Isolates of the Nematophagous Fungus Pochonia chlamydosporia Are Endophytic in Banana Roots and Promote Plant Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants and Fungi
2.2. Growth Chamber Experiments
2.3. Greenhouse Experiments
2.4. Pc Strain Variability and Banana Plant Growth Promotion Experiments
2.5. Root Colonization
2.6. Statistical Analysis
3. Results
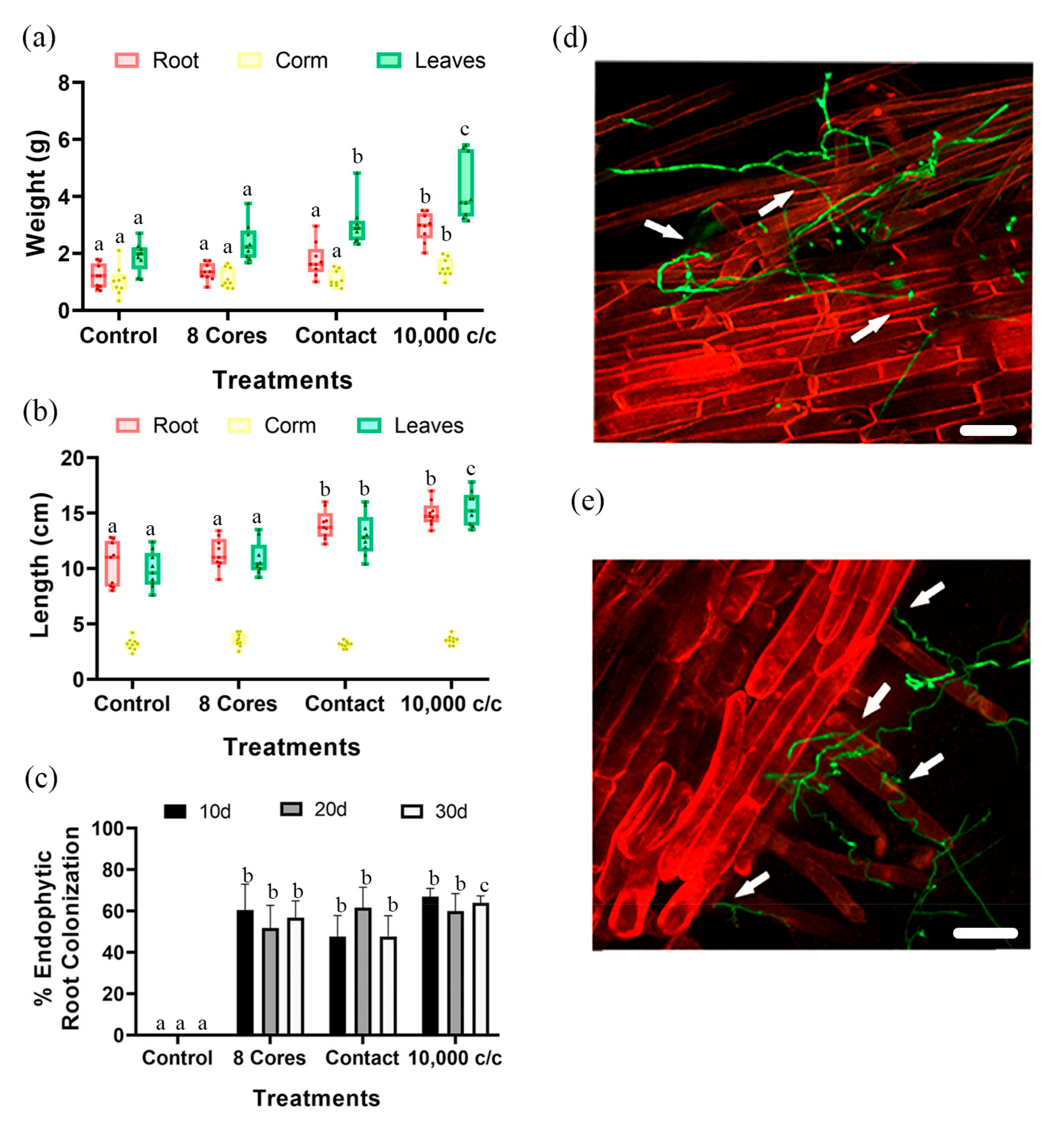
3.1. Effect of Pc Inoculation on Plant Growth
3.2. Pc Promotes the Growth of Banana Plants in the Greenhouse
3.3. Pc Strains Vary in Their Plant Growth Promotion Ability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gold, C.S.; Pena, J.E.; Karamura, E.B. Biology and Integrated Pest Management for the Banana Weevil Cosmopolites sordidus (Germar) (Coleoptera: Curculionidae). Integr. Pest Manag. Rev. 2001, 6, 79–155. [Google Scholar] [CrossRef]
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carreel, F.; Hippolyte, I.; Horry, J.-P.; Jenny, C.; et al. Multidisciplinary Perspectives on Banana (Musa spp.) Domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Pingali, P.L. Green Revolution: Impacts, Limits, And the Path Ahead. Proc. Natl. Acad. Sci. USA 2012, 109, 12302–12308. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, U.K.S.; Ganapathi, T.R. MusaWRKY71 Overexpression in Banana Plants Leads to Altered Abiotic and Biotic Stress Responses. PLoS ONE 2013, 8, e75506. [Google Scholar] [CrossRef]
- Gang, G.; Bizun, W.; Weihong, M.; Xiaofen, L.; Xiaolin, Y.; Chaohua, Z.; Jianhong, M.; Huicai, Z. Biocontrol of Fusarium Wilt of Banana: Key Influence Factors and Strategies. Afr. J. Microbiol. Res. 2013, 7, 4835–4843. [Google Scholar]
- Dubois, T.; Gold, C.S.; Coyne, D.; Paparu, P.; Mukwaba, E.; Athman, S.; Kapinduand, S.; Adipala, E. Merging Biotechnology with Biological Control: Banana Musa Tissue Culture Plants Enhanced by Endophytic Fungi. Uganda J. Agric. Sci. 2004, 9, 445–451. [Google Scholar]
- Khanal, C.; Desaeger, J.A. On-Farm Evaluations of Non-Fumigant Nematicides on Cucurbits. Crop. Prot. 2020, 133, 105152. [Google Scholar] [CrossRef]
- Li, J.; Meng, Z.; Li, N.; Dong, B.; Ji, X.; Zhang, S.; Qiao, K. Evaluating a New Non-Fumigant Nematicide Fluopimomide for Management of Southern Root-Knot Nematodes in Tomato. Crop. Prot. 2020, 129, 105040. [Google Scholar] [CrossRef]
- Wu, K.; Chen, W.; Yang, S.; Wen, Y.; Zheng, Y.; Anjago, W.M.; Yun, Y.; Wang, Z. Isolation and Identification of Fusarium oxysporum f. Sp. Cubense in Fujian Province, China. J. Integr. Agric. 2019, 18, 1905–1913. [Google Scholar] [CrossRef]
