Effects of Morphological and Anatomical Characteristics of Banana Crown Vascular Bundles on Cutting Mechanical Properties Using Multiple Imaging Methods
Abstract
:1. Introduction
2. Materials and Methods
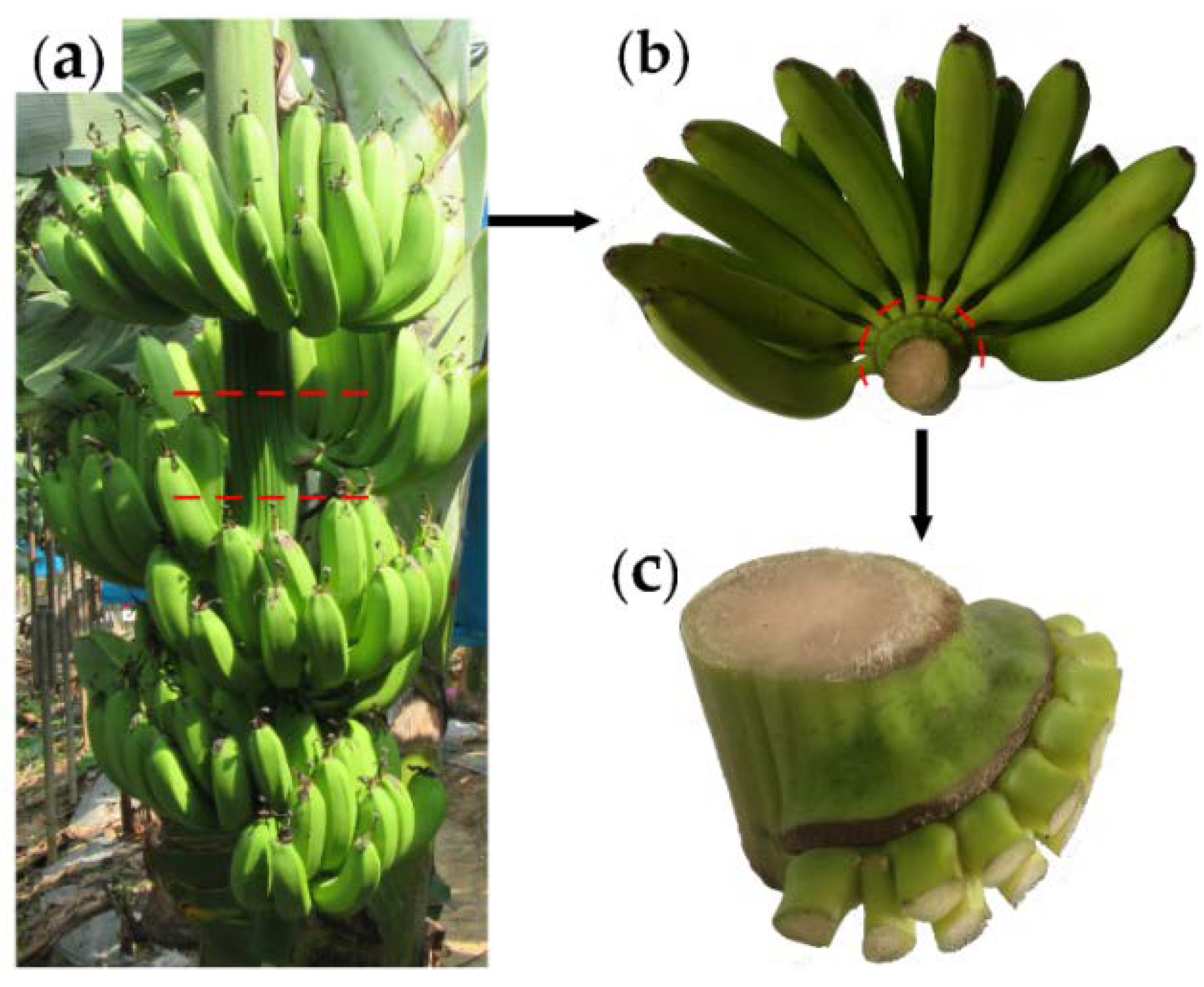
2.1. Banana Crown Preparation
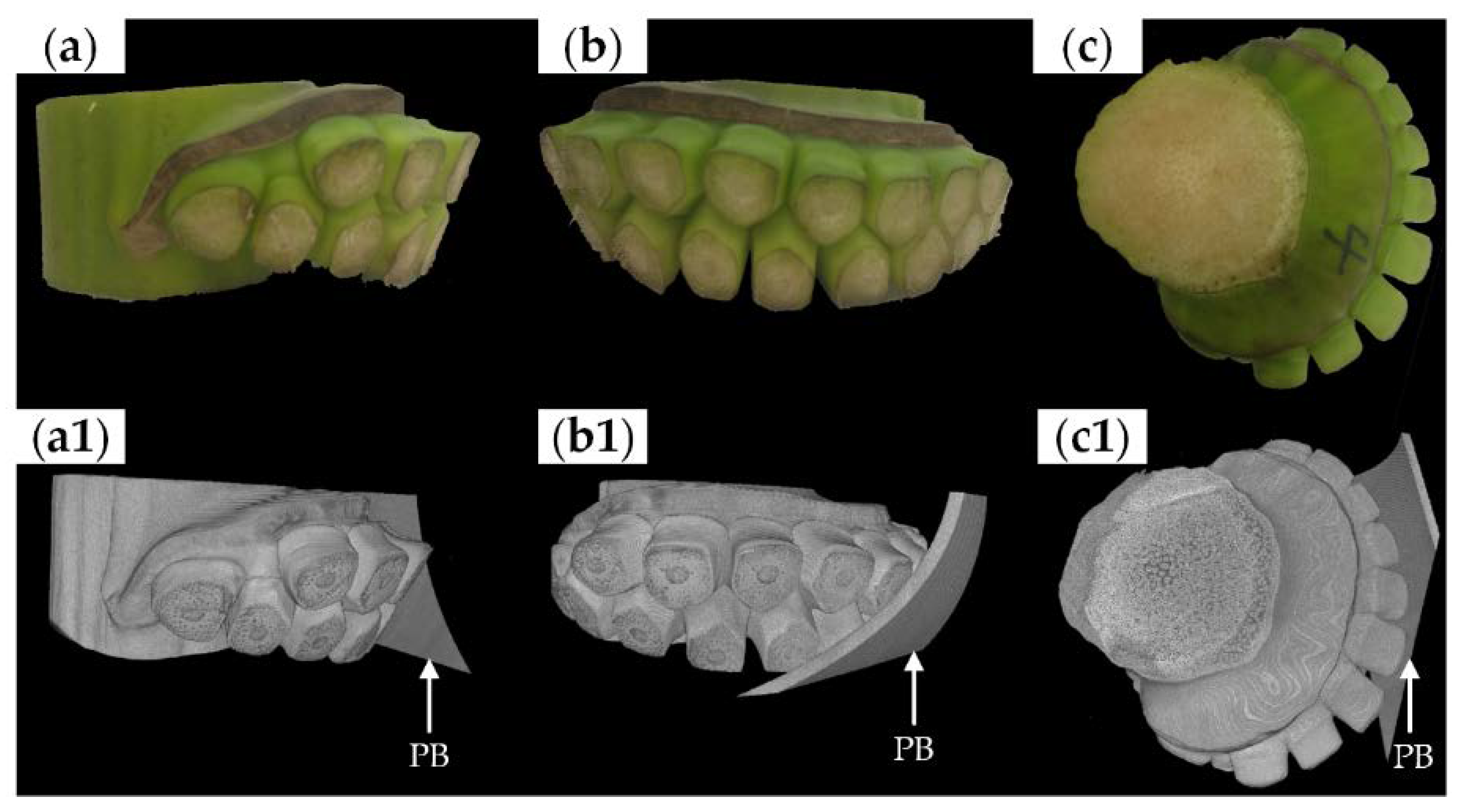
2.2. CT Scanning
2.3. Biological Microscopic Observations
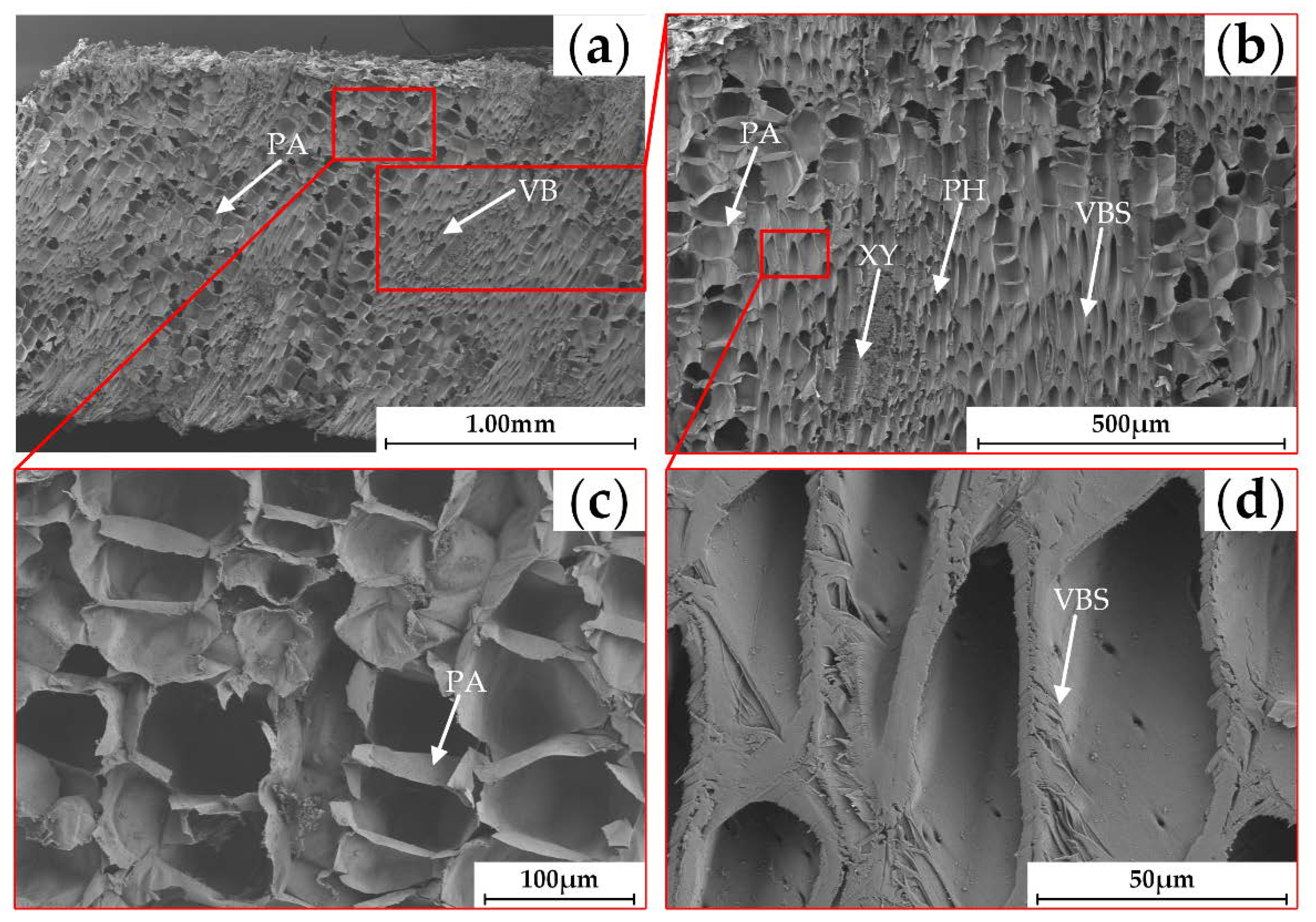
2.4. SEM Analysis
2.5. Cutting Tests
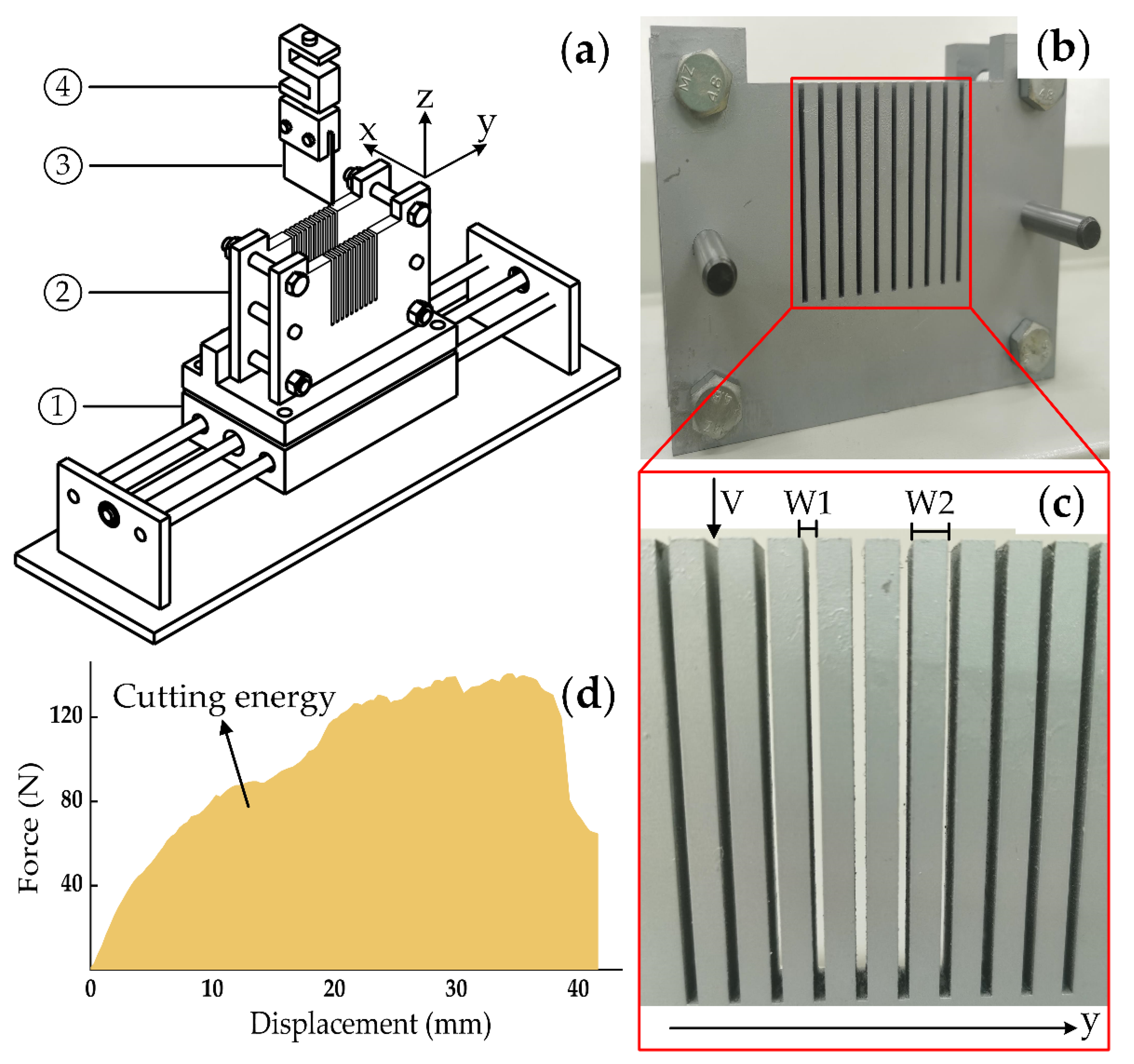
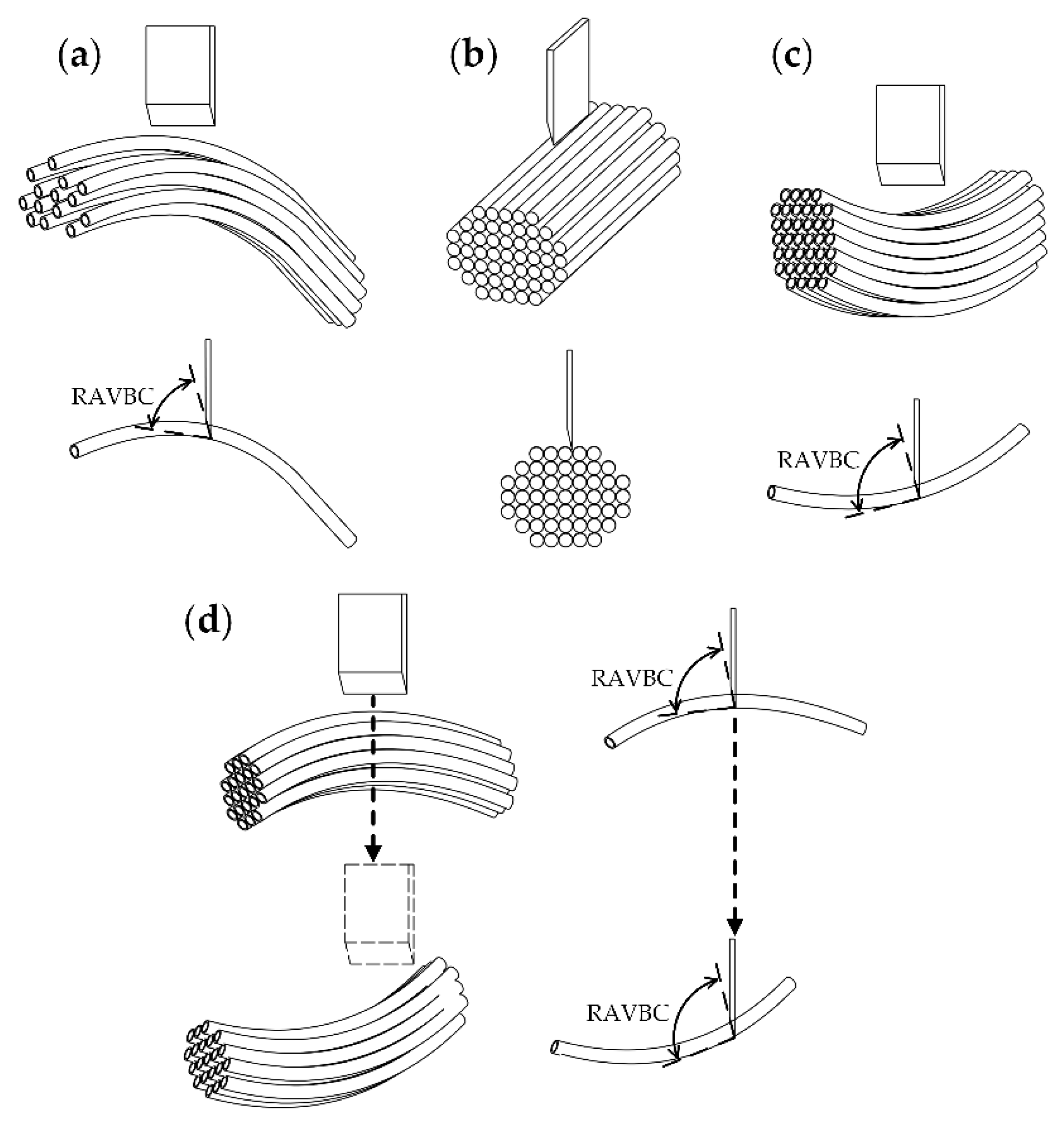
2.5.1. Apparatus Description and Cutting Mechanism
2.5.2. Experiment Design
2.6. Data Analysis
3. Results and Discussion
3.1. Morphological Characteristics
3.2. Anatomical Characteristics
3.3. Cutting Mechanic Properties
3.3.1. Force–Displacement Curves
3.3.2. Cutting Energy
4. Conclusions
- Based on the analysis of the morphological and anatomical characteristics, the banana crown vascular bundles are divided into three regions including: (1) CSCTR, the vascular bundles where the central stalk flows into the crown have a low density and are distributed longitudinally along the crown with a tubular structure; (2) CER, the vascular bundles in the region are distributed transversely at a high density, and the basic structure of the vascular bundle includes the xylem, phloem, and vascular bundle sheath; and (3) CFTR, in which the crown vascular bundles are dispersed in each finger and followed by collection in the fingers.
