Exogenous Gibberellic Acid Advances Reproductive Phenology and Increases Early-Season Yield in Subtropical Blackberry Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. GA3 Treatment
2.3. Phenology, Fruit Yield, and Fruit Quality Measurements
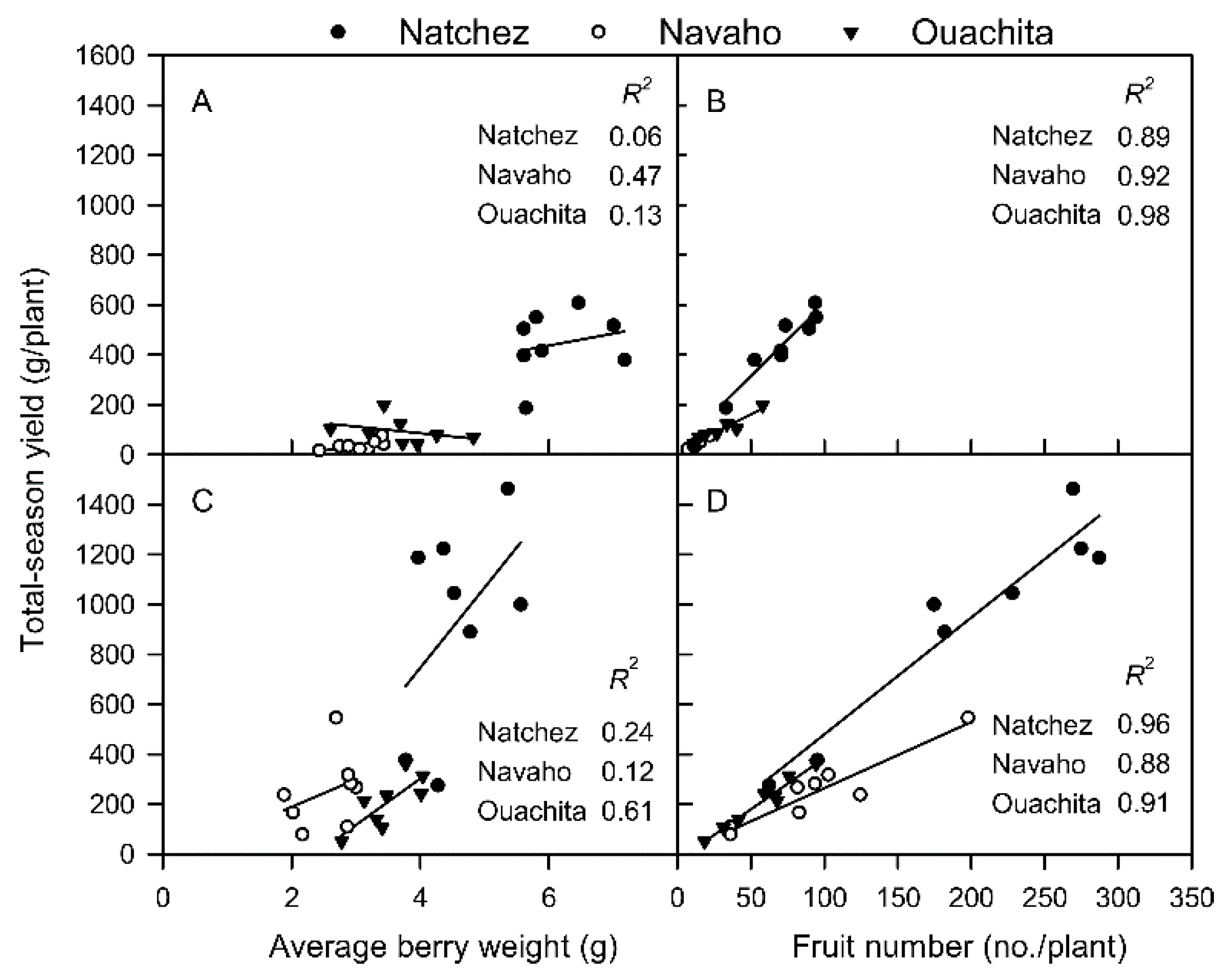
2.4. Regression Analysis
2.5. Experiment Design and Statistical Analysis
3. Results
3.1. Phenology
3.2. Marketable Yield (2015–2016 Season)
3.3. Marketable Yield (2016–2017 Season)
3.4. Fruit Number
3.5. Average Berry Weight
3.6. Regression Analysis
3.7. Fruit SSC
4. Discussion
4.1. Blackberry Reproductive Phenology in a Subtropical Climate
4.2. Exogenous GA3 Advances Blackberry Reproductive Phenology and Increases Early-Season Yield
4.3. Exogenous GA3 Improves Fruit Number and Total-Season Yield
4.4. Negative Side Effects of Exogenous GA3 on Fruit Quality
4.5. Practical Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clark, J.R.; Finn, C.E. Blackberry cultivation in the world. Rev. Bras. Frutic. 2014, 36, 46–57. [Google Scholar] [CrossRef]
- California Strawberry Commission Retail Category Trends—Total U.S. Available online: https://www.calstrawberry.com/Portals/2/Reports/RetailReports/RetailCategoryTrends/RetailCategoryTrends-TotalU.S.-12.03.17.pdf (accessed on 13 October 2019).