- Davies, L.J.; Elling, A.A. Resistance genes against plant-parasitic nematodes: A durable control strategy? Nematology 2015, 17, 249–263. [Google Scholar] [CrossRef]
- Dos Santos, M.C.V.; Abrantes, I.; Curtis, R.H. Priming Plant Defence Responses Can Enhance the Biological Control of Pochonia Chlamydosporia Against Root-Knot Nematodes. In Perspectives in Sustainable Nematode Management through Pochonia chlamydosporia Applications for Root and Rhizosphere Health; Springer: Berlin/Heidelberg, Germany, 2017; pp. 295–309. [Google Scholar]
- Manzanilla-López, R.H.; Esteves, I.; Finetti-Sialer, M.M.; Hirsch, P.R.; Ward, E.; Devonshire, J.; Hidalgo-Díaz, L. Pochonia chlamydosporia: Advances and Challenges to Improve Its Performance as a Biological Control Agent of Sedentary Endo-Parasitic Nematodes. J. Nematol. 2013, 45, 1–7. [Google Scholar]
- Escudero, N.; Ferreira, S.R.; Lopez-Moya, F.; Naranjo-Ortiz, M.A.; Marin-Ortiz, A.I.; Thornton, C.R.; Lopez-Llorca, L.V. Chitosan Enhances Parasitism of Meloidogyne javanica Eggs by the Nematophagous Fungus Pochonia chlamydosporia. Fungal Biol. 2016, 120, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Gams, W.; Zare, R. A Revision of Verticillium Sect. Prostrata. III. Generic Classification. Nova Hedwig. 2001, 72, 329–337. [Google Scholar] [CrossRef]
- Giné, A.; Bonmatí, M.; Sarro, A.; Stchiegel, A.; Valero, J.; Ornat, C.; Fernández, C.; Sorribas, F.J. Natural Occurrence of Fungal Egg Parasites of Root-Knot Nematodes, Meloidogyne spp. in Organic and Integrated Vegetable Production Systems in Spain. BioControl 2013, 58, 407–416. [Google Scholar] [CrossRef]
- Kerry, B.; Esteves, I.; Dos Santos, M.C.V.; Abrantes, I. Biology, Growth Parameters and Enzymatic Activity of Pochonia chlamydosporia Isolated from Potato Cyst and Root-Knot Nematodes. Nematology 2013, 15, 493–504. [Google Scholar] [CrossRef]
- Viggiano, J.R.; de Freitas, L.G.; Lopes, E.A. Use of Pochonia chlamydosporia to Control Meloidogyne javanica in Cucumber. Biol. Control 2014, 69, 72–77. [Google Scholar] [CrossRef]
- Ward, E.; Kerry, B.R.; Manzanilla-López, R.H.; Mutua, G.; Devonshire, J.; Kimenju, J.; Hirsch, P.R. The Pochonia chlamydosporia Serine Protease Gene Vcp1 Is Subject to Regulation by Carbon, Nitrogen and PH: Implications for Nematode Biocontrol. PLoS ONE 2012, 7, e35657. [Google Scholar] [CrossRef]
