Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem
Abstract
:1. Introduction
2. Materials and Methods
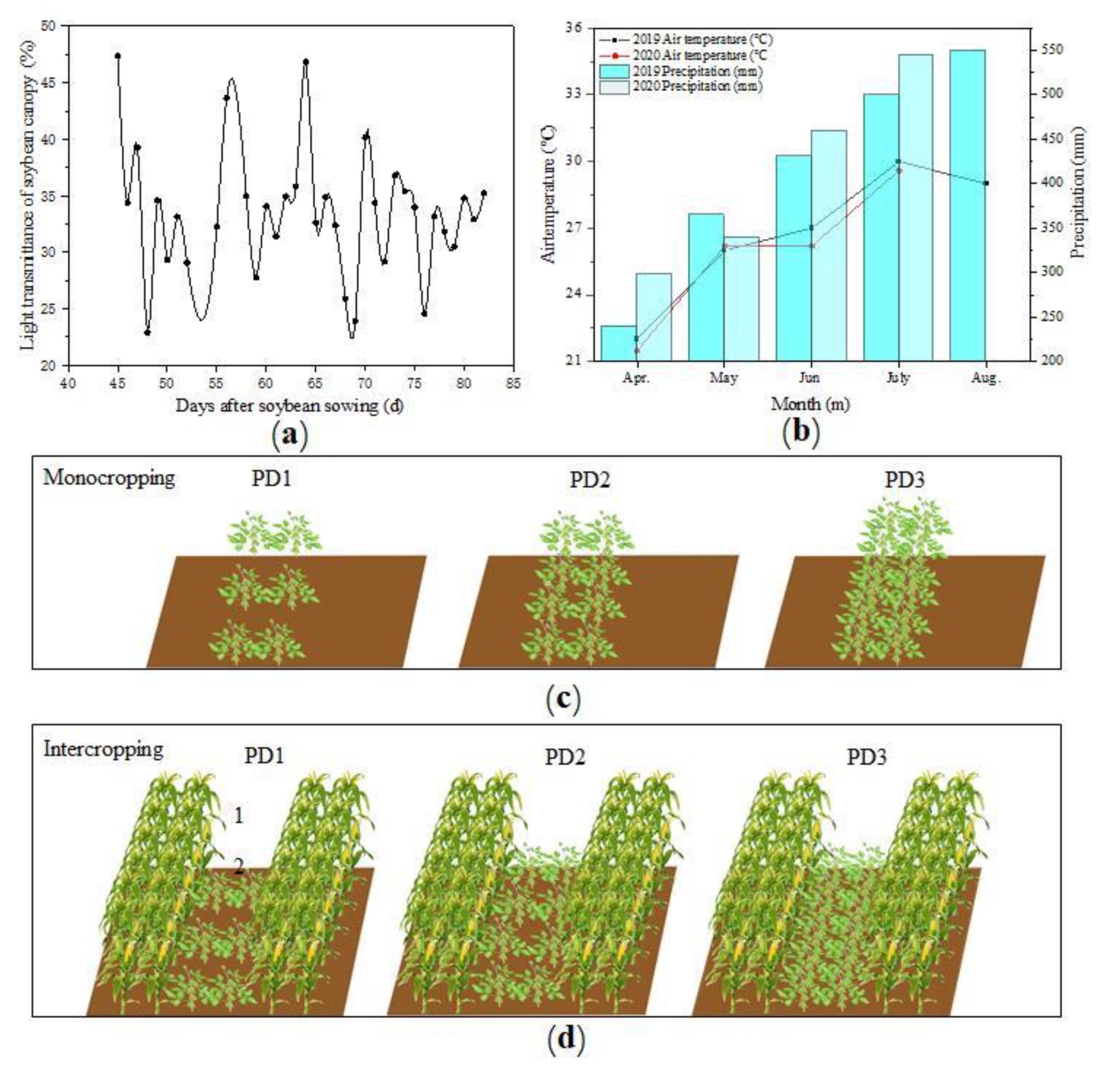
2.1. Site Description
2.2. Experimental Design
2.3. Data Collections
3. Sampling and Measurements
3.1. Plant Height and Stem Diameter
3.2. Lodging Percentage
3.3. Stem Strength and Lodging Resistance Index
3.4. Evaluation of Stem Carbohydrates Content
3.5. Lignin Content
3.6. Cellulose Content
3.7. Determination of Soluble Sugar and Starch
3.8. Determination of Sucrose Content
3.9. Key Enzymes of Lignin Synthesis Pathway
3.10. The Available Light of Soybean Plants
3.11. Photosynthetic Rate of Soybean Inverted Trifoliate
3.12. Statistical Analysis
4. Results
4.1. Effect of Multiple Planting Densities on Lodging Resistance Index of Soybean
4.2. Effect of Multiple Planting Densities on the Available Light for Soybean Plants
4.3. Effect of MultiplePlanting Densities on the Net Photosynthetic Rate
4.4. Effects of Multiple Planting Densities on Non-Structural Carbohydrates Content of Soybean Stem
4.5. Effect of Multiple Planting Densities on Structural Carbohydrates Contents of Soybean Stem
4.6. Effect of Planting Densities on Key Enzymes of the Lignin Synthesis Pathway
4.7. Effect of Planting Densities on Plant Height and Stem Diameter
4.8. The Contribution Rate of Carbohydrate Contents and Lignin Synthesis Related Enzyme Activities to Stem Strength
5. Correlation Analysis
6. Discussion
6.1. Light Environment and Photosynthesis Characteristics
6.2. Lodging Resistance and Stem Morphology
6.3. Lodging Resistance and Carbohydrates Contents
6.4. Lodging Resistance, Lignin Content, and Activities of Enzymes Related to Lignin Synthesis
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Wen, J.; Wu, W. Yield increasing and water saving effect under different soil fertility improvements in wheat-corn intercropping field in Huabei Plain. Ying Yong Sheng Tai Xue Bao 2000, 11, 219–222. [Google Scholar] [PubMed]
- Chen, P.; Song, C.; Liu, X.M.; Zhou, L.; Yang, H.; Zhang, X.; Zhou, Y.; Du, Q.; Pang, T.; Fu, Z.D.; et al. Yield advantage and nitrogen fate in an additive maize-soybean relay intercropping system. Sci. Total Environ. 2019, 657, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Tang, M.M.; Zhang, W.P.; Bao, X.G.; Wang, Y.; Christie, P.; Li, L. Temporal Differentiation of Crop Growth as One of the Drivers of Intercropping Yield Advantage. Sci. Rep. 2018, 8, 3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.J.; Wang, P.; Chen, X.X.; Feng, H.; Zhu, L.X. Effects of Platycodon grandiflorum/pepper intercropping on root growth, yield and quality of Platycodon grandiflorum. Zhongguo Zhong Yao Za Zhi 2018, 43, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Yong, T.W.; Chen, P.; Dong, Q.; Du, Q.; Yang, F.; Wang, X.C.; Liu, W.G.; Yang, W.Y. Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize-soybean relay intercropping system. J. Integr. Agric. 2018, 17, 664–676. [Google Scholar] [CrossRef]