- Finn, C.E.; Clark, J.R. Cultivar development and selection. In Blackberries and Their Hybrids; Hall, H.K., Funt, R.C., Eds.; CABI: Boston, MA, USA, 2017; Volume 26, pp. 63–92. ISBN 1780646682. [Google Scholar]
- Strik, B.C.; Clark, J.R.; Finn, C.E.; Ban, M.P. Comprehensive crop reports worldwide blackberry production. HortTechnology 2007, 17, 205–213. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Brennan, R.M.; Jones, H.G. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Takeda, F.; Strik, B.C.; Peacock, D.; Clark, J.R. Cultivar differences and the effect of winter temperature on flower bud development in blackberry. J. Am. Soc. Hortic. Sci. 2002, 127, 495–501. [Google Scholar] [CrossRef]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R. Endo-, para-, and ecodormancy: Physiological terminology and classification for dormancy research. HortScience 1987, 22, 371–377. [Google Scholar]
- Carter, P.M.; Clark, J.R.; Particka, C.D.; Crowne, D.Y. Chilling response of Arkansas blackberry cultivars. J. Am. Pomol. Soc. 2006, 60, 187–197. [Google Scholar]
- Drake, C.A.; Clark, J.R. Determination of the chilling requirement of Arkansas thornless blackberry cultivars. Discovery 2000, 1, 30–32. [Google Scholar]
- McWhirt, A. Blackberry Variety Selection. Available online: http://extension.missouri.edu/greene/documents/Horticulture/Blackberry/BlackberryCultivars%2CMcWhirtNov_15%2C2016.pdf (accessed on 29 January 2018).
- Cook, N.C.; Jacobs, G. Suboptimal winter chilling impedes development of acrotony in apple shoots. HortScience 1999, 34, 1213–1216. [Google Scholar] [CrossRef]
- Fear, C.D.; Meyer, M.-D.L. Breeding and variation in Rubus germplasm for low winter chill requirement. Acta Hortic. 1993, 352, 295–304. [Google Scholar] [CrossRef]
- Hall, H.K.; Brewer, L.R. Breeding Rubus cultivars for warm temperate climates. Acta Hortic. 1989, 262, 65–74. [Google Scholar] [CrossRef]
- Jones, H.G.; Gordon, S.L.; Brennan, R.M. Chilling requirement of Ribes cultivars. Front. Plant Sci. 2015, 5, 767. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-Y.; Agehara, S. Foliar spray of gibberellic acid improves the onset of bud break and fruit set of blackberries in Florida. Proc. Fla. State Hortic. Soc. 2017, 130, 11–13. [Google Scholar]
- Edgley, M.; Close, D.C.; Measham, P.F. Effects of climatic conditions during harvest and handling on the postharvest expression of red drupelet reversion in blackberries. Sci. Hortic. 2019, 253, 399–404. [Google Scholar] [CrossRef]
- Lawrence, B.; Melgar, J.C. Harvest, handling, and storage recommendations for improving postharvest quality of blackberry cultivars. HortTechnology 2018, 28, 578–583. [Google Scholar] [CrossRef]
- Mcwhirt, A. What is Going on with My Blackberry Fruit? Identifying Blackberry Fruit Disorders. Available online: https://www.uaex.edu/farm-ranch/crops-commercial-horticulture/horticulture/ar-fruit-veg-nut-update-blog/posts/fruitdisorders.aspx (accessed on 21 May 2018).
