Assessing Soil Organic Carbon in Soils to Enhance and Track Future Carbon Stocks
Abstract
:1. Introduction
2. Materials and Methods
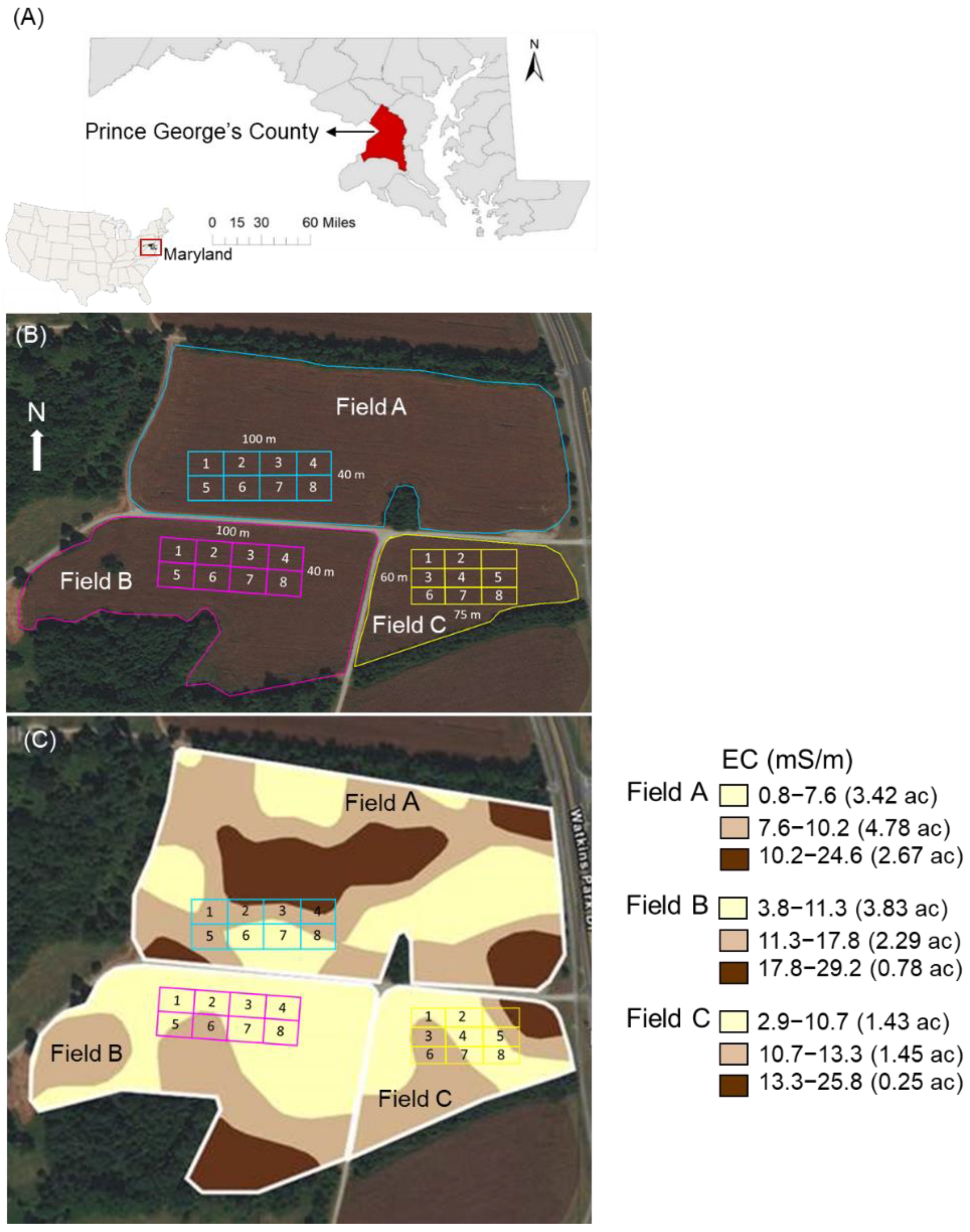
2.1. Study Sites
2.2. Soil Sampling
2.3. Soil Analysis and Soil Organic Carbon Stocks Calculation
2.4. Statistical Analysis
3. Results and Discussion
3.1. Soil pH across Depths and Fields
3.2. Depthwise Distribution of Soil Organic Carbon
3.3. Carbon to Nitrogen Ratios and Stable Isotopes in Agricultural Fields
3.4. Magnitude of Total Soil Organic Carbon Stocks
3.5. Spatial Variability and Guidance for Determining Soil Organic Carbon Stocks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vicente-Vicente, J.L.; García-Ruiz, R.; Francaviglia, R.; Aguilera, E.; Smith, P. Soil carbon sequestration rates under Mediterranean woody crops using recommended management practices: A meta-analysis. Agric. Ecosyst. Environ. 2016, 235, 204–214. [Google Scholar] [CrossRef] [Green Version]
- Batjes, N. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Scharlemann, J.P.W.; Tanner, E.V.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2018; United States Environmental Protection Agency: Washington, DC, USA, 2020.
- Zomer, R.J.; Bossio, D.A.; Sommer, R.; Verchot, L.V. Global Sequestration Potential of Increased Organic Carbon in Cropland Soils. Sci. Rep. 2017, 7, 15554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyle, F.C.; O’Leary, R.A.; Murphy, D.V. Spatially governed climate factors dominate management in determining the quantity and distribution of soil organic carbon in dryland agricultural systems. Sci. Rep. 2016, 6, 31468. [Google Scholar] [CrossRef]
- Lawrence, C.R.; Harden, J.; Xu, X.; Schulz, M.S.; Trumbore, S. Long-term controls on soil organic carbon with depth and time: A case study from the Cowlitz River Chronosequence, WA USA. Geoderma 2015, 247, 73–87. [Google Scholar] [CrossRef] [Green Version]
- VandenBygaart, B.; Gregorich, E.; Angers, D.A. Influence of agricultural management on soil organic carbon: A compendium and assessment of Canadian studies. Can. J. Soil Sci. 2003, 83, 363–380. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Chang. Boil. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.J. Soil carbon debt of 12,000 years of human land use. Proc. Natl. Acad. Sci. USA 2017, 114, 9575–9580. [Google Scholar] [CrossRef] [Green Version]
