Comparison of Two Inoculation Methods of Endophytic Bacteria to Enhance Phytodegradation Efficacy of an Aged Petroleum Hydrocarbons Polluted Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Bacterial Strains and Selection of Rifampicin-Resistant Mutants
2.3. Inoculum Preparation
2.4. Experimental Design and Inoculation
- (1)
- Polluted soil (S);
- (2)
- Polluted soil with ryegrass grains (R);
- (3)
- Polluted soil with ryegrass grains inoculated with the 5WKrif strain (SI + 5WK);
- (4)
- Polluted soil with ryegrass grains inoculated with the 10WKrif strain (SI + 10WK);
- (5)
- Polluted soil with ryegrass grains inoculated with a consortium of the 5WKrif and 10WKrif strains (SI + 5WK + 10WK);
- (6)
- Polluted soil with ryegrass grains pre-inoculated with the 5WKrif strain followed by soil inoculation with the 5WKrif strain (PI + 5WK);
- (7)
- Polluted soil with ryegrass grains pre-inoculated with the 10WKrif strain followed by soil inoculation with the 10WKrif strain (PI + 10WK);
- (8)
- Polluted soil with ryegrass grains pre-inoculated with a consortium of the 5WKrif and 10WKrif strains followed by soil inoculation with the consortium of 5WKrif and 10WKrif strains (PI + 5WK + 10WK).
2.5. Petroleum Hydrocarbons (PHC)
2.6. Establishment of Inoculated Endophytic Bacteria
2.7. Plant Weight
2.8. Real-Time PCR
2.9. Plant Colonization by Gfp-Labelled Endophytic Bacteria
2.10. Statistical Analysis
3. Results
3.1. PHC Concentration
3.2. Detection and Quantification of Inoculated Endophytic Bacteria
3.3. Plant Biomass Production
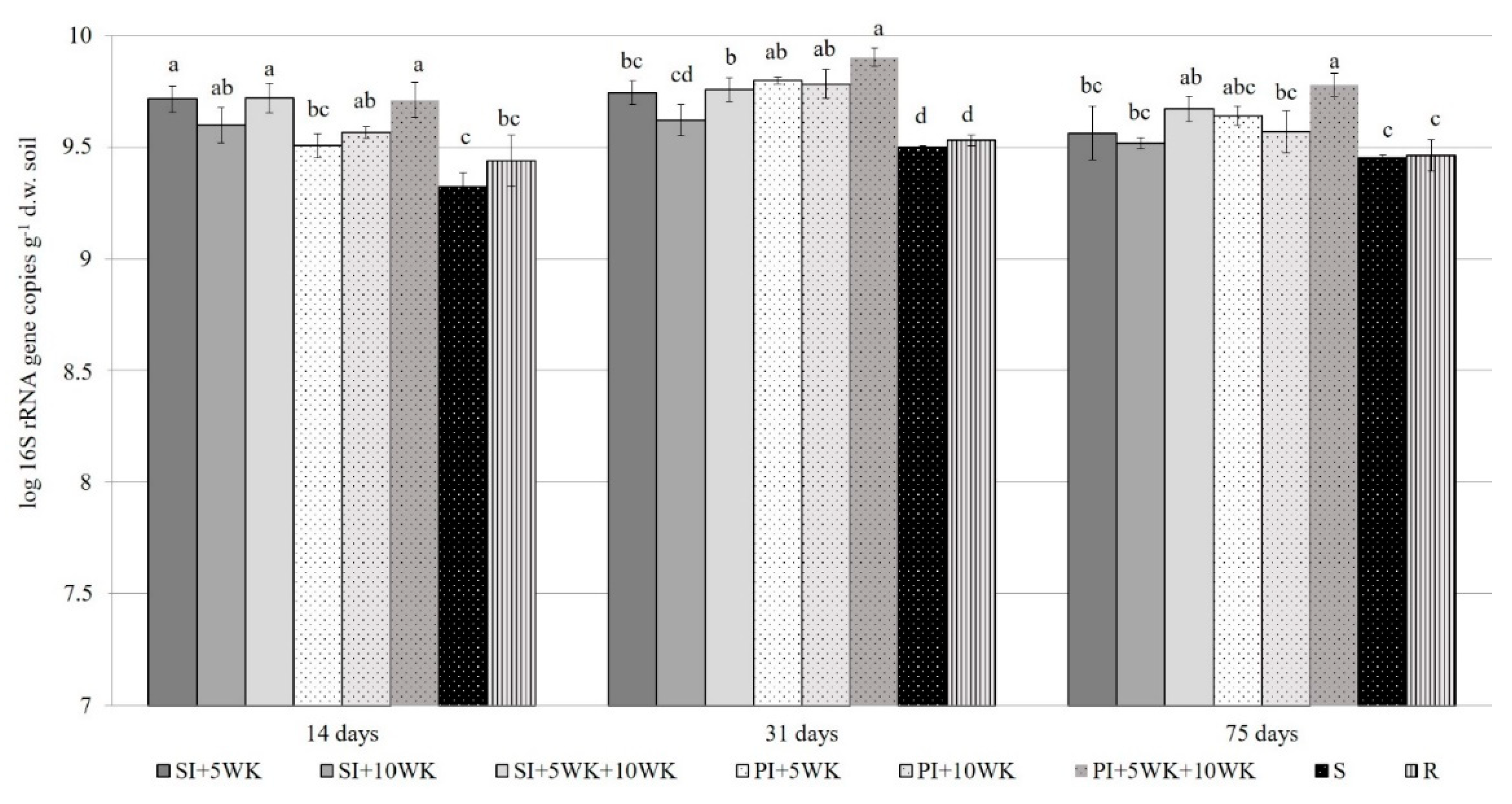
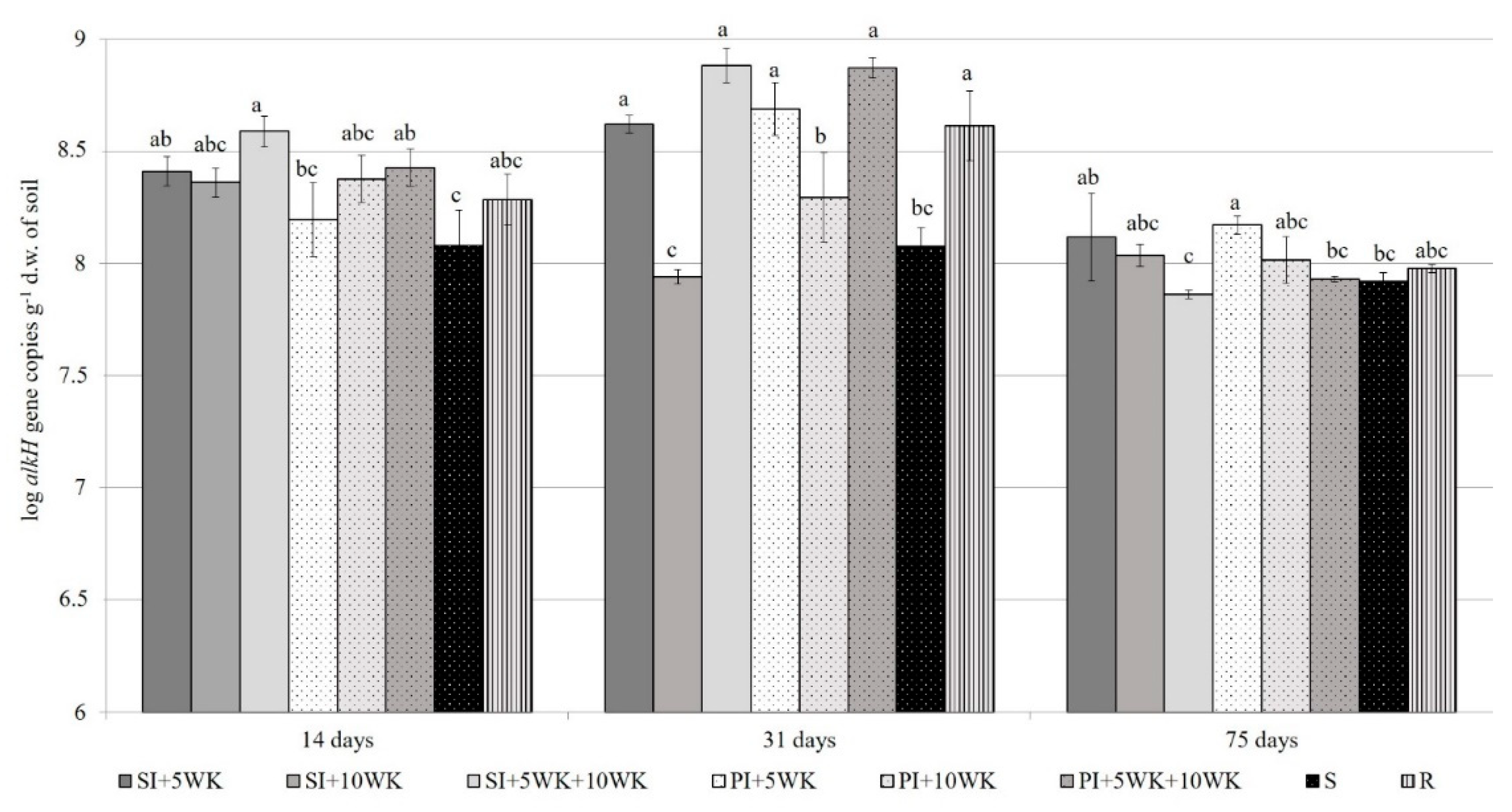
3.4. Abundance of Autochthonous Bacteria and Alkane-Degrading Bacteria in the Soil
3.5. Plant Colonization by Gfp-Tagged Endophytic Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USEPA (U.S. Environmental Protection Agency). Guidelines for carcinogen risk assessment. Fed. Regist. 1986, 51, 33992–34003. [Google Scholar]
- USEPA (U.S. Environmental Protection Agency). Guidelines for mutagenicity risk assessment. Fed. Regist. 1986, 51, 34006–34012. [Google Scholar]
- Balseiro-Romero, M.; Gkorezis, P.; Kidd, P.S.; Vangronsveld, J.; Monterroso, C. Enhanced degradation of diesel in the rhizosphere of Lupinus luteus after inoculation with diesel-degrading and plant growth-promoting bacterial strains. J. Environ. Q. 2016, 45, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Balseiro-Romero, M.; Gkorezis, P.; Kidd, P.S.; van Hammed, J.; Weyens, N.; Monterroso, C.; Vangronsveld, J. Use of plant growth promoting bacterial strains to improve Cytisus striatus and Lupinus luteus development for potential application in phytoremediation. Sci. Total Environ. 2017, 581, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. Int. 2009, 16, 765–794. [Google Scholar] [CrossRef]