- Escudero, N.; Marhuenda-Egea, F.C.; Ibanco-Cañete, R.; Zavala-Gonzalez, E.A.; Lopez-Llorca, L.V. A Metabolomic Approach to Study the Rhizodeposition in the Tritrophic Interaction: Tomato, Pochonia chlamydosporia and Meloidogyne javanica. Metabolomics 2014, 10, 788–804. [Google Scholar] [CrossRef]
- Bordallo, J.J.; Lopez-Llorca, L.V.; Jansson, H.-B.; Salinas, J.; Persmark, L.; Asensio, L. Colonization of Plant Roots by Egg-Parasitic and Nematode-Trapping Fungi. New Phytol. 2002, 154, 491–499. [Google Scholar] [CrossRef]
- Zavala-Gonzalez, E.A.; Rodríguez-Cazorla, E.; Escudero, N.; Aranda-Martinez, A.; Martínez-Laborda, A.; Ramírez-Lepe, M.; Vera, A.; Lopez-Llorca, L.V. Arabidopsis thaliana Root Colonization by the Nematophagous Fungus Pochonia chlamydosporia Is Modulated by Jasmonate Signaling and Leads to Accelerated Flowering and Improved Yield. New Phytol. 2017, 213, 351–364. [Google Scholar] [CrossRef]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The Root Endophytic Fungus Serendipita Indica Improves Resistance of Banana to Fusarium oxysporum f. Sp. Cubense Tropical Race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Monfort, E.; Lopez-Llorca, L.V.; Jansson, H.-B.; Salinas, J.; Park, J.O.; Sivasithamparam, K. Colonisation of Seminal Roots of Wheat and Barley by Egg-Parasitic Nematophagous Fungi and Their Effects on Gaeumannomyces graminis Var. Tritici and Development of Root-Rot. Soil Biol. Biochem. 2005, 37, 1229–1235. [Google Scholar] [CrossRef]
- Dallemole-Giaretta, R.; Freitas, L.G.; Lopes, E.A.; Silva, M.D.C.S.D.; Kasuya, M.C.M.; Ferraz, S. Pochonia chlamydosporia promotes the growth of tomato and lettuce plants. Acta Sci. Agron. 2015, 37, 417–423. [Google Scholar] [CrossRef]
- Monteiro, T.S.A.; Valadares, S.V.; de Mello, I.N.K.; Moreira, B.C.; Kasuya, M.C.M.; de Araújo, J.V.; de Freitas, L.G. Nematophagus Fungi Increasing Phosphorus Uptake and Promoting Plant Growth. Biol. Control 2018, 123, 71–75. [Google Scholar] [CrossRef]
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Zavala-Gonzalez, E.A.; Escudero, N.; Lopez-Moya, F.; Aranda-Martinez, A.; Exposito, A.; Ricaño-Rodríguez, J.; Naranjo-Ortiz, M.A.; Ramírez-Lepe, M.; Lopez-Llorca, L.V. Some Isolates of the Nematophagous Fungus Pochonia chlamydosporia Promote Root Growth and Reduce Flowering Time of Tomato. Ann. Appl. Biol. 2015, 166, 472–483. [Google Scholar] [CrossRef]
- Larriba, E.; Jaime, M.D.L.A.; Nislow, C.; Martín-Nieto, J.; Lopez-Llorca, L.V. Endophytic Colonization of Barley (Hordeum vulgare) Roots by the Nematophagous Fungus Pochonia chlamydosporia Reveals Plant Growth Promotion and a General Defense and Stress Transcriptomic Response. J. Plant Res. 2015, 128, 665–678. [Google Scholar] [CrossRef]