- Jian, J.; Du, X.; Reiter, M.S.; Stewart, R.D. A meta-analysis of global cropland soil carbon changes due to cover cropping. Soil Boil. Biochem. 2020, 143, 107735. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, H.D. Using cover crops to mitigate and adapt to climate change. A review. Agron. Sustain. Dev. 2017, 37, 4. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Jha, P.; Hati, K.; Dalal, R.C.; Dang, Y.P.; Kopittke, P.M.; Menzies, N.W. Soil carbon and nitrogen dynamics in a Vertisol following 50 years of no-tillage, crop stubble retention and nitrogen fertilization. Geoderma 2020, 358, 113996. [Google Scholar] [CrossRef]
- Bai, Z.; Caspari, T.; Gonzalez, M.R.; Batjes, N.; Mäder, P.; Bünemann, E.K.; De Goede, R.G.; Brussaard, L.; Xu, M.; Ferreira, C.; et al. Effects of agricultural management practices on soil quality: A review of long-term experiments for Europe and China. Agric. Ecosyst. Environ. 2018, 265, 1–7. [Google Scholar] [CrossRef]
- Aziz, I.; Mahmood, T.; Islam, K.R. Effect of long term no-till and conventional tillage practices on soil quality. Soil Tillage Res. 2013, 131, 28–35. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agric. Ecosyst. Environ. 2010, 139, 224–231. [Google Scholar] [CrossRef]
- Angers, D.A.; Eriksen-Hamel, N.S. Full-Inversion Tillage and Organic Carbon Distribution in Soil Profiles: A Meta-Analysis. Soil Sci. Soc. Am. J. 2008, 72, 1370–1374. [Google Scholar] [CrossRef]
- Mondal, S.; Chakraborty, D.; Bandyopadhyay, K.; Aggarwal, P.; Rana, D.S. A global analysis of the impact of zero-tillage on soil physical condition, organic carbon content, and plant root response. Land Degrad. Dev. 2020, 31, 557–567. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Adl, S.M.; Coleman, D.C.; Read, F. Slow recovery of soil biodiversity in sandy loam soils of Georgia after 25 years of no-tillage management. Agric. Ecosyst. Environ. 2006, 114, 323–334. [Google Scholar] [CrossRef]
- Stockmann, U.; Adams, M.A.; Crawford, J.W.; Field, D.; Henakaarchchi, N.; Jenkins, M.; Minasny, B.; McBratney, A.B.; Courcelles, V.D.R.D.; Singh, K.; et al. The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agric. Ecosyst. Environ. 2013, 164, 80–99. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Xu, M.; Feng, G.; Zhang, W.; Yang, X.; Huang, S. Contributions of wheat and maize residues to soil organic carbon under long-term rotation in north China. Sci. Rep. 2015, 5, 11409. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, X.; Xu, M.; Zhang, H.; Luo, Y. Characteristics of soil C:N ratio and δ13C in wheat-maize cropping system of the North China Plain and influences of the Yellow River. Sci. Rep. 2017, 7, 16854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, D. Department of Legislative Services, Maryland General Assembly 2017 Session. House Bill 1063. 2017. Available online: http://mgaleg.maryland.gov/2017RS/fnotes/bil_0003/hb1063.pdf (accessed on 1 May 2020).
- Ellert, B.H.; Janzen, H.H.; Entz, T. Assessment of a Method to Measure Temporal Change in Soil Carbon Storage. Soil Sci. Soc. Am. J. 2002, 66, 1687–1695. [Google Scholar] [CrossRef]
- Conant, R.T.; Smith, G.R.; Paustian, K. Spatial variability of soil carbon in forested and cultivated sites: Implications for change detection. J. Environ. Qual. 2003, 32, 278–286. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture, Web Soil Survey. 2019. Available online: https://websoilsurvey.sc.egov.usda.gov/App/HomePage.htm (accessed on 1 May 2020).
- National Weather Service. 2020. Available online: https://www.weather.gov/ (accessed on 1 May 2020).
- Eckert, D.; Sims, J.T. Recommended Soil pH and Lime Requirement Tests. In Recommended Soil Testing Procedures for the Northeastern United States. Northeast Regional Bulletin #493, 3rd ed.; Sims, J.T., Wolf, A., Eds.; Agricultural Experiment Station, University of Delaware: Newark, DE, USA, 2011; pp. 19–25. [Google Scholar]
- Hantsoo, K.G.; Kaufman, A.J.; Cui, H.; Plummer, R.E.; Narbonne, G.M. Effects of bioturbation on carbon and sulfur cycling across the Ediacaran–Cambrian transition at the GSSP in Newfoundland, Canada. Can. J. Earth Sci. 2018, 55, 1240–1252. [Google Scholar] [CrossRef]
- Funes, I.; Savé, R.; Rovira, P.; Molowny-Horas, R.; Alcañiz, J.M.; Ascaso, E.; Herms, I.; Herrero, C.; Boixadera, J.; Vayreda, J. Agricultural soil organic carbon stocks in the north-eastern Iberian Peninsula: Drivers and spatial variability. Sci. Total. Environ. 2019, 668, 283–294. [Google Scholar] [CrossRef]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Daniels, W.; Haering, K. The Mid-Atlantic Nutrient Management Handbook; Chapter 3. Concepts of Basic Soil Science; Virginia Cooperative Extension Service: Blacksburg, VA, USA, 2015.