- Liu, J.; Sherif, S.M. Hormonal orchestration of bud dormancy cycle in deciduous woody perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Horvath, D.P.; Anderson, J.V.; Chao, W.S.; Foley, M.E. Knowing when to grow: Signals regulating bud dormancy. Trends Plant Sci. 2003, 8, 534–540. [Google Scholar] [CrossRef]
- Rodrigues, C.; Vandenberghe, L.P.d.S.; de Oliveira, J.; Soccol, C.R. New perspectives of gibberellic acid production: A review. Crit. Rev. Biotechnol. 2012, 32, 263–273. [Google Scholar] [CrossRef]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef]
- Duan, C.; Li, X.; Gao, D.; Liu, H.; Li, M. Studies on regulations of endogenous ABA and GA3 in sweet cherry flower buds on dormancy. Acta Hortic. Sin. 2004, 31, 149–154. [Google Scholar]
- Wen, L.H.; Zhong, W.J.; Huo, X.M.; Zhuang, W.B.; Ni, Z.J.; Gao, Z.H. Expression analysis of ABA- and GA-related genes during four stages of bud dormancy in Japanese apricot (Prunus mume Sieb. et Zucc). J. Hortic. Sci. Biotechnol. 2016, 91, 362–369. [Google Scholar] [CrossRef]
- Chauhan, K.S.; Biggs, R.H.; Sites, J.W. Influence of gibberellic acid, naphthaleneacetic acid, indole-3-acetic acid and maleic hydrazide on peach bud dormancy. Proc. Fla. State Hortic. Soc. 1961, 74, 374–377. [Google Scholar]
- Donoho, C.W.; Walker, D.R. Effect of gibberellic acid on breaking of rest period in Elberta peach. Science 1957, 126, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Tzoutzoukou, C.G.; Pontikis, C.A.; Tolia-Marioli, A. Effects of gibberellic acid on bloom advancement in female pistachio (Pistacia vera L.). J. Hortic. Sci. Biotechnol. 1998, 73, 517–526. [Google Scholar] [CrossRef]
- Elsabagh, A.S. Influences of potassium nitrate, gibberellin and benzyl adenine on bud break, fruit set and branch induction of almond trees. Acta Hortic. 2014, 1028, 359–366. [Google Scholar] [CrossRef]
- Galindo-Reyes, M.A.; González-Hernández, V.A.; Muratalla-Lúa, A.; Soto-Hernández, R.M.; Livera-Muñoz, M. Forced production of blackberry ‘Comanche’ through growth regulators. Rev. Chapingo Ser. Hortic. 2004, 10, 205–209, (In Spanish with English abstract). [Google Scholar] [CrossRef]
- Luedeling, E.; Girvetz, E.H.; Semenov, M.A.; Brown, P.H. Climate change affects winter chill for temperate fruit and nut trees. PLoS ONE 2011, 6, e20155. [Google Scholar] [CrossRef]
- Luedeling, E.; Zhang, M.; Girvetz, E.H. Climatic changes lead to declining winter chill for fruit and nut trees in California during 1950–2099. PLoS ONE 2009, 4, e6166. [Google Scholar] [CrossRef]
- Clark, J.R.; Moore, J.N. ‘Natchez’ thornless blackberry. HortScience 2008, 43, 1897–1899. [Google Scholar] [CrossRef]
- 2008 Southeast Bramble Production Guide. Available online: http://content.ces.ncsu.edu/southeast-regional-caneberry-production-guide (accessed on 28 April 2015).
- U.S. Department of Agriculture United States Standards for Grades of Dewberries and Blackberries. Available online: https://www.ams.usda.gov/sites/default/files/media/DewberriesBlackberriesStandard.pdf (accessed on 26 August 2015).
