Moore, K.J.; Moser, L.E.; Fritz, J.O.; Redfearn, D.D. Predicting developmental morphology in switchgrass and big bluestem. Agron. J. 1997, 89, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Moser, L.E.; Vogel, K.P.; Waller, S.S.; Johnson, B.E.; Pedersen, J.F. Describing and quantifying growth stages of perennial forage grasses. Agron. J. 1991, 83, 1073–1077. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Moser, L.E. Quantifying developmental morphology of perennial grasses. Crop Sci. 1995, 35, 37–43. [Google Scholar] [CrossRef]
- Massey, J.R.; Antonangelo, J.A.; Zhang, H. Nitrogen affecting switchgrass yield, nitrogen removal and use efficiency. Agrosyst. Geosci. Environ. 2020, 3. [Google Scholar] [CrossRef]
- Jones, J.B.; Case, V.W. Sampling, Handling, and Analyzing Plant Tissue Samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; SSSA: Madison, WI, USA, 1990; pp. 404–410. [Google Scholar] [CrossRef]
- Undersander, D.; Mertens, D.R.; Thiex, N. Forage Analysis Procedures; National Forage Testing Association: Omaha, NE, USA, 1993. [Google Scholar]
- Antonangelo, J.A.; Zhang, H. Heavy metal phytoavailability in a contaminated soil of northeastern Oklahoma as affected by biochar amendment. Environ. Sci. Pollut. Res. 2019, 26, 33582–33593. [Google Scholar] [CrossRef]
- Mulkey, V.R.; Owens, V.N.; Lee, D.K. Management of switchgrass-dominated Conservation Reserve Program lands for biomass production in South Dakota. Crop Sci. 2006, 46, 712–720. [Google Scholar] [CrossRef]
- Hong, C.O.; Owens, V.N.; Bransby, D.; Farris, R.; Fike, J.; Heaton, E.; Kim, S.; Mayton, H.; Mitchell, R.; Viands, D. Switchgrass response to nitrogen fertilizer across diverse environments in the USA: A regional feedstock partnership report. Bioenergy Res. 2014, 7, 777–788. [Google Scholar] [CrossRef]
- Aurangzaib, M.; Moore, K.J.; Lenssen, A.W.; Archontoulis, S.V.; Heaton, E.A.; Fei, S. Developmental morphology and biomass yield of upland and lowland switchgrass ecotypes grown in Iowa. Agronomy 2018, 8, 61. [Google Scholar] [CrossRef] [Green Version]
- Dubeux, J.C.B., Jr.; Sollenberger, L.E.; Mathews, B.W.; Scholberg, J.M.; Santos, H.Q. Nutrient cycling in warm-Climate grasslands. Crop Sci. 2007, 47, 915–928. [Google Scholar] [CrossRef]
- Yang, J.; Worley, E.; Ma, Q.; Li, J.; Torres-Jerez, I.; Li, G.; Zhao, P.X.; Xu, Y.; Tang, Y.; Udvardi, M. Nitrogen remobilization and conservation, and underlying senescence-Associated gene expression in the perennial switchgrass Panicum virgatum. New Phytol. 2016, 211, 75–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell, C.J.; Williams, M.A.; Rice, C.W. Partitioning of nitrogen over five growing seasons in tallgrass prairie. Ecology 2005, 86, 1280–1287. [Google Scholar] [CrossRef] [Green Version]
- Bausenwein, U.; Millard, P.; Raven, J.A. Remobilized old-Leaf nitrogen predominates for spring growth in two temperate grasses. New Phytol. 2001, 152, 283–290. [Google Scholar] [CrossRef]
- Dohleman, F.G.; Heaton, E.A.; Arundale, R.A.; Long, S.P. Seasonal dynamics of above- and below-Ground biomass and nitrogen partitioning in Miscanthus x giganteus and Panicum virgatum across three growing seasons. GCB Bioenergy 2012, 4, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.M.; Heaton, E.A.; Liebman, M.; Moore, K.J. Intraseasonal changes in switchgrass nitrogen distribution compared with corn. Agron. J. 2013, 105, 285–294. [Google Scholar] [CrossRef]
- Thomason, W.E.; Raun, W.R.; Johnson, G.V.; Taliaferro, C.M.; Freeman, K.W.; Wynn, K.J.; Mullen, R.W. Switchgrass response to harvest frequency and time and rate of applied nitrogen. J. Plant Nutr. 2005, 27, 1199–1226. [Google Scholar] [CrossRef]