- Islam, K.R.; Weil, R.R. Soil quality indicator properties in mid-Atlantic soils as influenced by conservation management. J. Soil Water Conserv. 2000, 55, 69–78. [Google Scholar]
- De Queiroz, J.F.; Nicolella, G.; Wood, C.W.; Boyd, C.E. Lime application methods, water and bottom soil acidity in fresh water fish ponds. Sci. Agric. 2004, 61, 469–475. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Lobsey, C.R.; Sharman, C.; Flick, P.; McLachlan, G. Novel proximal sensing for monitoring soil organic C stocks and condition. Environ. Sci. Technol. 2017, 51, 5630–5641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, D.; Lemon, J. Changes in soil pH as a result of lime addition as affected by rates, time and incorporation method. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010. [Google Scholar]
- Kumar, S.; Lal, R.; Liu, D.; Rafiq, R. Estimating the spatial distribution of organic carbon density for the soils of Ohio, USA. J. Geogr. Sci. 2013, 23, 280–296. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Hübner, R.; Barthold, F.; Spörlein, P.; Geuß, U.; Hangen, E.; Reischl, A.; Schilling, B.; Von Lützow, M.; Kögel-Knabner, I. Amount, distribution and driving factors of soil organic carbon and nitrogen in cropland and grassland soils of southeast Germany (Bavaria). Agric. Ecosyst. Environ. 2013, 176, 39–52. [Google Scholar] [CrossRef]
- Mclauchlan, K.K. Effects of soil texture on soil carbon and nitrogen dynamics after cessation of agriculture. Geoderma 2006, 136, 289–299. [Google Scholar] [CrossRef]
- Li, Q.; Li, A.; Dai, T.; Fan, Z.; Luo, Y.; Li, S.; Yuan, D.; Zhao, B.; Tao, Q.; Wang, C.; et al. Depth-dependent soil organic carbon dynamics of croplands across the Chengdu Plain of China from the 1980s to the 2010s. Glob. Chang. Boil. 2020, 26, 4134–4146. [Google Scholar] [CrossRef]
- Tsozué, D.; Nghonda, J.P.; Tematio, P.; Basga, S.D. Changes in soil properties and soil organic carbon stocks along an elevation gradient at Mount Bambouto, Central Africa. Catena 2019, 175, 251–262. [Google Scholar] [CrossRef]
- Chen, C.P.; Juang, K.W.; Cheng, C.H.; Pai, C.W. Effects of adjacent land-use types on the distribution of soil organic carbon stocks in the montane area of central Taiwan. Bot. Stud. 2016, 57, 32. [Google Scholar] [CrossRef] [Green Version]
- Parras-Alcántara, L.; García, B.L.; Galán-Espejo, A. Soil organic carbon along an altitudinal gradient in the Despeñaperros Natural Park, southern Spain. Solid Earth 2015, 6, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Bambrick, A.D.; Whalen, J.K.; Bradley, R.L.; Cogliastro, A.; Gordon, A.M.; Olivier, A.; Thevathasan, N.V. Spatial heterogeneity of soil organic carbon in tree-based intercropping systems in Quebec and Ontario, Canada. Agrofor. Syst. 2010, 79, 343–353. [Google Scholar] [CrossRef]
- Wendt, J.W.; Hauser, S. An equivalent soil mass procedure for monitoring soil organic carbon in multiple soil layers. Eur. J. Soil Sci. 2013, 64, 58–65. [Google Scholar] [CrossRef]
- Hassink, J. Effects of soil texture and grassland management on soil organic C and N and rates of C and N mineralization. Soil Boil. Biochem. 1994, 26, 1221–1231. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, P.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Haruna, S.I.; Anderson, S.H.; Nkongolo, N.V.; Zaibon, S. Soil Hydraulic Properties: Influence of Tillage and Cover Crops. Pedosphere 2018, 28, 430–442. [Google Scholar] [CrossRef]