- Bowley, S.R. Hitchhiker’s Guide to Statistics in Biology. Generalized Linear Mixed Model Edition; Plant et al., Inc.: Kincardine, ON, Canada, 2015; pp. 163–194. ISBN 9780968550045. [Google Scholar]
- Sprugel, D.G. Correcting for bias in log-transformed allometric equations. Ecology 1983, 64, 209–210. [Google Scholar] [CrossRef]
- Florida Automated Weather Network. Available online: https://fawn.ifas.ufl.edu/ (accessed on 26 August 2017).
- Black, B.; Frisby, J.; Lewers, K.; Takeda, F.; Finn, C. Heat unit model for predicting bloom dates in Rubus. HortScience 2008, 43, 2000–2004. [Google Scholar] [CrossRef]
- Clark, J.R. ‘Osage’ thornless blackberry. HortScience 2013, 48, 909–912. [Google Scholar] [CrossRef]
- Clark, J.R.; Moore, J.N. ‘Ouachita ’ thornless blackberry. HortScience 2005, 40, 258–260. [Google Scholar] [CrossRef]
- Moore, J.N.; Clark, J.R. ‘Navajo’ erect thornless blackberry. HortScience 1989, 24, 863–865. [Google Scholar]
- Moore, J.N. Blackberry—Navaho Cultivar. U.S. Patent 6679, 21 March 1989. [Google Scholar]
- Clark, J.R. Blackberry Plant Named ‘Natchez’. U.S. Patent PP 20,891 P3, 30 March 2010. [Google Scholar]
- Clark, J.R.; Moore, J.N. Blackberry Plant Named ‘Ouachita’. U.S. Patent PP 17,162 P3, 24 October 2006. [Google Scholar]
- Hussain, I.; Roberto, S.R.; Fonseca, I.C.B.; de Assis, A.M.; Koyama, R.; Antunes, L.E.C. Phenology of ‘Tupy’ and ‘Xavante’ blackberries grown in a subtropical area. Sci. Hortic. 2016, 201, 78–83. [Google Scholar] [CrossRef]
- Vimont, N.; Schwarzenberg, A.; Domijan, M.; Beauvieux, R.; Arkoun, M.; Yvin, J.-C.; Cortijo, S.; Wigge, P.A.; Dirlewanger, E.; Wenden, B. Hormonal balance finely tunes dormancy status in sweet cherry flower buds. bioRxiv 2018. [Google Scholar] [CrossRef]
- López-Galarza, S.; Pascual, B.; Algarda, J.; Maroto, J.V. The influence of winter gibberellic acid applications on earliness, productivity and other parameters of quality in strawberry cultivation (Fragaria X Ananassa Duch.) on the Spanish Mediterranean coast. Acta Hortic. 1989, 265, 217–222. [Google Scholar] [CrossRef]
- Schuch, U.K.; Fuchigami, L.H.; Nagao, M.A. Gibberellic acid causes earlier flowering and synchronizes fruit ripening of coffee. Plant Growth Regul. 1990, 9, 59–64. [Google Scholar] [CrossRef]
- Southwick, S.M.; Glozer, K. Reducing flowering with gibberellins to increase fruit size in stone fruit tees: Applications and implications in fruit production. HortTechnology 2000, 10, 744–751. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture NASS—Quick Stats. Available online: https://www.ers.usda.gov/webdocs/DataFiles/54499/FruitYearbookBerries_DTables.xlsx?v=42671 (accessed on 6 June 2017).