- Zhang, R.; Mu, Y.; Li, X.; Li, S.; Sang, P.; Wang, X.; Wu, H.; Xu, N. Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates. Sci. Total Environ. 2020, 740, 139810. [Google Scholar] [CrossRef]
- Chen, P.; Du, Q.; Zhou, L.; Yang, H.; Dong, Q.; Song, C.; Yang, W.Y.; Yong, T.W. Effects of N application reduction and fertilizing distance on saving fertilizer and improving yield in maize/soybean intercropping system. Ying Yong Sheng Tai Xue Bao 2016, 27, 3247–3256. [Google Scholar] [CrossRef]
- Liu, W.G.; Ren, M.L.; Liu, T.; Du, Y.L.; Zhou, T.; Liu, X.M.; Liu, J.; Hussain, S.; Yang, W.Y. Effect of shade stress on lignin biosynthesis in soybean stems. J. Integr. Agric. 2018, 17, 1594–1604. [Google Scholar] [CrossRef]
- Gao, Y.; Duan, A.; Sun, J.; Li, F.; Liu, Z.; Liu, H.; Liu, Z. Crop coefficient and water-use efficiency of winter wheat/spring maize strip intercropping. Field Crops Res. 2009, 111, 65–73. [Google Scholar] [CrossRef]
- Raza, M.A.; Khalid, M.H.B.; Zhang, X.; Feng, L.Y.; Khan, I.; Hassan, M.J.; Ahmed, M.; Ansar, M.; Chen, Y.K.; Fan, Y.F. Effect of planting patterns on yield, nutrient accumulation and distribution in maize and soybean under relay intercropping systems. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Chen, J.; Cheng, Y.; Raza, M.A.; Wu, X.; Wang, Z.; Liu, Q.; Wang, R.; Wang, X.; Yong, T. Effect of shading and light recovery on the growth, leaf structure, and photosynthetic performance of soybean in a maize-soybean relay-strip intercropping system. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.G.; Wen, B.X.; Zhou, T.; Wang, L.; Gao, Y.; Li, S.X.; Qin, S.S.; Liu, J.; Yang, W.Y. iTRAQ protein profile analysis of soybean stems reveals new aspects critical for lodging in intercropping systems. J. Integr. Agric. 2019, 18, 2029–2040. [Google Scholar] [CrossRef]
- Liu, W.G.; Deng, Y.C.; Hussain, S.; Zou, J.L.; Yuan, J.; Luo, L.; Yang, C.Y.; Yuan, X.Q.; Yang, W.Y. Relationship between cellulose accumulation and lodging resistance in the stem of relay intercropped soybean [Glycine max (L.) Men.]. Field Crops Res. 2016, 196, 261–267. [Google Scholar] [CrossRef]
- Wan, Y.; Yan, Y.H.; Xiang, D.B.; Ye, M.Y.; Yang, W.Y.; Liu, J. Isoflavonoid Accumulation Pattern as Affected by Shading from Maize in Soybean (Glycine max (L.) Merr.) in Relay Strip Intercropping System. Plant Prod. Sci. 2015, 18, 302–313. [Google Scholar] [CrossRef] [Green Version]
- Su, B.Y.; Song, Y.X.; Song, C.; Cui, L.; Yong, T.W.; Yang, W.Y. Growth and photosynthetic responses of soybean seedlings to maize shading in relay intercropping system in Southwest China. Photosynthetica 2014, 52, 332–340. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, R.; Hou, Z.F.; Yan, C.; Xia, X.; Ma, C.M.; Dong, S.K.; Gong, Z.P. Mechanical properties of soybean plants under various plant densities. Crop Pasture Sci. 2020, 71, 249–259. [Google Scholar] [CrossRef]