- Sainju, U.M.; Whitehead, W.F.; Singh, B.P. Biculture Legume-Cereal Cover Crops for Enhanced Biomass Yield and Carbon and Nitrogen. Agron. J. 2005, 97, 1403–1412. [Google Scholar] [CrossRef] [Green Version]
- Haruna, S.I.; Nkongolo, N.V. Tillage, Cover Crop and Crop Rotation Effects on Selected Soil Chemical Properties. Sustainability 2019, 11, 2770. [Google Scholar] [CrossRef] [Green Version]
- Rumpel, C.; Kögel-Knabner, I. Deep soil organic matter—A key but poorly understood component of terrestrial C cycle. Plant Soil 2010, 338, 143–158. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.; Tang, Z.; Shangguan, Z. Soil organic carbon dynamics following natural vegetation restoration: Evidence from stable carbon isotopes (δ13C). Agric. Ecosyst. Environ. 2016, 221, 235–244. [Google Scholar] [CrossRef]
- Schiedung, H.; Tilly, N.; Hütt, C.; Welp, G.; Brüggemann, N.; Amelung, W. Spatial controls of topsoil and subsoil organic carbon turnover under C3–C4 vegetation change. Geoderma 2017, 303, 44–51. [Google Scholar] [CrossRef]
- Schaefer, M.V.; Bogie, N.A.; Rath, D.; Marklein, A.R.; Garniwan, A.; Haensel, T.; Lin, Y.; Avila, C.C.E.; Nico, P.S.; Scow, K.M.; et al. Effect of cover crop on carbon distribution in size and density separated soil aggregates. Soil Syst. 2020, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fang, N.; Tong, L.; Shi, Z. Source identification and budget evaluation of eroded organic carbon in an intensive agricultural catchment. Agric. Ecosyst. Environ. 2017, 247, 290–297. [Google Scholar] [CrossRef]
- Cavigelli, M.A.; Nash, P.R.; Gollany, H.T.; Rasmann, C.; Polumsky, R.W.; Le, A.N.; Conklin, A.E. Simulated soil organic carbon changes in maryland are affected by tillage, climate change, and crop yield. J. Environ. Qual. 2018, 47, 588–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobley, E.U.; Wilson, B. The depth distribution of organic carbon in the soils of eastern Australia. Ecosphere 2016, 7, e01214. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, K.; Hartemink, A.E.; Minasny, B.; Kheir, R.B.; Greve, M.B.; Greve, M.H. Digital mapping of soil organic carbon contents and stocks in denmark. PLoS ONE 2014, 9, e105519. [Google Scholar] [CrossRef]
- Chakan, A.A.; Taghizadeh-Mehrjardi, R.; Kerry, R.; Kumar, S.; Khordehbin, S.; Khanghah, S.Y. Spatial 3D distribution of soil organic carbon under different land use types. Environ. Monit. Assess. 2017, 189, 131. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, M.; Minasny, B.; McBratney, A.; Michot, D.; Viaud, V.; Walter, C. High resolution 3D mapping of soil organic carbon in a heterogeneous agricultural landscape. Geoderma 2014, 213, 296–311. [Google Scholar] [CrossRef]
- Rabbi, S.M.F.; Tighe, M.; Delgado-Baquerizo, M.; Cowie, A.; Robertson, F.; Dalal, R.; Page, K.; Crawford, D.; Wilson, B.R.; Schwenke, G.; et al. Climate and soil properties limit the positive effects of land use reversion on carbon storage in Eastern Australia. Sci. Rep. 2015, 5, 17866. [Google Scholar] [CrossRef] [Green Version]