- Sønsteby, A.; Stavang, J.A.; Heide, O.M. Production of high-yielding raspberry long canes: The way to 3 kg of fruit per cane. J. Hortic. Sci. Biotechnol. 2013, 88, 591–599. [Google Scholar] [CrossRef]
- Clark, J.R.; Finn, C.E. New trends in blackberry breeding. Acta Hortic. 2008, 777, 41–47. [Google Scholar] [CrossRef]
- Carvalho, C.P.; Betancur, J.A. Quality characterization of Andean blackberry fruits (Rubus glaucus Benth.) in different maturity stages in Antioquia, Colombia. Agron. Colomb. 2015, 33, 74–83. [Google Scholar] [CrossRef]
- Vergara, M.F.; Vargas, J.; Acuña, J.F. Physicochemical characteristics of blackberry (Rubus glaucus Benth.) fruits from four production zones of Cundinamarca, Colombia. Agron. Colomb. 2016, 34, 336–345. [Google Scholar] [CrossRef]
- Betts, R.A.; Collins, M.; Hemming, D.L.; Jones, C.D.; Lowe, J.A.; Sanderson, M.G. When could global warming reach 4 °C? Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Baldocchi, D.; Wong, S. Accumulated winter chill is decreasing in the fruit growing regions of California. Clim. Chang. 2008, 87, 153–166. [Google Scholar] [CrossRef]

| 2015–2016 Season | 2016–2017 Season | ||||||
|---|---|---|---|---|---|---|---|
| Cultivar | GA3 z (g·ha−1) | Budbreak | Flowering | Harvest | Budbreak | Flowering | Harvest |
| Natchez | 0 | 11 March 2016 | NA | 16 May 2016 | 16 March 2017 | 26 March 2017 | 13 May 2017 |
| 49 | 31 December 2015 | NA | 16 May 2016 | 4 March 2017 | 22 March 2017 | 13 May 2017 | |
| Navaho | 0 | 24 March 2016 | NA | 3 June 2016 | 27 March 2017 | 23 April 2017 | 10 June 2017 |
| 49 | 2 January 2016 | NA | 12 June 2016 | 2 March 2017 | 3 April 2017 | 26 May 2017 | |
| Ouachita | 0 | 17 March 2016 | NA | 7 June 2016 | 3 April 2017 | 28 April 2017 | 11 June 2017 |
| 49 | 31 December 2015 | NA | 2 June 2016 | 4 March 2017 | 10 April 2017 | 26 May 2017 | |
| GA3 z | Marketable Yield (g/plant) | ||||||
|---|---|---|---|---|---|---|---|
| Cultivar | (g·ha−1) | Early y | Late x | Total | |||
| Natchez | 0 | 243.5 | a w | 174.7 | ab | 372.5 | a |
| 49 | 264.9 | a | 321.4 | a | 538.9 | a | |
| Navaho | 0 | 3.6 | c | 39.3 | de | 43.0 | cd |
| 49 | 3.3 | c | 27.9 | e | 29.9 | d | |
| Ouachita | 0 | 6.9 | c | 54.1 | cd | 61.2 | c |
| 49 | 41.3 | b | 93.6 | bc | 129.7 | b | |
| Pooled data | |||||||
| Natchez | 254.0 | a | 236.9 | a | 448.0 | a | |
| Navaho | 3.4 | c | 33.1 | c | 35.8 | c | |
| Ouachita | 16.8 | b | 71.1 | b | 89.1 | b | |
| 0 | 18.1 | b | 71.9 | b | 99.3 | ||
| 49 | 33.1 | a | 94.3 | a | 127.8 | ||
| p value | |||||||
| Cultivar | 0.0001 | 0.0001 | 0.0001 | ||||
| GA3 | 0.0923 | 0.0485 | 0.1015 | ||||
| Cultivar × GA3 | 0.0738 | 0.0166 | 0.0259 | ||||
| GA3 z | Marketable Fruit Yield (g/plant) | ||||||
|---|---|---|---|---|---|---|---|
| Cultivar | (g·ha−1) | Early y | Late x | Total | |||
| Natchez | 0 | 330.2 | a w | 643.0 | a | 916.2 | a |
| 49 | 402.3 | a | 528.6 | a | 880.4 | a | |
| Navaho | 0 | 12.6 | ab | 151.0 | b | 153.1 | b |