- Paulsen, M.R.; Pinto, F.D.D.; de Sena, D.G.; Zandonadi, R.S.; Ruffato, S.; Costa, A.G.; Ragagnin, V.A.; Danao, M.G.C. Measurement of Combine Losses for Corn and Soybeans in Brazil. Appl. Eng. Agric. 2014, 30, 841–855. [Google Scholar]
- Ray, C.L.; Shipe, E.R.; Bridges, W.C. Planting date influence on soybean agronomic traits and seed composition in modified fatty acid breeding lines. Crop Sci. 2008, 48, 181–188. [Google Scholar] [CrossRef]
- Kitabatake, T.; Yoshihira, T.; Suzuki, H.; Yamaguchi, N. Yield and related traits for a soybean breeding line ‘Tokei 1122′ with QTLs for long terminal racemes under high planting density conditions. Plant Prod. Sci. 2020, 23. [Google Scholar] [CrossRef]
- Echezona, B.C. Corn-stalk lodging and borer damage as influenced by varying corn densities and planting geometry with soybean (Glycine max L. Merrill). Int. Agrophys. 2007, 21, 133–143. [Google Scholar]
- Deng, Y.C.; Liu, W.G.; Yuan, X.Q.; Yuan, J.; Zou, J.L.; Du, J.B.; Yang, W.Y. Relationship between cellulose synthesis metabolism and lodging resistance in intercropping soybean at seedling stage. Ying Yong Sheng Tai Xue Bao 2016, 27, 469–476. [Google Scholar] [PubMed]
- Hussain, S.; Liu, T.; Iqbal, N.; Brestic, M.; Pang, T.; Mumtaz, M.; Shafiq, I.; Li, S.; Wang, L.; Gao, Y.; et al. Effects of lignin, cellulose, hemicellulose, sucrose and monosaccharide carbohydrates on soybean physical stem strength and yield in intercropping. Photochem. Photobiol. Sci. 2020, 19, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, S.; Gao, R.C.; Liu, W.G.; Yong, T.W.; Wang, X.C.; Wu, X.L.; Yang, W.Y. Growth of soybean seedlings in relay strip intercropping systems in relation to light quantity and red:far-red ratio. Field Crops Res. 2014, 155, 245–253. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Shi, W.B.; Xia, X.; Tang, Y.H.; Tao, J. Microstructural and lignin characteristics in herbaceous peony cultivars with different stem strengths. Postharvest Biol. Technol. 2020, 159, 111043. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, Z.; Ma, X.; Ma, H.; Zhao, Y. Characterising the morphological characters and carbohydrate metabolism of oat culms and their association with lodging resistance. Plant Biol. 2020, 22, 267–276. [Google Scholar] [CrossRef]
- Hu, D.; Liu, X.B.; She, H.Z.; Gao, Z.; Ruan, R.W.; Wu, D.Q.; Yi, Z.L. The lignin synthesis related genes and lodging resistance of Fagopyrum esculentum. Biol. Plant. 2017, 61, 138–146. [Google Scholar] [CrossRef]
- Su, G.; An, Z.; Zhang, W.; Liu, Y. Light promotes the synthesis of lignin through the production of H2O2 mediated by diamine oxidases in soybean hypocotyls. J. Plant Physiol. 2005, 162, 1297–1303. [Google Scholar] [CrossRef]
- Soares, A.R.; Siqueira-Soares, R.D.; Salvador, V.H.; Ferrarese, M.D.L.; Ferrarese, O. The effects of L-DOPA on root growth, lignification and enzyme activity in soybean seedlings. Acta Physiol. Plant. 2012, 34, 1811–1817. [Google Scholar] [CrossRef]
- Dos Santos, W.D.; Ferrarese, M.L.L.; Nakamura, C.V.; Mourao, K.S.M.; Mangolin, C.A.; Ferrarese-Filho, O. Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. J. Chem. Ecol. 2008, 34, 1230–1241. [Google Scholar] [CrossRef]