- Khan, S.; Afzal, M.; Iqbal, S.; Khan, Q.M. Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere 2013, 4, 1317–1332. [Google Scholar] [CrossRef]
- Ma, Y.; Oliveira, R.S.; Freitas, H.; Zhang, C. Biochemical and molecular mechanisms of plant-microbe-metal interactions: Relevance for phytoremediation. Front. Plant Sci. 2016, 7, 918. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Artois, T.; Smeets, K.; Taghavi, S.; Newman, L.; Carleer, R.; Vangronsveld, J. Bioaugmentation with engineered endophytic bacteria improves phytoremediation. Environ. Sci. Technol. 2009, 43, 9413–9418. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Newman, L.; Vangronsveld, J. Exploiting plant–microbe partnerships for improving biomass production and remediation. Trends Biotechnol. 2009, 27, 591–598. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant-endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 2, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an enhanced understanding of plant–microbiome interactions to improve phytoremediation: Engineering the metaorganism. Front. Microbiol. 2016, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, M.; Cania, B.; Thijs, S.; Vngronsveld, J.; Piotrowska-Seget, Z. Hydrocarbon degradation potential and plant growth-promoting activity of culturable endophytic bacteria of Lotus corniculatus and Oenothera biennis from a long-term polluted site. Environ. Sci. Pollut. Res. 2017, 24, 19640–19652. [Google Scholar] [CrossRef] [PubMed]
- Barac, T.; Taghavi, S.; Borremans, B.; Provoost, A.; Oeyen, A.; Colpaert, J.V.; Vangronsveld, J.; van der Lelie, D. Engineered endophytic bacteria improve phytoremediation of water–soluble, volatile, organic pollutants. Nat. Biotechnol. 2004, 22, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Barac, T.; Weyens, N.; Oeyen, L.; Taghavi, S.; van der Lelie, D.; Dubin, D.; Spliet, M.; Vangronsveld, J. Application of poplar and its associated microorganisms for the in situ remediation of a BTEX contaminated groundwater plume. Int. J. Phytorem. 2009, 11, 416–424. [Google Scholar] [CrossRef]