- The cutting mechanical properties are significantly affected by the vascular bundle direction and density. In CSCTR, the cutting mechanical properties are unstable during the transition from central stalk to crown, and the cutting energy decreased from 4.3 to 2.57 J. For vascular bundles distributed transversely along with the crown in CER, the cutting mechanical properties remain stable, and the cutting energy varies from 2.83 to 2.92 J. The largest cutting energy (4.84 J) among the whole crown is found to be in the CFTR.
- The appropriate mechanized cutting region for dehanding is ascertained to be between CSCTR and CER, which can help to reduce the energy needed in the cutting process.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Souza, A.V.; Bonini Neto, A.; Cabrera Piazentin, J.; Dainese Junior, B.J.; Perin Gomes, E.; dos Santos Batista Bonini, C.; Ferrari Putti, F. Artificial neural network modelling in the prediction of bananas’ harvest. Sci. Hortic. 2019, 257, 108724. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 October 2019).
- Lassois, L.; Jijakli, M.H.; Chillet, M.; de Lapeyre de Bellaire, L. Crown Rot of Bananas: Preharvest Factors Involved in Postharvest Disease Development and Integrated Control Methods. Plant Dis. 2010, 94, 648–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, E.P.; Odilo, D. Tropical Fruits, 2nd ed.; CABI: Oxfordshire, UK, 2011; Volume 1, pp. 214–216. [Google Scholar]