- Ahmed, M.; Akhtar, S.; Fanglu, M.; Hasan, M.M.; Shahid, A.A.; Yanang, X.; Sarwar, M.B.; Rau, A.Q.; Husnain, T.; Wang, X. Sucrose Synthase (SuSy) Gene Expression: An Indicator for Cotton Fiber Initiation and Early Development. Russ. J. Plant Physl. 2019, 66, 41–49. [Google Scholar] [CrossRef]
- Cook, F.R.; Fahy, B.; Trafford, K. A rice mutant lacking a large subunit of ADP-glucose pyrophosphorylase has drastically reduced starch content in the culm but normal plant morphology and yield. Funct. Plant Biol. 2012, 39, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.J.; Magalhaes, J.R.; Sales, C.R.G.; Pires, R.C.D.; Machado, E.C. Source-sink relationships in two soybean cultivars with indeterminate growth under water deficit. Bragantia 2018, 77, 23–35. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Feng, L.; Liu, Q.; Wu, X.; Fan, Y.; Raza, M.A.; Cheng, Y.; Chen, J.; Wang, X.; Yong, T. Effect of interactions between light intensity and red-to-far-red ratio on the photosynthesis of soybean leaves under shade condition. Environ. Exp. Bot. 2018, 150, 79–87. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.F.; Galstyan, A.; Salla-Martret, M.; Cifuentes-Esquivel, N.; Gallemi, M.; Bou-Torrent, J. Regulatory components of shade avoidance syndrome. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 53, pp. 65–116. [Google Scholar]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Zhang, W.; Ding, Y.; Zhang, J.; Cambula, E.D.; Weng, F.; Liu, Z.; Ding, C.; Tang, S.; Chen, L. Shading contributes to the reduction of stem mechanical strength by decreasing cell wall synthesis in japonica rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Rahman, T.; Song, C.; Yang, F.; Su, B.; Cui, L.; Bu, W.; Yang, W. Relationships among light distribution, radiation use efficiency and land equivalent ratio in maize-soybean strip intercropping. Field Crops Res. 2018, 224, 91–101. [Google Scholar] [CrossRef]
- Asghar, M.A.; Du, J.B.; Jiang, H.K.; Li, Y.; Sun, X.; Shang, J.; Liu, J.; Liu, W.G.; Imran, S.; Iqbal, N.; et al. Shade pretreatment enhanced drought resistance of soybean. Environ. Exp. Bot. 2020, 171, 103952. [Google Scholar] [CrossRef]
- Song, D.G.; Tariq, A.; Pan, K.W.; Khan, S.U.; Saleh, T.A.; Gong, S.X.; Zhang, A.P.; Wu, X.G. Influence of planting distance and density on the yield and photosynthetic traits of sweet potato (Ipomoea balatas L.) under an intercropping system with walnut (Juglans regia) saplings. Soil Till. Res. 2020, 196. [Google Scholar] [CrossRef]
- Toyota, M.; Maitree, L.; Chomsang, K. Changes in radiation interception and R:FR over time and with canopy depth of two soybean cultivars with different branching characteristics. Plant Prod. Sci. 2017, 20, 205–214. [Google Scholar] [CrossRef]