- Dadrasnia, A.; Usman, M.M.; Omar, R.; Ismail, S.; Abdullah, R. Potential Use of Bacillus Genus to Control of Bananas Diseases: Approaches toward High Yield Production and Sustainable Management. J. King Saud Univ.-Sci. 2020, 32, 2336–2342. [Google Scholar] [CrossRef]
- MacIá-Vicente, J.G.; Rosso, L.C.; Ciancio, A.; Jansson, H.B.; Lopez-Llorca, L.V. Colonisation of Barley Roots by Endophytic Fusarium equiseti and Pochonia chlamydosporia: Effects on Plant Growth and Disease. Ann. Appl. Biol. 2009, 155, 391–401. [Google Scholar] [CrossRef]
- Olivares-Bernabeu, C.M.; López-Llorca, L.V. Fungal Egg-Parasites of Plant-Parasitic Nematodes from Spanish Soils. Rev. Iberoam. Micol. 2002, 19, 104–110. [Google Scholar]
- Maciá-Vicente, J.G.; Ferraro, V.; Burruano, S.; Lopez-Llorca, L.V. Fungal Assemblages Associated with Roots of Halophytic and Non-Halophytic Plant Species Vary Differentially Along a Salinity Gradient. Microb. Ecol. 2012, 64, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Llorca, L.V.; Duncan, J.M. New Media for the Estimation of Fungal Infection in Eggs of the Cereal Cyst Nematode, Heterodera avenae Woll. Nematologica 1986, 32, 486–489. [Google Scholar] [CrossRef]
- Escudero, N.; Lopez-Llorca, L.V. Effects on Plant Growth and Root-Knot Nematode Infection of an Endophytic GFP Transformant of the Nematophagous Fungus Pochonia chlamydosporia. Symbiosis 2012, 57, 33–42. [Google Scholar] [CrossRef]
- O’Donnell, K.; Cigelnik, E.; Nirenberg, H.I. Molecular Systematics and Phylogeography of the Gibberella Fujikuroi Species Complex. Mycologia 1998, 90, 465–493. [Google Scholar] [CrossRef]
- Lopez-Llorca, L.V.; Gómez-Vidal, S.; Monfort, E.; Larriba, E.; Casado-Vela, J.; Elortza, F.; Jansson, H.B.; Salinas, J.; Martín-Nieto, J. Expression of Serine Proteases in Egg-Parasitic Nematophagous Fungi during Barley Root Colonization. Fungal Genet. Biol. 2010, 47, 342–351. [Google Scholar] [CrossRef]
- Maciá-Vicente, J.G.; Jansson, H.-B.; Talbot, N.J.; Lopez-Llorca, L.V. Real-Time PCR Quantification and Live-Cell Imaging of Endophytic Colonization of Barley (Hordeum vulgare) Roots by Fusarium equiseti and Pochonia chlamydosporia. New Phytol. 2009, 182, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ferri, D.V.; Munhoz, C.F.; Neves, P.M.O.; Ferracin, L.M.; Sartori, D.; Vieira, M.L.C.; Fungaro, M.H.P. Genetic Variability of Beauveria bassiana and a DNA Marker for Environmental Monitoring of a Highly Virulent Isolate Against Cosmopolites sordidus. Indian J. Microbiol. 2012, 52, 569–574. [Google Scholar] [CrossRef]