- Shah, D.U.; Reynolds, T.P.S.; Ramage, M.H. The strength of plants: Theory and experimental methods to measure the mechanical properties of stems. J. Exp. Bot. 2017, 68, 4497–4516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, L.J. Biomechanics of cellular solids. J. Biomech. 2005, 38, 377–399. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Robertson, D.; Smith, S.; Gardunia, B.; Cook, D. An Improved Method for Accurate Phenotyping of Corn Stalk Strength. Crop Sci. 2014, 54, 2038–2044. [Google Scholar] [CrossRef] [Green Version]
- Gomez, F.E.; Muliana, A.H.; Niklas, K.J.; Rooney, W.L. Identifying Morphological and Mechanical Traits Associated with Stem Lodging in Bioenergy Sorghum (Sorghum bicolor). Bioenergy Res. 2017, 10, 635–647. [Google Scholar] [CrossRef]
- Shahbazi, F.; Galedar, M.N. Bending and Shearing Properties of Safflower Stalk. J. Agric. Sci. Technol. 2012, 14, 743–754. [Google Scholar]
- Seflek, A.Y. Determining the Physico-Mechanical Characteristics of Maize Stalks Fordesigning Harvester. J. Anim. Plant Sci. 2017, 27, 855–860. [Google Scholar]
- Leblicq, T.; Vanmaercke, S.; Ramon, H.; Saeys, W. Mechanical analysis of the bending behaviour of plant stems. Biosyst. Eng. 2015, 129, 87–99. [Google Scholar] [CrossRef]
- Leblicq, T.; Smeets, B.; Vanmaercke, S.; Ramon, H.; Saeys, W. A discrete element approach for modelling bendable crop stems. Comput. Electron. Agric. 2016, 124, 141–149. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Yang, Z.; Zhu, Z.B. Parameter optimization of banana crown-cutting machine using combined cutters. Curr. Sci. 2019, 117, 492–497. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Yang, Z.; Duan, J.L.; Jin, M.H.; Mo, J.S.; Zhao, L.; Guo, J.; Yao, H.L. Design and Experiment of Symmetrical Shape Deployable Arc Profiling Mechanism Based on Composite Multi-Cam Structure. Symmetry 2019, 11, 958. [Google Scholar] [CrossRef] [Green Version]
- Hesse, L.; Bunk, K.; Leupold, J.; Speck, T.; Masselter, T. Structural and functional imaging of large and opaque plant specimens. J. Exp. Bot. 2019, 70, 3659–3678. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, F.; Schurr, U. Future Scenarios for Plant Phenotyping. In Annual Review of Plant Biology; Merchant, S.S., Ed.; Annual Reviews: Palo Alto, CA, USA, 2013; Volume 64, pp. 267–291. [Google Scholar]
- Brodersen, C.R.; Roddy, A.B. New frontiers in the three-dimensional visualization of plant structure and function. Am. J. Bot. 2016, 103, 184–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasaya, A.; Zhang, X.Y.; Fang, Q.; Yan, Z.Z. Root Phenotyping for Drought Tolerance: A Review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Valdes, C.; Clarke, J. Existing and Potential Statistical and Computational Approaches for the Analysis of 3D CT Images of Plant Roots. Agronomy 2018, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Paya, A.M.; Silverberg, J.L.; Padgett, J.; Bauerle, T.L. X-ray computed tomography uncovers root-root interactions: Quantifying spatial relationships between interacting root systems in three dimensions. Front. Plant Sci. 2015, 6, 274. [Google Scholar] [CrossRef]