- Liu, B.; Liu, X.B.; Wang, C.; Li, Y.S.; Jin, J.; Herbert, S.J. Soybean yield and yield component distribution across the main axis in response to light enrichment and shading under different densities. Plant Soil Environ. 2010, 56, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Sarabi, V.; Mahallati, M.N.; Nezami, A.; Mohassel, M.H.R. Effects of the relative time of emergence and the density of common lambsquarters (Chenopodium album) on corn (Zea mays) yield. Weed Biol. Manag. 2011, 11, 127–136. [Google Scholar] [CrossRef]
- Echarte, L.; Della Maggiora, A.; Cerrudo, D.; Gonzalez, V.H.; Abbate, P.; Cerrudo, A.; Sadras, V.O.; Calvino, P. Yield response to plant density of maize and sunflower intercropped with soybean. Field Crops Res. 2011, 121, 423–429. [Google Scholar] [CrossRef]
- Zheng, M.J.; Chen, J.; Shi, Y.H.; Li, Y.X.; Yin, Y.P.; Yang, D.Q.; Luo, Y.L.; Pang, D.W.; Xu, X.; Li, W.Q.; et al. Manipulation of lignin metabolism by plant densities and its relationship with lodging resistance in wheat. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ruan, R.W.; Yuan, X.H.; Hu, D.; Yang, H.; Li, Y.; Yi, Z.L. Effects of Nitrogen Fertilizer and Planting Density on the Lignin Synthesis in the Culm in Relation to Lodging Resistance of Buckwheat. Plant Prod. Sci. 2015, 18, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Sher, A.; Khan, A.; Ashraf, U.; Liu, H.H.; Li, J.C. Characterization of the Effect of Increased Plant Density on Canopy Morphology and Stalk Lodging Risk. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- San-Oh, Y.; Kond, M.; Ookawa, T.; Hirasawa, T. Ecophysiological analysis on effect of planting pattern on biomass production and grain yield in rice. Jpn. Agric. Res. Q. 2008, 42, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Anwar, S.; Kuai, J.; Noman, A.; Shahid, M.; Din, M.; Ali, A.; Zhou, G.S. Alteration in yield and oil quality traits of winter rapeseed by lodging at different planting density and nitrogen rates. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Z.; Liao, D.; Raza, M.; Wang, B.; Zhang, J.; Chen, J.; Feng, L.; Wu, X.; Liu, C.; et al. Uptake and utilization of nitrogen, phosphorus and potassium as related to yield advantage in maize-soybean intercropping under different row configurations. Sci. Rep. 2020, 10, 9504. [Google Scholar] [CrossRef]
- Xiang, D.B.; Zhao, G.; Wan, Y.; Tan, M.L.; Song, C.; Song, Y. Effect of planting density on lodging-related morphology, lodging rate, and yield of tartary buckwheat (Fagopyrum tataricum). Plant Prod. Sci. 2016, 19, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Wen, B.; Zhang, Y.; Hussain, S.; Wang, S.; Zhang, X.; Yang, J.; Xu, M.; Qin, S.; Yang, W.; Liu, W. Slight Shading Stress at Seedling Stage Does not Reduce Lignin Biosynthesis or Affect Lodging Resistance of Soybean Stems. Agronomy 2020, 10, 544. [Google Scholar] [CrossRef] [Green Version]
- Leng, F.; Sun, S.; Jing, Y.; Wang, F.; Wei, Q.; Wang, X.; Zhu, X. A rapid and sensitive method for determination of trace amounts of glucose by anthrone-sulfuric acid method. Bulg. Chem. Commun. 2016, 48, 109–113. [Google Scholar]