| 49 | 97.0 | ab | 289.9 | ab | 359.1 | ab | |
| Ouachita | 0 | 6.4 | b | 143.9 | b | 146.2 | b |
| 49 | 36.4 | ab | 225.0 | ab | 264.8 | b | |
| Pooled data | |||||||
| Natchez | 364.5 | a | 612.1 | a | 898.1 | a | |
| Navaho | 35.0 | b | 209.2 | b | 234.5 | b | |
| Ouachita | 15.3 | b | 180.0 | b | 196.7 | b | |
| 0 | 29.9 | b | 240.9 | 273.7 | b | ||
| 49 | 112.4 | a | 336.2 | 437.5 | a | ||
| p value | |||||||
| Cultivar | 0.0064 | 0.0180 | 0.0052 | ||||
| GA3 | 0.0559 | 0.1131 | 0.0247 | ||||
| Cultivar × GA3 | 0.3080 | 0.2977 | 0.1543 | ||||
| 2015–2016 Season | 2016–2017 Season | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GA3 z | Fruit Number | Avg. Berry wt. | SSC y | Fruit Number | Avg. Berry wt. | SSC | |||||||
| Cultivar | (g·ha−1) | (no./plant) | (g) | (°Brix) | (no./plant) | (g) | (°Brix) | ||||||
| Natchez | 0 | 58.8 | ab x | 6.43 | a | 9.99 | bc | 180.2 | 4.85 | a | 8.46 | b | |
| 49 | 87.1 | ab | 5.89 | a | 9.54 | c | 214.0 | 4.31 | ab | 7.96 | b | ||
| Navaho | 0 | 13.5 | d | 3.20 | c | 11.50 | a | 59.1 | 2.50 | d | 11.79 | a | |
| 49 | 10.2 | d | 2.91 | c | 9.83 | c | 129.7 | 2.58 | cd | 11.17 | a | ||
| Ouachita | 0 | 14.1 | cd | 4.19 | b | 11.65 | a | 43.6 | 3.42 | bc | 11.88 | a | |
| 49 | 39.5 | bc | 3.23 | c | 10.60 | b | 69.9 | 3.57 | ab | 11.29 | a | ||
| Pooled data | |||||||||||||
| Natchez | 70.3 | a | 6.16 | a | 9.76 | b | 196.4 | a | 4.57 | a | 8.20 | b | |
| Navaho | 11.8 | c | 3.05 | c | 10.63 | a | 87.5 | ab | 2.54 | c | 11.47 | a | |
| Ouachita | 23.6 | b | 3.68 | b | 11.11 | a | 55.2 | b | 3.49 | b | 11.58 | a | |
| 0 | 22.1 | b | 4.42 | a | 11.02 | a | 77.4 | 3.46 | 10.58 | a | |||
| 49 | 32.8 | a | 3.81 | b | 9.98 | b | 124.0 | 3.41 | 10.01 | b | |||
| p value | |||||||||||||
| Cultivar | 0.0001 | 0.0001 | 0.0001 | 0.0191 | 0.0002 | 0.0001 | |||||||
| GA3 | 0.0221 | 0.0054 | 0.0001 | 0.1155 | 0.7792 | 0.0079 | |||||||
| Cultivar × GA3 | 0.1012 | 0.1905 | 0.0090 | 0.6087 | 0.3619 | 0.9800 | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-Y.; Agehara, S. Exogenous Gibberellic Acid Advances Reproductive Phenology and Increases Early-Season Yield in Subtropical Blackberry Production. Agronomy 2020, 10, 1317. https://doi.org/10.3390/agronomy10091317
Lin S-Y, Agehara S. Exogenous Gibberellic Acid Advances Reproductive Phenology and Increases Early-Season Yield in Subtropical Blackberry Production. Agronomy. 2020; 10(9):1317. https://doi.org/10.3390/agronomy10091317
Chicago/Turabian StyleLin, Syuan-You, and Shinsuke Agehara. 2020. "Exogenous Gibberellic Acid Advances Reproductive Phenology and Increases Early-Season Yield in Subtropical Blackberry Production" Agronomy 10, no. 9: 1317. https://doi.org/10.3390/agronomy10091317
APA StyleLin, S.-Y., & Agehara, S. (2020). Exogenous Gibberellic Acid Advances Reproductive Phenology and Increases Early-Season Yield in Subtropical Blackberry Production. Agronomy, 10(9), 1317. https://doi.org/10.3390/agronomy10091317