- Schillaci, C.; Acutis, M.; Lombardo, L.; Lipani, A.; Fantappiè, M.; Märker, M.; Saia, S. Spatio-temporal topsoil organic carbon mapping of a semi-arid Mediterranean region: The role of land use, soil texture, topographic indices and the influence of remote sensing data to modelling. Sci. Total. Environ. 2017, 601, 821–832. [Google Scholar] [CrossRef]
- Piccoli, I.; Chiarini, F.; Carletti, P.; Furlan, L.; Lazzaro, B.; Nardi, S.; Berti, A.; Sartori, L.; Dalconi, M.C.; Morari, F. Disentangling the effects of conservation agriculture practices on the vertical distribution of soil organic carbon. Evidence of poor carbon sequestration in North- Eastern Italy. Agric. Ecosyst. Environ. 2016, 230, 68–78. [Google Scholar] [CrossRef]
- Johnson, J.M.F.; Allmaras, R.R.; Reicosky, D.C. estimating source carbon from crop residues, roots and rhizodeposits using the national grain-yield database. Agron. J. 2006, 98, 622–636. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Soussana, J.; Angers, D.; Schipper, L.A.; Chenu, C.; Rasse, D.; Batjes, N.; Van Egmond, F.; McNeill, S.; Kuhnert, M.; et al. How to measure, report and verify soil carbon change to realize the potential of soil carbon sequestration for atmospheric greenhouse gas removal. Glob. Chang. Boil. 2019, 26, 219–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Site | Area | Soil Type | Bulk Density (g/cm3) | Previous Cropping History | Future Cropping History (for 5 Years) | Future Cropping Practices |
|---|---|---|---|---|---|---|
| Field A | 4.3 ha | Combination of, AdA Adelphia-Holmdel Complex, Moderately well drained, Slope: 0–2%, Class 11, Non-Hydric CnB Collington-Wist Complex, Well Drained, Slope 0–2%, Class I, Non-Hydric | 0–20 cm: 1.56 20–40 cm: 1.6 40–60 cm: 1.7 | 2015. Corn; 2017 Wheat, DC Soybean | Continuous No-Till Soybean-multispecies Cover Crop-Corn-Wheat-Cover Crop-Sorghum-Rye-Soybean | New soil health practices |
| Field B | 2.8 ha | AdA Adelphia-Holmdel Complex, Moderately well drained, Slope: 0–2%, Class II, Non-Hydric | 0–20 cm: 1.56 20–40 cm: 1.6 40–60 cm: 1.7 | 2015 NT Soybean; 2016 NT Corn; 2017 Conventional Wheat, NT DC Soybean | Continuous Clean-Till Soybean. No Cover Crop. | Typical of prior modern farming practices. |
| Field C | 1.25 ha | Combination of SrA Shrewsberry Loam, Poorly drained, Slope 0–2%, Class IV, Hydric AfB Annapolis Fine Sandy Loam, Slope 2–5%, Well drained, Class II, Non-Hydric | 0–20 cm: 1.42 20–40 cm: 1.6 40–60 cm: 1.6 | 2015. Corn; 2017 Wheat, DC Soybean | Continuous No-Till Soybean. No Cover Crop. | Typical of today’s farming techniques |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-Y.; Goldsmith, A.; Herold, I.; Lecha, S.; Toor, G.S. Assessing Soil Organic Carbon in Soils to Enhance and Track Future Carbon Stocks. Agronomy 2020, 10, 1139. https://doi.org/10.3390/agronomy10081139
Yang Y-Y, Goldsmith A, Herold I, Lecha S, Toor GS. Assessing Soil Organic Carbon in Soils to Enhance and Track Future Carbon Stocks. Agronomy. 2020; 10(8):1139. https://doi.org/10.3390/agronomy10081139
Chicago/Turabian StyleYang, Yun-Ya, Avi Goldsmith, Ilana Herold, Sebastian Lecha, and Gurpal S. Toor. 2020. "Assessing Soil Organic Carbon in Soils to Enhance and Track Future Carbon Stocks" Agronomy 10, no. 8: 1139. https://doi.org/10.3390/agronomy10081139