- John, K.M.M.; Enkhtaivan, G.; Lee, J.H.; Thiruvengadam, M.; Keum, Y.S.; Kim, D.H. Spectroscopic determination of metabolic and mineral changes of soya-chunk mediated by Aspergillus sojae. Food Chem. 2015, 170, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.J.; Nogueira, M.A.; Hungria, M. Feasibility of Lowering Soybean Planting Density without Compromising Nitrogen Fixation and Yield. Agron. J. 2014, 106, 2118–2124. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.B.; Xu, H.S.; Bi, H.X.; Xi, W.M.; Bao, B.; Wang, X.Y.; Bi, C.; Chang, Y.F. Intercropping Competition between Apple Trees and Crops in Agroforestry Systems on the Loess Plateau of China. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jain, A.K.; McIsaac, G.F. Implementation of dynamic crop growth processes into a land surface model: Evaluation of energy, water and carbon fluxes under corn and soybean rotation. Biogeosciences 2013, 10, 8039–8066. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.C.R.; Carvalho, S.J.P.; Marcolini, L.W.; Melo, M.S.C.; Christoffoleti, P.J. Competitiveness of Alexandergrass or Bengal Dayflower with Soybean. Planta Daninha 2010, 28, 515–522. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, Y.; Zhang, M.; Hong, A.; Yang, H.; Liu, Y. Shade effects on growth, photosynthesis and chlorophyll fluorescence parameters of three Paeonia species. PeerJ 2020, 8, e9316. [Google Scholar] [CrossRef]
- Drouet, J.L.; Kiniry, J.R. Does spatial arrangement of 3D plants affect light transmission and extinction coefficient within maize crops? Field Crops Res. 2008, 107, 62–69. [Google Scholar] [CrossRef]
- Dong, W.L.; Yu, H.; Zhang, L.Z.; Wang, R.N.; Wang, Q.; Xue, Q.W.; Pan, Z.H.; Sun, Z.G.; Pan, X.B. Asymmetric Ridge-Furrow and Film Cover Improves Plant Morphological Traits and Light Utilization in Rain-Fed Maize. J. Meteorol. Res. 2018, 32, 829–838. [Google Scholar] [CrossRef]
- Moreira, A.S.F.P.; de Lemos, J.P.; Isaias, R.M.D. Structural adaptations of two sympatric epiphytic orchids (Orchidaceae) to a cloudy forest environment in rocky outcrops of Southeast Brazil. Rev. Biol. Trop. 2013, 61, 1053–1065. [Google Scholar]
- Shahinnia, F.; Le Roy, J.; Laborde, B.; Sznajder, B.; Kalambettu, P.; Mahjourimajd, S.; Tilbrook, J.; Fleury, D. Genetic association of stomatal traits and yield in wheat grown in low rainfall environments. BMC Plant Biol. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L.; Catoni, R.; Pirone, G.; Frattaroli, A.R.; Varone, L. Physiological and morphological leaf trait variations in two Apennine plant species in response to different altitudes. Photosynthetica 2012, 50, 15–23. [Google Scholar] [CrossRef]
- Chen, H.F.; Yang, Z.L.; Chen, L.M.; Zhang, C.J.; Yuan, S.L.; Zhang, X.J.; Qiu, D.Z.; Wan, Q.; Zhan, Y.; Chen, S.; et al. Combining QTL and candidate gene analysis with phenotypic model to unravel the relationship between lodging and related traits in soybean. Mol. Breed. 2017, 37, 43. [Google Scholar] [CrossRef]