- Germaine, K.J.; Keogh, E.; Ryan, D.; Dowling, D.N. Bacterial endophyte-mediated naphthalene phytoprotection and phytoremediation. FEMS Microbiol. Lett. 2009, 296, 226–234. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, S.; Afzal, M.; Reichenauer, T.G.; Brady, C.L.; Sessitsch, A. Hydrocarbon degradation, plant colonization and gene expression of alkane degradation genes by endophytic Enterobacter ludwigii strains. Environ. Pollut. 2011, 10, 2675–2683. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.; Khan, S.; Iqbal, S.; Mirza, M.S.; Khan, Q.M. Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int. Biodeterior. Biodegrad. 2013, 85, 331–336. [Google Scholar] [CrossRef]
- Peng, A.; Liu, J.; Gao, Y.; Chen, Z. Distribution of endophytic bacteria in Alopecurus aequalis Sobol and Oxalis corniculata L. from soils contaminated by polycyclic aromatic hydrocarbons. PLoS ONE 2013, 8, 12. [Google Scholar] [CrossRef]
- Alvarez-Lopez, V.; Prieto-Fernandez, A.; Janssen, J.; Herzig, R.; Vangronsveld, J.; Kidd, P.S. Inoculation methods using Rhodococcus erythropolis strain P30 affects bacterial assisted phytoextraction capacity of Nicotiana tabacum. Int. J. Phytorem. 2016, 18, 406–415. [Google Scholar] [CrossRef]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1836. [Google Scholar] [CrossRef] [PubMed]
- Tardif, S.; Yergeau, É.; Tremblay, J.; Legendre, P.; Whyte, L.G.; Greer, C.W. The willow microbiome is influenced by soil petroleum-hydrocarbon concentration with plant compartment-specific effects. Front. Microbiol. 2016, 7, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Kuffner, M.; Sessitsch, A. Soil type affects plant colonization, activity and catabolic gene expression of inoculated bacterial strains during phytoremediation of diesel. J. Hazard. Mater. 2011, 186, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The inoculation method affects colonization and performance of bacterial inoculant strains in the phytoremediation of soil contaminated with diesel oil. Int. J. Phytorem. 2012, 14, 35–47. [Google Scholar] [CrossRef]
- Andreolli, M.; Lampis, S.; Poli, M.; Gullner, G.; Biró, B.; Vallini, G. Endophytic Burkholderia fungorum DBT1 can improve phytoremediation efficiency of polycyclic aromatic hydrocarbons. Chemosphere 2013, 92, 688–694. [Google Scholar] [CrossRef]
- Arslan, M.; Afzal, M.; Amin, I.; Iqbal, S.; Khan, Q.M. Nutrients can enhance the abundance and expression of alkane hydroxylase CYP153 gene in the rhizosphere of ryegrass planted in hydrocarbon-polluted soil. PLoS ONE 2014, 9, e111208. [Google Scholar] [CrossRef]
- Sun, K.; Liu, J.; Jin, L.; Gao, Y. Utilizing pyrene-degrading endophytic bacteria to reduce the risk of plant pyrene contamination. Plant Soil 2014, 374, 251–262. [Google Scholar] [CrossRef]