- Bauerle, T.L.; Centinari, M. Assessment of Root System Development among Four Ornamental Tree Species through Time via X-ray Computed Tomography. HortScience 2014, 49, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Hsu, H.C.; Wang, C.N.; Liang, C.H.; Wang, C.C.; Kuo, Y.F. Association between Petal Form Variation and CYC2-like Genotype in a Hybrid Line of Sinningia speciosa. Front. Plant Sci. 2017, 8, 558. [Google Scholar] [CrossRef] [Green Version]
- Van der Niet, T.; Zollikofer, C.P.; Leon, M.S.; Johnson, S.D.; Linder, H.P. Three-dimensional geometric morphometrics for studying floral shape variation. Trends Plant Sci. 2010, 15, 423–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donis-Gonzalez, I.R.; Guyer, D.E.; Fulbright, D.W.; Pease, A. Postharvest noninvasive assessment of fresh chestnut (Castanea spp.) internal decay using computer tomography images. Postharvest Biol. Technol. 2014, 94, 14–25. [Google Scholar] [CrossRef]
- Herremans, E.; Verboven, P.; Verlinden, B.E.; Cantre, D.; Abera, M.; Wevers, M.; Nicolai, B.M. Automatic analysis of the 3-D microstructure of fruit parenchyma tissue using X-ray micro-CT explains differences in aeration. BMC Plant Biol. 2015, 15, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herremans, E.; Verboven, P.; Defraeye, T.; Rogge, S.; Ho, Q.T.; Hertog, M.L.A.T.M.; Verlinden, B.E.; Bongaers, E.; Wevers, M.; Nicolai, B.M. X-ray CT for quantitative food microstructure engineering: The apple case. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. Atoms 2014, 324, 88–94. [Google Scholar] [CrossRef]