- Yan, Y.H.; Gong, W.Z.; Yang, W.Y.; Wan, Y.; Chen, X.L.; Chen, Z.Q.; Wang, L.Y. Seed Treatment with Uniconazole Powder Improves Soybean Seedling Growth under Shading by Corn in Relay Strip Intercropping System. Plant Prod. Sci. 2010, 13, 367–374. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wu, L.W.; Wu, X.R.; Ding, Y.F.; Li, G.H.; Li, J.Y.; Weng, F.; Liu, Z.H.; Tang, S.; Ding, C.Q.; et al. Lodging Resistance of Japonica Rice (Oryza Sativa L.): Morphological and Anatomical Traits due to top-Dressing Nitrogen Application Rates. Rice 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Hu, D.; Liu, X.B.; She, H.Z.; Ruan, R.W.; Yang, H.; Yi, Z.L.; Wu, D.Q. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Li, X.; Yan, X.; Yu, T. Effects of color films shading on Phellodendron amurense seedlings biomass and primary nitrogen-assimilation enzyme activities. Ying Yong Sheng Tai Xue Bao 2006, 17, 2020–2023. [Google Scholar]
- Tang, L.; Ma, W.; Noor, M.A.; Li, L.; Hou, H.; Zhang, X.; Zhao, M. Density resistance evaluation of maize varieties through new “Density-Yield Model” and quantification of varietal response to gradual planting density pressure. Sci. Rep. 2018, 8, 17281. [Google Scholar] [CrossRef]
- Fang, J.; Lutz, J.A.; Shugart, H.H.; Yan, X.D. A physiological model for predicting dynamics of tree stem-wood non-structural carbohydrates. J. Ecol. 2020, 108, 702–718. [Google Scholar] [CrossRef]
- Gent, M.P.N.; Seginer, I. A carbohydrate supply and demand model of vegetative growth: Response to temperature and light. Plant Cell Environ. 2012, 35, 1274–1286. [Google Scholar] [CrossRef]
- Rebetzke, G.J.; van Herwaarden, A.F.; Jenkins, C.; Weiss, M.; Lewis, D.; Ruuska, S.; Tabe, L.; Fettell, N.A.; Richards, R.A. Quantitative trait loci for water-soluble carbohydrates and associations with agronomic traits in wheat. Aust. J. Agric. Res. 2008, 59, 891–905. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M.; Weston, C. Reductions in biomass accumulation, photosynthesis in situ and net carbon balance are the costs of protecting Vitis vinifera ‘Semillon’ grapevines from heat stress with shade covering. AoB Plants 2011. [Google Scholar] [CrossRef] [PubMed]
- Closa, I.; Irigoyen, J.J.; Goicoechea, N. Microclimatic conditions determined by stem density influence leaf anatomy and leaf physiology of beech (Fagus sylvatica L.) growing within stands that naturally regenerate from clear-cutting. Trees 2010, 24, 1029–1043. [Google Scholar] [CrossRef]
- Goncalves, B.; Correia, C.M.; Silva, A.P.; Bacelar, E.A.; Santos, A.; Moutinho-Pereira, J.M. Leaf structure and function of sweet cherry tree (Prunus avium L.) cultivars with open and dense canopies. Sci. Hortic. 2008, 116, 381–387. [Google Scholar] [CrossRef]