- Sun, K.; Liu, L.; Gao, Y.; Jin, L.; Gu, Y.; Wang, W. Isolation, plant colonization potential, and phenanthrene degradation performance of the endophytic bacterium Pseudomonas sp. Ph6-gfp. Sci. Rep. 2014, 4, 5462. [Google Scholar] [CrossRef] [Green Version]
- Su, X.; Sun, F.; Wang, Y.; Hashmi, M.Z.; Guo, L.; Ding, L.; Shen, C. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci. Rep. 2015, 5, 18590. [Google Scholar] [CrossRef]
- Bodor, A.; Bounedjoum, N.; Vincze, G.E.; Kis, A.E.; Laczi, K.; Bende, G.; Szilagyi, A.; Kovacs, T.; Perei, K.; Rakhely, G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Biotechnol. 2020, 19, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Płociniczak, T.; Fic, E.; Pacwa-Płociniczak, M.; Pawlik, M.; Piotrowska-Seget, Z. Improvement of phytoremediation of an aged petroleum hydrocarbon-contaminated soil by Rhodococcus erythropolis CD 106 strain. Int. J. Phytorem. 2017, 19, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z. Monitoring the changes in a bacterial community in petroleum-polluted soil bioaugmented with hydrocarbon-degrading strains. Appl. Soil Ecol. 2016, 105, 76–85. [Google Scholar] [CrossRef]
- Edwards, U.; Rogall, T.; Blöcker, H.; Emde, M.; Böttger, E.C. Isolation and direct complete nucleotide determination of entire genes: Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989, 17, 7843–7853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, L.A.; Germida, J.J.; Farrell, R.E.; Greer, C.W. Hydrocarbon degradation potential and activity of endophytic bacteria associated with prairie plants. Soil Biol. Biochem. 2008, 12, 3054–3064. [Google Scholar] [CrossRef]
- Lagendijk, E.L.; Validov, S.; Lamers, G.E.M.; De Weert, S.; Bloemberg, G.V. Genetic tools for tagging Gram-negative bacteria with mCherry for visualization in vitro and in natural habitats, biofilm and pathogenicity studies. FEMS Microbiol. Lett. 2010, 305, 81–90. [Google Scholar] [CrossRef]
- Sánchez-López, A.S.; Pintelon, I.; Stevens, V.; Imperato, V.; Timmermans, J.P.; González-Chávez, C.; Carrillo, R.; van Hamme, J.D.; Vangronsveld, J.; Thijs, S. Seed endophyte microbiome of Crotalaria pumila unpeeled: Identification of plant-beneficial methylobacteria. Int. J. Mol. Sci. 2018, 19, 291. [Google Scholar] [CrossRef] [Green Version]
- Syranidou, E.; Thijs, S.; Avramidou, M.; Weyens, N.; Venieri, D.; Pintelon, I.; Vangronsveld, J.; Kalogerakis, N. Responses of the endophytic bacterial communities of Juncus acutus to pollution with metals, emerging organic pollutants and to bioaugmentation with indigenous strains. Front. Plant Sci. 2018, 9, 1526. [Google Scholar] [CrossRef] [Green Version]