- Perez-Torres, E.; Kirchgessner, N.; Pfeifer, J.; Walter, A. Assessing potato tuber diel growth by means of X-ray computed tomography. Plant Cell Environ. 2015, 38, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Sumardi, I.; Wulandari, M. Anatomy and morphology character of five Indonesian banana cultivars (Musa spp.) of different ploidy level. Biodiversitas J. Biol. Divers. 2010, 11, 167–175. [Google Scholar] [CrossRef]
- Boydas, M.G.; Comakli, M.; Sayinci, B.; Kara, M. Effects of moisture content, internode region, and oblique angle on the mechanical properties of sainfoin stem. Turk. J. Agric. For. 2019, 43, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Galedar, M.N.; Tabatabaeefar, A.; Jafari, A.; Sharifi, A.; Rafiee, S. Bending and Shearing Characteristics of Alfalfa Stems. Agric. Eng. Int. CIGR Ejournal 2008, X, FP 08 001. [Google Scholar]
- Robinson, J.C.; Saúco, V.G. Bananas and Plantains, 2nd ed.; CABI: Oxfordshire, UK, 2010; pp. 248–253. [Google Scholar]
- Zhang, Y.; Du, J.; Wang, J.; Ma, L.; Lu, X.; Pan, X.; Guo, X.; Zhao, C. High-throughput micro-phenotyping measurements applied to assess stalk lodging in maize (Zea mays L.). Biol. Res. 2018, 51, 40. [Google Scholar] [CrossRef]
- Du, J.J.; Zhang, Y.; Guo, X.Y.; Ma, L.M.; Shao, M.; Pan, X.D.; Zhao, C.J. Micron-scale phenotyping quantification and three-dimensional microstructure reconstruction of vascular bundles within maize stalks based on micro-CT scanning. Funct. Plant Biol. 2017, 44, 10–22. [Google Scholar] [CrossRef]