- Wesseling, C.; Keifer, M.; Ahlbom, A.; McConnell, R.; Moon, J.-D.; Rosenstock, L.; Hogstedt, C. Long-Term Neurobehavioral Effects of Mild Poisonings with Organophosphate and n-Methyl Carbamate Pesticides among Banana Workers. Int. J. Occup. Environ. Health 2002, 8, 27–34. [Google Scholar] [CrossRef]
- Diepens, N.J.; Pfennig, S.; Brink, P.J.V.D.; Gunnarsson, J.S.; Ruepert, C.; Castillo, L.E. Effect of Pesticides Used in Banana and Pineapple Plantations on Aquatic Ecosystems in Costa Rica. J. Environ. Biol. 2014, 35, 73–84. [Google Scholar]
- Vézina, A.; Van den Bergh, I. Pesticide-Redicing Practices. Available online: http://www.promusa.org/Pesticidereducing+practices+portal (accessed on 15 March 2020).
- Life, T. Short Communication: Molecular Characterisation of Endophytic Fungi from Roots of Wild Banana. Trop. Life Sci. Res. 2016, 27, 153–162. [Google Scholar]
- Ciancio, A.; Colagiero, M.; Rosso, L.; Pentimone, I.; Cepero, J.L. A Metagenomic Study of Banana Nematode Antagonists in Canary Island. Nematropica 2020, 49. [Google Scholar]
- Chakraborty, B.N.; Chakraborty, U.; Sunar, K. Induced Immunity Developed by Trichoderma Species in Plants. In Trichoderma: Host Pathogen Interactions and Applications; Springer: Singapore, 2020; pp. 125–147. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.A.; Socorro, M.Á.H.; Piedra, A.L.; de Martínez, N.M.O.; Hidalgo-Díaz, L. Efecto de Pochonia chlamydosporia Var. Catenulata (Goddard) Zare y Gams Como Endófito Facultativo En Frijol (Phaseolus vulgaris L.). Rev. Prot. Veg. 2019, 34. [Google Scholar]
- Barbosa, R.T.; Monteiro, T.S.A.; Coutinho, R.R.; Silva, J.G.; Freitas, L.G.; Gerais, M.; Gerais, M.; Monteiro, T.S.A.; Coutinho, R.R.; Silva, J.G. Pochonia chlamydosporia No Controle. Nematropica 2019, 49, 99–106. [Google Scholar]
- Hernández-Socorro, M.A.; Arévalo-Ortega, J.; Marrero-Roque, D.; Hidalgo-Díaz, L. Efecto de KlamiC® En La Estimulación Del Crecimiento de Vitroplantas de Plátanos y Bananos. Cultiv. Trop. 2016, 37, 168–172. [Google Scholar]
- Maciá-Vicente, J.G.; Jansson, H.B.; Mendgen, K.; Lopez-Llorca, L.V. Colonization of Barley Roots by Endophytic Fungi and Their Reduction of Take-All Caused by Gaeumannomyces graminis Var. Tritici. Can. J. Microbiol. 2008, 54, 600–609. [Google Scholar] [CrossRef]
- Moonjely, S.; Bidochka, M.J. Generalist and Specialist Metarhizium Insect Pathogens Retain Ancestral Ability to Colonize Plant Roots. Fungal Ecol. 2019, 41, 209–217. [Google Scholar] [CrossRef]