- Staley, T.E.; Stout, W.L.; Jung, G.A. Nitrogen use by tall fescue and switchgrass on acidic soils of varying water holding capacity. Agron. J. 1991, 83, 732–738. [Google Scholar] [CrossRef]
- Stout, W.L.; Jung, G.A. Biomass and nitrogen accumulation in switchgrass: Effects of soil and environment. Agron. J. 1995, 87, 663–669. [Google Scholar] [CrossRef]
- Garten, C.T., Jr.; Smith, J.; Tyler, D.; Amonette, J.; Bailey, V.; Brice, D.; Castro, H.; Graham, R.; Gunderson, C.; Izaurralde, R. Intra-Annual changes in biomass, carbon, and nitrogen dynamics at 4-year old switchgrass field trials in west Tennessee, USA. Agric. Ecosyst. Environ. 2010, 136, 177–184. [Google Scholar] [CrossRef]
- Silver, W.L.; Miya, R.K. Global patterns in root decomposition: Comparisons of climate and litter quality effects. Oecologia 2001, 129, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wood, C.; Bransby, D. Impacts of soil management on root characteristics of switchgrass. Biomass Bioenergy 2000, 18, 105–112. [Google Scholar] [CrossRef]
- Adler, P.R.; Sanderson, M.A.; Boateng, A.A.; Weimer, P.J.; Jung, H.-J.G. Biomass yield and biofuel quality of switchgrass harvested in fall or spring. Agron. J. 2006, 98, 1518. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H. Senescence, ageing, and death of the whole plant. New Phytol. 2012, 197, 696–711. [Google Scholar] [CrossRef]
- Blagosklonny, M.V.; Hall, M.N. Growth and aging: A common molecular mechanism. Aging 2009, 1, 357–362. Available online: www.impactaging.com (accessed on 10 April 2019). [CrossRef] [Green Version]
- Masclaux, C.; Quillere, I.; Gallais, A.; Hirel, B. The challenge of remobilisation in plant nitrogen economy. A survey of physio-Agronomic and molecular approaches. Ann. Appl. Biol. 2001, 138, 69–81. [Google Scholar] [CrossRef]
- Gregersen, P.L.; Culetic, A.; Boschian, L.; Krupinska, K. Plant senescence and crop productivity. Plant Mol. Biol. 2013, 82, 603–622. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Wynia, R.L. Latitudinal adaptation of switchgrass populations. Crop Sci. 2004, 44, 293–303. [Google Scholar] [CrossRef]
- Sanderson, M.; Reed, R.; Ocumpaugh, W.; Hussey, M.; Esbroeck, G.V.; Read, J.; Tischler, C.; Hons, F. Switchgrass cultivars and germplasm for biomass feedstock production in Texas. Bioresour. Technol. 1999, 67, 209–219. [Google Scholar] [CrossRef]
- Aravindhakshan, S.C.; Epplin, F.M.; Taliaferro, C.M. Switchgrass, bermudagrass, flaccidgrass, and lovegrass biomass yield response to nitrogen for single and double harvest. Biomass Bioenergy 2011, 35, 308–319. [Google Scholar] [CrossRef]
| Year | pH | NO3-N | P | K | SO4-S | Ca | Mg | Fe | Zn | B | Cu |
|---|---|---|---|---|---|---|---|---|---|---|---|
| kg ha−1 | mg kg−1 | ||||||||||
| 2008 kg N ha−1 | 6.3 ± 0.2 | 3 ± 0.8 | 16 ± 1 | 118 ± 16 | 8.4 ± 0.2 | 1563 ± 125 | 317 ± 32 | 48.5 ± 11 | 0.7 ± 0.1 | 0.34 ± 0.0 | 1.4 ± 0.1 |
| 2009 | 6.2 ± 0.2 | 6.4 ± 2.7 | 17 ± 2 | 109 ± 14 | 5.7 ± 0.3 | 1538 ± 172 | 309 ± 31 | 60.3 ± 9 | 0.8 ± 0.1 | 0.36 ± 0.0 | 1.5 ± 0.0 |
| 2010 | 6.4 ± 0.1 | 2.5 ± 1.5 | 14.9 ± 3 | 110 ± 8 | 5.9 ± 0.5 | 1560 ± 107 | 310.7 ± 16 | 58.4 ± 8 | 0.7 ± 0.2 | 0.27 ± 0.0 | 1.5 ± 0.3 |
| Year | Date | AGDD §§ | MSC | Growth Stage | Description |
|---|---|---|---|---|---|
| 2008 | 12 June 2008 | 755 | 2.2 | E1: Elongation-Stem elongation | First node palpable/visible |
| 2008 | 24 July 2008 | 1452 | 3.3 | R2: Reproductive-Floral development | Spikelets fully emerged/peduncle not emerged |