- Huang, P.X.; Chang, W.S.; Ansell, M.P.; Chew, Y.M.J.; Shea, A. Density distribution profile for internodes and nodes of Phyllostachys edulis (Moso bamboo) by computer tomography scanning. Constr. Build. Mater. 2015, 93, 197–204. [Google Scholar] [CrossRef]
- Venard, C.; Vaillancourt, L. Colonization of Fiber Cells by Colletotrichum graminicola in Wounded Maize Stalks. Phytopathology 2007, 97, 438–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aydin, I.; Arslan, S. Mechanical properties of cotton shoots for topping. Ind. Crop. Prod. 2018, 112, 396–401. [Google Scholar] [CrossRef]
- Ozbek, O.; Seflek, A.Y.; Carman, K. Some Mechanical Properties of Safflower Stalk. Appl. Eng. Agric. 2009, 25, 619–625. [Google Scholar] [CrossRef]
- Chen, Y.; Gratton, J.L.; Liu, J. Power requirements of hemp cutting and conditioning. Biosyst. Eng. 2004, 87, 417–424. [Google Scholar] [CrossRef]
- İnce, A.; Uğurluay, S.; Güzel, E.; Özcan, M.T. Bending and Shearing Characteristics of Sunflower Stalk Residue. Biosyst. Eng. 2005, 92, 175–181. [Google Scholar] [CrossRef]



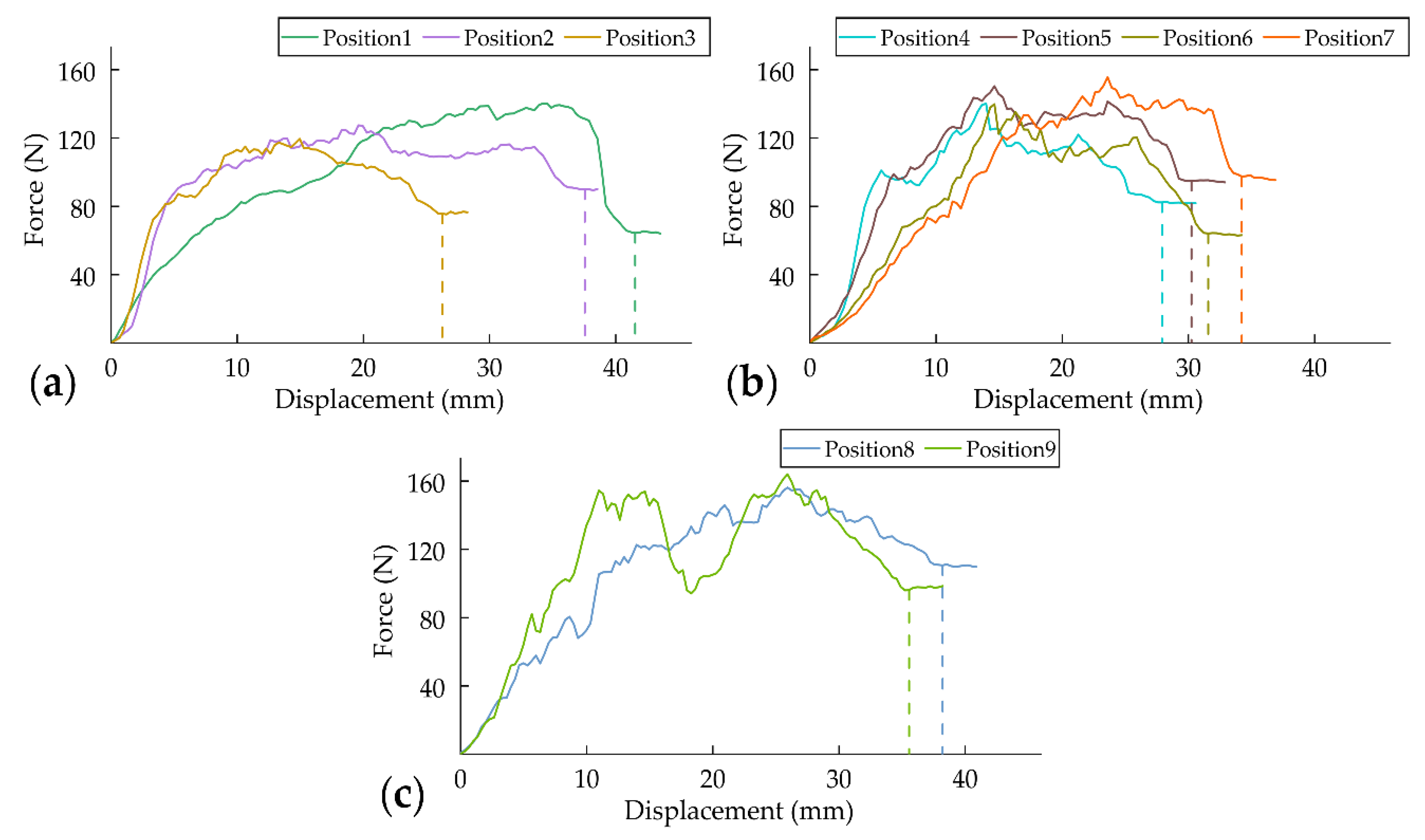

| Crown Region | Cutting Position | Cutter Displacement (mm) | Cutting Energy (J) |
|---|---|---|---|
| CSCTR | 1 | 41.96 ± 1.76 | 4.30 ± 0.23 c |
| 2 | 37.81 ± 1.92 | 3.66 ± 0.16 d | |
| 3 | 26.18 ± 1.06 | 2.57± 0.17 g | |
| CER | 4 | 27.98 ± 1.72 | 2.85 ± 0.20 f |
| 5 | 30.40 ± 1.15 | 2.92 ± 0.14 f | |