- Taghavi, S.; Barac, T.; Greenberg, B.; Borremans, B.; Vangronsveld, J.; van der Lelie, D. Horizontal gene transfer to endogenous endophytic bacteria from poplar improves phytoremediation of toluene. Appl. Environ. Microbiol. 2005, 71, 8500–8505. [Google Scholar] [CrossRef] [Green Version]
- Chuluun, B.; Shah, S.H.; Rhee, J.S. Bio-augmented phytoremediation: A strategy for reclamation of diesel oil-contaminated soils. Int. J. Agric. Biol. 2014, 16, 624–628. [Google Scholar]
- Heinaru, E.; Merimaa, M.; Viggor, S.; Lehiste, M.; Leito, I.; Truu, J.; Heinaru, A. Biodegradation efficiency of functionally important populations selected for bioaugmentation in phenol- and oil-polluted area. FEMS Microbiol. Ecol. 2005, 51, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Kirk, J.L.; Klironomos, J.N.; Lee, H.; Trevors, J.T. The effects of perennial ryegrass and alfalfa on microbial abundance and diversity in petroleum contaminated soil. Environ. Pollut. 2005, 133, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, R.; Niu, X.; Zhou, Q. Enhancement of soil petroleum remediation by using a combination of ryegrass (Lolium perenne) and different microorganisms. Soil Tillage Res. 2010, 110, 87–93. [Google Scholar] [CrossRef]
- Porteous-Moore, F.P.; Barac, T.; Borremans, B.; Oeyen, L.; Vangronsveld, J.; van der Lelie, D.; Campbell, C.D.; Moore, E.R. Endophytic bacterial diversity in poplar trees growing on a BTEX-contaminated site: The characterization of isolates with potential to enhance phytoremediation. Syst. Appl. Microbiol. 2006, 7, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Jamal, M.A.H.M.; Nasrin, S. Sterilization factors affect seed germination and proliferation of Achyranthes aspera cultured in vitro. Environ. Exp. Biol. 2013, 11, 119–123. [Google Scholar]
- Barampuram, S.; Allen, G.; Krasnyanski, S. Effect of various sterilization procedures on the in vitro germination of cotton seeds. Plant Cell Tissue Organ Cult. 2014, 118, 179–185. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 10, 463–471. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Freitas, H. Inoculation of plant growth promoting bacterium Achromobacter xylosoxidans strain Ax10 for the improvement of copper phytoextraction by Brassica juncea. J. Environ. Manag. 2009, 90, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Płociniczak, T.; Chodór, M.; Pacwa-Płociniczak, M.; Piotrowska-Seget, Z. Metal-tolerant endophytic bacteria associated with Silene vulgaris support the Cd and Zn phytoextraction in non-host plants. Chemosphere 2019, 219, 250–260. [Google Scholar] [CrossRef]