| 2008 | 5 September 2008 | 2175 | 4.6 | S3: Seed development and ripening | Hard dough |
| 2008 | 30 October 2008 | 2611 | 5.3 | -- Postripening/senescence | -- |
| 2008 | 4 December 2008 | 2672 | 5.4 | -- Postripening/senescence | -- |
| 2009 | 28 February 2009 | 2748 | 1.0 | V0: Vegetative-Leaf development | Emergence of 1st leaf |
| 2009 | 3 July 2009 | 1180 | 2.9 | E4: Elongation-Stem elongation | 4th node palpable/visible |
| 2009 | 9 August 2009 | 1822 | 4.0 | S0: Seed development and ripening | Caryopsis visible |
| 2009 | 25 September 2009 | 2426 | 5.0 | S5: Seed development and ripening | Endosperm dry/seed ripe |
| 2009 | 19 November 2009 | 2627 | 5.3 | -- Postripening/senescence | -- |
| 2010 | 26 January 2010 | 2642 | 0.9 | G5: Germination | Coleoptile emergence from soil |
| 2010 | 2 March 2010 | 2642 | 0.9 | G5: Germination | Coleoptile emergence from soil |
| 2010 | 15 July 2010 | 1323 | 3.1 | R1: Reproductive-Floral development | Inflorescence emergence/1st spikelet visible |
| 2010 | 3 September 2010 | 2240 | 4.7 | S4: Seed development and ripening | Endosperm hard/physiological maturity |
| 2010 | 28 October 2010 | 2803 | 5.6 | -- Postripening/senescence | -- |
| 2010 | 2 December 2010 | 2878 | 5.8 | -- Postripening/senescence | -- |
| 2011 | 7 January 2011 | 2888 | 0.9 | G5: Germination | Coleoptile emergence from soil |
| 2011 | 25 March 2011 | 3031 | 1.1 | V1: Vegetative-Leaf development | First leaf collared |
| 2008 | 2009 | 2010 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yield | N | P | K | N | P | K | N | P | K |
| 2008 | −0.75 *** | −0.67 *** | −0.74 *** | -- | -- | -- | -- | -- | -- |
| 2009 | -- | -- | -- | −0.08 NS | −0.20 NS | −0.26 NS | -- | -- | -- |
| 2010 | -- | -- | -- | -- | -- | -- | −0.01 NS | -- | −0.18 NS |
| Regression | Plant | Ca | Mg | S | Na | Cu | Fe | Zn | Mn | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Portion | p-Value | ||||||||
| 2008 | AG | 0.001 | <0.0001 | <0.0001 | NS | NS | NS | <0.0001 | <0.0001 | 0.008 |
| Linear | BG | NS | 0.0013 | <0.001 | NS | 0.001 | <0.001 | NS | 0.0095 | 0.0003 |
| 2008 | AG | 0.006 | <0.001 | <0.0001 | NS | NS | NS | <0.0001 | <0.0001 | 0.015 |
| Quadratic | BG | NS | 0.002 | <0.0001 | NS | NS | <0.0001 | NS | <0.0001 | <0.0001 |
| 2009 | AG | 0.032 | <0.001 | <0.0001 | NS | <0.0001 | NS | <0.001 | 0.005 | NS |
| Linear | BG | 0.002 | 0.032 | 0.012 | NS | 0.0005 | 0.0037 | NS | 0.022 | <0.0001 |
| 2009 | AG | NS | <0.001 | <0.0001 | NS | <0.0001 | NS | <0.0001 | 0.017 | NS |
| Quadratic | BG | 0.001 | NS | 0.004 | NS | 0.0027 | 0.0049 | NS | 0.037 | <0.001 |
| 2010 | AG | 0.03 | 0.0126 | --- | --- | 0.001 | NS | 0.012 | --- | --- |
| Linear | BG | NS | --- | --- | NS | NS | NS | NS | --- | --- |
| 2010 | AG | 0.044 | 0.0107 | --- | --- | 0.0036 | NS | 0.032 | --- | --- |
| Quadratic | BG | NS | --- | --- | NS | NS | NS | NS | --- | --- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massey, J.; Antonangelo, J.; Zhang, H. Nutrient Dynamics in Switchgrass as a Function of Time. Agronomy 2020, 10, 940. https://doi.org/10.3390/agronomy10070940
Massey J, Antonangelo J, Zhang H. Nutrient Dynamics in Switchgrass as a Function of Time. Agronomy. 2020; 10(7):940. https://doi.org/10.3390/agronomy10070940
Chicago/Turabian StyleMassey, Joshua, João Antonangelo, and Hailin Zhang. 2020. "Nutrient Dynamics in Switchgrass as a Function of Time" Agronomy 10, no. 7: 940. https://doi.org/10.3390/agronomy10070940
APA StyleMassey, J., Antonangelo, J., & Zhang, H. (2020). Nutrient Dynamics in Switchgrass as a Function of Time. Agronomy, 10(7), 940. https://doi.org/10.3390/agronomy10070940