| 6 | 31.69 ± 1.56 | 2.83 ± 0.13 f | |
| 7 | 34.43 ± 2.20 | 3.37 ± 0.29 e | |
| CFTR | 8 | 38.49 ± 2.09 | 4.84 ± 0.46 a |
| 9 | 35.77 ± 1.24 | 4.48 ± 0.41 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Yang, H.; Xie, H.; Duan, J.; Jin, M.; Fu, H.; Guo, J.; Xu, Z.; Jiang, T.; Yang, Z. Effects of Morphological and Anatomical Characteristics of Banana Crown Vascular Bundles on Cutting Mechanical Properties Using Multiple Imaging Methods. Agronomy 2020, 10, 1199. https://doi.org/10.3390/agronomy10081199
Zhao L, Yang H, Xie H, Duan J, Jin M, Fu H, Guo J, Xu Z, Jiang T, Yang Z. Effects of Morphological and Anatomical Characteristics of Banana Crown Vascular Bundles on Cutting Mechanical Properties Using Multiple Imaging Methods. Agronomy. 2020; 10(8):1199. https://doi.org/10.3390/agronomy10081199
Chicago/Turabian StyleZhao, Lei, Haijun Yang, Heng Xie, Jieli Duan, Mohui Jin, Han Fu, Jie Guo, Zeyu Xu, Tingting Jiang, and Zhou Yang. 2020. "Effects of Morphological and Anatomical Characteristics of Banana Crown Vascular Bundles on Cutting Mechanical Properties Using Multiple Imaging Methods" Agronomy 10, no. 8: 1199. https://doi.org/10.3390/agronomy10081199
APA StyleZhao, L., Yang, H., Xie, H., Duan, J., Jin, M., Fu, H., Guo, J., Xu, Z., Jiang, T., & Yang, Z. (2020). Effects of Morphological and Anatomical Characteristics of Banana Crown Vascular Bundles on Cutting Mechanical Properties Using Multiple Imaging Methods. Agronomy, 10(8), 1199. https://doi.org/10.3390/agronomy10081199