- Errampalli, D.; Leung, K.; Cassidy, M.B.; Kostrzynska, M.; Blears, M.; Lee, H.; Trevors, J.T. Applications of the green fluorescent protein as a molecular marker in environmental microorganisms. J. Microbiol. Methods 1999, 35, 187–199. [Google Scholar] [CrossRef]
- Compant, S.; Clement, C.; Sessitach, A. Plant growth–promoting bacteria in the rhizo–and endosphere of plants: Their role, colonization, mechanisms involved and prospects for utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Fortin, N.; Mihoc, A.; Wisse, G.; Labelle, S.; Beaumier, D.; Ouellette, D.; Roy, R.; Whyte, L.G.; Banks, M.K.; et al. Selection of specific endophytic bacterial genotypes by plants in response to soil contamination. Appl. Environ. Microbiol. 2001, 6, 2469–2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.L.M.; de Lima Canuto, E.; Reis, V.M.; Baldani, J.I. Response of micropropagated sugarcane varieties to inoculation with endophytic diazotrophic bacteria. Braz. J. Microbiol. 2003, 34, 59–61. [Google Scholar] [CrossRef] [Green Version]
- Vargas, L.; Santa Brígida, A.B.; Mota Filho, J.P.; de Carvalho, T.G.; Rojas, C.A.; Vaneechoutte, D.; van Bel, M.; Farrinelli, L.; Ferreira, P.C.G.; Vandepoele, K.; et al. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [Green Version]
- Andria, V.; Reichenauer, T.G.; Sessitsch, A. Expression of alkane monooxygenase (alkB) genes by plant-associated bacteria in the rhizosphere and endosphere of Italian ryegrass (Lolium multiflorum L.) grown in diesel contaminated soil. Environ. Pollut. 2009, 157, 3347–3350. [Google Scholar] [CrossRef]
- Yousaf, S.; Andria, V.; Reichenauer, T.G.; Smalla, K.; Sessitsch, A. Phylogenetic and functional diversity of alkane degrading bacteria associated with Italian ryegrass (Lolium multiflorum) and Birdsfoot trefoil (Lotus corniculatus) in a petroleum oil-contaminated environment. J. Hazard. Mater. 2010, 184, 523–532. [Google Scholar] [CrossRef]
- Yousaf, S.; Ripka, K.; Reichenauer, T.G.; Andria, V.; Afzal, M.; Sessitsch, A. Hydrocarbon degradation and plant colonization by selected bacterial strains isolated from Italian ryegrass and birdsfoot trefoil. J. Appl. Microbiol. 2010, 4, 1389–1401. [Google Scholar] [CrossRef]
- Han, J.I.; Choi, H.K.; Lee, S.W.; Orwin, P.M.; Kim, J.; Laroe, S.L.; Kim, T.G.; O’Neil, J.; Leadbetter, J.R.; Lee, S.Y.; et al. Complete genome sequence of the metabolically versatile plant growth-promoting endophyte Variovorax paradoxus S110. J. Bacteriol. 2011, 193, 1183–1190. [Google Scholar] [CrossRef] [Green Version]