- Ghahremani, Z.; Escudero, N.; Saus, E.; Gabaldón, T.; Sorribas, F.J. Pochonia chlamydosporia Induces Plant-Dependent Systemic Resistance to Meloidogyne incognita. Front. Plant Sci. 2019, 10, 945. [Google Scholar] [CrossRef]
- Larriba, E.; Jaime, M.D.L.A.; Carbonell-Caballero, J.; Conesa, A.; Dopazo, J.; Nislow, C.; Martín-Nieto, J.; Lopez-Llorca, L.V. Sequencing and Functional Analysis of the Genome of a Nematode Egg-Parasitic Fungus, Pochonia chlamydosporia. Fungal Genet. Biol. 2014, 65, 69–80. [Google Scholar] [CrossRef]
- Lin, R.; Qin, F.; Shen, B.; Shi, Q.; Liu, C.; Zhang, X.; Jiao, Y.; Lu, J.; Gao, Y.; Suarez-Fernandez, M.; et al. Genome and Secretome Analysis of Pochonia chlamydosporia Provide New Insight into Egg-Parasitic Mechanisms. Sci. Rep. 2018, 8, 1123. [Google Scholar] [CrossRef]
- Nandhini, M.; Rajini, S.; Udayashankar, A.; Niranjana, S.; Lund, O.S.; Shetty, H.S.; Prakash, H.S. Diversity, plant growth promoting and downy mildew disease suppression potential of cultivable endophytic fungal communities associated with pearl millet. Biol. Control. 2018, 127, 127–138. [Google Scholar] [CrossRef]
- Xue, C.; Penton, C.R.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef] [PubMed]


| Name | Sequence (5′→3′) | Target | Accession Number | Amplicon Size (pb) |
|---|---|---|---|---|
| VCP1-1F | CGCTGGCTCTCTCACTAAGG | P. chlamydosporia vcp1 | AJ427460 | 281 |
| VCP1-2R | TGCCAGTGTCAAGGACGTAG |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mingot-Ureta, C.; Lopez-Moya, F.; Lopez-Llorca, L.V. Isolates of the Nematophagous Fungus Pochonia chlamydosporia Are Endophytic in Banana Roots and Promote Plant Growth. Agronomy 2020, 10, 1299. https://doi.org/10.3390/agronomy10091299
Mingot-Ureta C, Lopez-Moya F, Lopez-Llorca LV. Isolates of the Nematophagous Fungus Pochonia chlamydosporia Are Endophytic in Banana Roots and Promote Plant Growth. Agronomy. 2020; 10(9):1299. https://doi.org/10.3390/agronomy10091299
Chicago/Turabian StyleMingot-Ureta, Cristina, Federico Lopez-Moya, and Luis Vicente Lopez-Llorca. 2020. "Isolates of the Nematophagous Fungus Pochonia chlamydosporia Are Endophytic in Banana Roots and Promote Plant Growth" Agronomy 10, no. 9: 1299. https://doi.org/10.3390/agronomy10091299
APA StyleMingot-Ureta, C., Lopez-Moya, F., & Lopez-Llorca, L. V. (2020). Isolates of the Nematophagous Fungus Pochonia chlamydosporia Are Endophytic in Banana Roots and Promote Plant Growth. Agronomy, 10(9), 1299. https://doi.org/10.3390/agronomy10091299