- Xu, G.L.; Singh, S.K.; Reddy, V.R.; Barnaby, J.Y.; Sicher, R.C.; Li, T. Soybean grown under elevated CO2 benefits more under low temperature than high temperature stress: Varying response of photosynthetic limitations, leaf metabolites, growth, and seed yield. J. Plant Physiol. 2016, 205, 20–32. [Google Scholar] [CrossRef]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Mohapatra, P.K.; Fujita, K. Differences in the responses of stem diameter and pod thickness to drought stress during the grain filling stage in soybean plants. Acta Physiol. Plant. 2009, 31, 271–277. [Google Scholar] [CrossRef]
- Tanaka, Y.; Shiraiwa, T.; Nakajima, A.; Sato, J.; Nakazaki, T. Leaf gas exchange activity in soybean as related to leaf traits and stem growth habit. Crop Sci. 2008, 48, 1925–1932. [Google Scholar] [CrossRef]
- Wang, Y.W.; Sun, L.G.; Xu, J.G.; Yu, J.; Zhang, J.J.; Wu, M.; Ma, J.; Chen, G.X. Physiological and growth characteristics of Ginkgo biloba L. exposed to open field and shade enclosure during the reproductive stage. Acta Physiol. Plant. 2014, 36, 2671–2681. [Google Scholar] [CrossRef]
- Jiang, H.K.; Shui, Z.W.; Xu, L.; Yang, Y.H.; Li, Y.; Yuan, X.Q.; Shang, J.; Asghar, M.A.; Wu, X.L.; Yu, L.; et al. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol. Biochem. 2020, 150, 209–221. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wu, X.; Liu, T.; Ding, R.; Han, Q. Application of paclobutrazol: A strategy for inducing lodging resistance of wheat through mediation of plant height, stem physical strength, and lignin biosynthesis. Environ. Sci. Pollut. Res. Int. 2018, 25, 29366–29378. [Google Scholar] [CrossRef]
- Lu, J.; Shi, Y.; Li, W.; Chen, S.; Wang, Y.; He, X.; Yin, X. RcPAL, a key gene in lignin biosynthesis in Ricinus communis L. BMC Plant Biol. 2019, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Eisvand, H.R.; Tavakkol-Afshari, R.; Sharifzadeh, F.; Arefi, H.M.; Hejazi, S.M.H. Effects of hormonal priming and drought stress on activity and isozyme profiles of antioxidant enzymes in deteriorated seed of tall wheatgrass (Agropyron elongatum Host). Seed Sci. Technol. 2010, 38, 280–297. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, B.; Raza, A.; Wang, L.; Xu, M.; Lu, J.; Gao, Y.; Qin, S.; Zhang, Y.; Ahmad, I.; Zhou, T.; et al. Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem. Agronomy 2020, 10, 1177. https://doi.org/10.3390/agronomy10081177
Cheng B, Raza A, Wang L, Xu M, Lu J, Gao Y, Qin S, Zhang Y, Ahmad I, Zhou T, et al. Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem. Agronomy. 2020; 10(8):1177. https://doi.org/10.3390/agronomy10081177
Chicago/Turabian StyleCheng, Bin, Ali Raza, Li Wang, Mei Xu, Junji Lu, Yang Gao, Sisi Qin, Yi Zhang, Irshan Ahmad, Tao Zhou, and et al. 2020. "Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem" Agronomy 10, no. 8: 1177. https://doi.org/10.3390/agronomy10081177
APA StyleCheng, B., Raza, A., Wang, L., Xu, M., Lu, J., Gao, Y., Qin, S., Zhang, Y., Ahmad, I., Zhou, T., Wen, B., Yang, W., & Liu, W. (2020). Effects of Multiple Planting Densities on Lignin Metabolism and Lodging Resistance of the Strip Intercropped Soybean Stem. Agronomy, 10(8), 1177. https://doi.org/10.3390/agronomy10081177