- Mitter, B.; Petric, A.; Shin, M.W.; Chain, P.S.; Hauberg-Lotte, L.; Reinhold-Hurek, B.; Nowak, J.; Sessitsch, A. Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front. Plant Sci. 2013, 4, 120. [Google Scholar] [CrossRef] [Green Version]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef]
- Shen, Y.; Ji, Y.; Li, C.; Luo, P.P.; Wang, W.; Zhang, Y.; Nover, D. Effects of phytoremediation treatment on bacterial community structure and diversity in different petroleum-contaminated soils. Int. J. Environ. Res. Public Health 2018, 15, 2168. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Treatment | PHC Removal (%) | |
|---|---|---|
| Polluted soil with ryegrass grains inoculated with strain | SI + 5WK | 2.2 ± 0.4cd |
| SI + 10WK | 1.6 ± 0.6d | |
| SI + 5WK + 10WK | 2.1 ± 1.8d | |
| Polluted soil with ryegrass grains pre-inoculated with strain followed by soil inoculation with strain | PI + 5WK | 8.9 ± 2.6b |
| PI + 10WK | 9.7 ± 1.3b | |
| PI + 5WK + 10WK | 19.1 ± 2.5a | |
| Polluted soil | S | 1.7 ± 0.5d |
| Polluted soil with ryegrass grains | R | 4.7 ± 1.4c |
| Numbers of Endophytic Bacteria in Soil and Ryegrass Tissues | ||||
|---|---|---|---|---|
| Treatment | SOIL (log cfu g−1 d.w.) | ROOT (log cfu g−1 f.w.) | SHOOT (log cfu g−1 f.w.) | |
| Polluted soil with ryegrass grains inoculated with strain | SI + 5WK | 0 | 5.84 ± 0.08bc | 4.84 ± 0.18cde |
| SI + 10WK | 8.34 ± 0.12a | 0 | 0 | |
| SI + 5WK + 10WK | 4.56 ± 0.28e | 6.55 ± 0.07b | 0 | |
| Polluted soil with ryegrass grains pre-inoculated with strain followed by soil inoculation with strain | PI + 5WK | 0 | 5.94 ± 0.52b | 0 |
| PI + 10WK | 0 | 4.72 ± 0.12de | 4.8 ± 0.17de | |
| PI + 5WK + 10WK | 5.7 ± 0.05bcd | 4.48 ± 0.21e | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, M.; Płociniczak, T.; Thijs, S.; Pintelon, I.; Vangronsveld, J.; Piotrowska-Seget, Z. Comparison of Two Inoculation Methods of Endophytic Bacteria to Enhance Phytodegradation Efficacy of an Aged Petroleum Hydrocarbons Polluted Soil. Agronomy 2020, 10, 1196. https://doi.org/10.3390/agronomy10081196
Pawlik M, Płociniczak T, Thijs S, Pintelon I, Vangronsveld J, Piotrowska-Seget Z. Comparison of Two Inoculation Methods of Endophytic Bacteria to Enhance Phytodegradation Efficacy of an Aged Petroleum Hydrocarbons Polluted Soil. Agronomy. 2020; 10(8):1196. https://doi.org/10.3390/agronomy10081196
Chicago/Turabian StylePawlik, Małgorzata, Tomasz Płociniczak, Sofie Thijs, Isabel Pintelon, Jaco Vangronsveld, and Zofia Piotrowska-Seget. 2020. "Comparison of Two Inoculation Methods of Endophytic Bacteria to Enhance Phytodegradation Efficacy of an Aged Petroleum Hydrocarbons Polluted Soil" Agronomy 10, no. 8: 1196. https://doi.org/10.3390/agronomy10081196






