Gene Expression and miRNA Regulation Changes in Leaves of Rice Backcross Introgression Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Construction of mRNA Libraries and Small RNA Libraries, Illumina Sequencing and Data Processing
2.3. Analysis of Differential Expression of Genes and miRNAs
2.4. Function Annotation of Genes and Target Genes of miRNA
2.5. Real-Time Quantitative PCR (RT-qPCR)
3. Results
3.1. Gene Expression and Functional Classification of Commonly Expressed (Co-Expressed) Genes
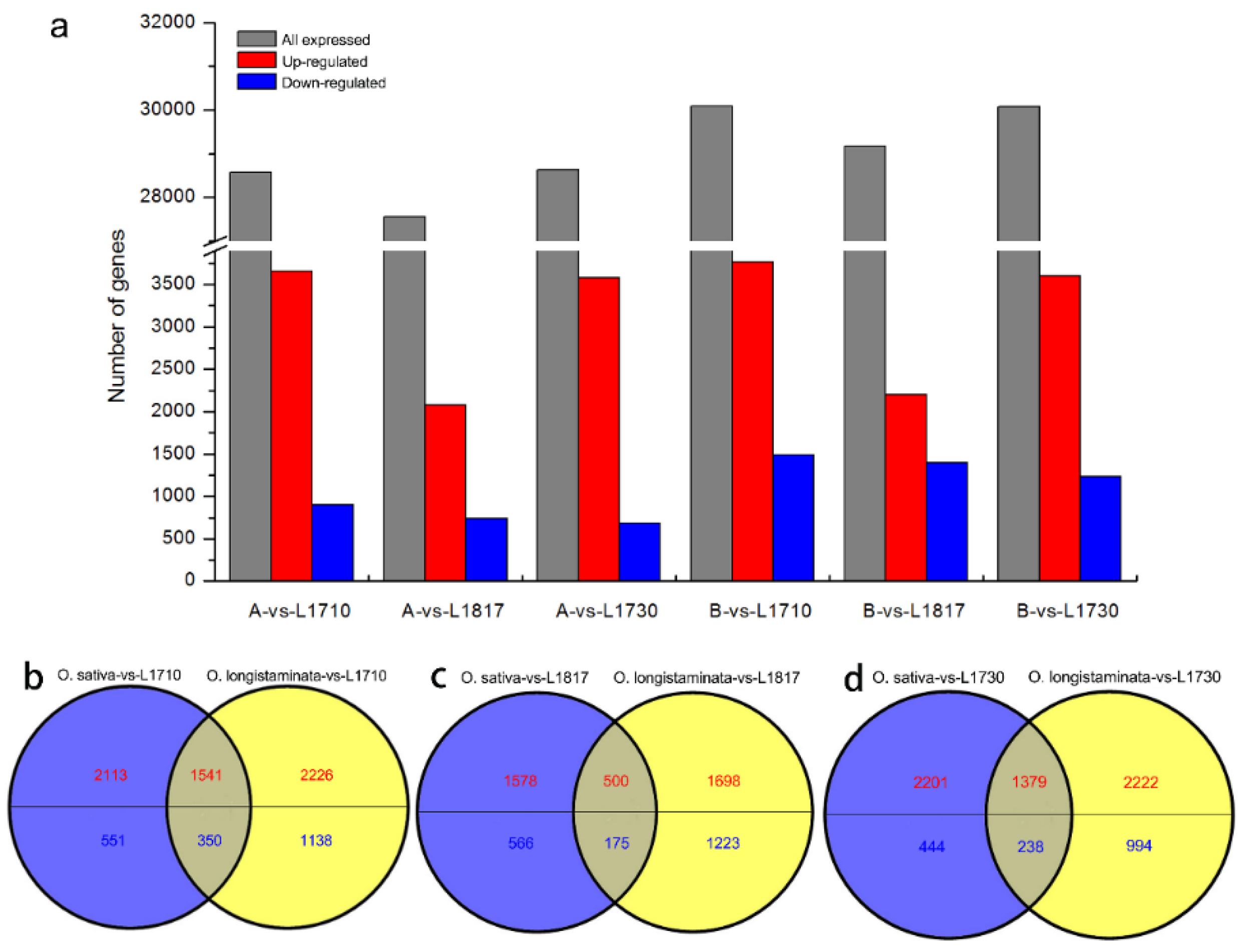
3.2. More Differentially Expressed Genes Were Discovered in Three Progenies Compared with O. longistaminata
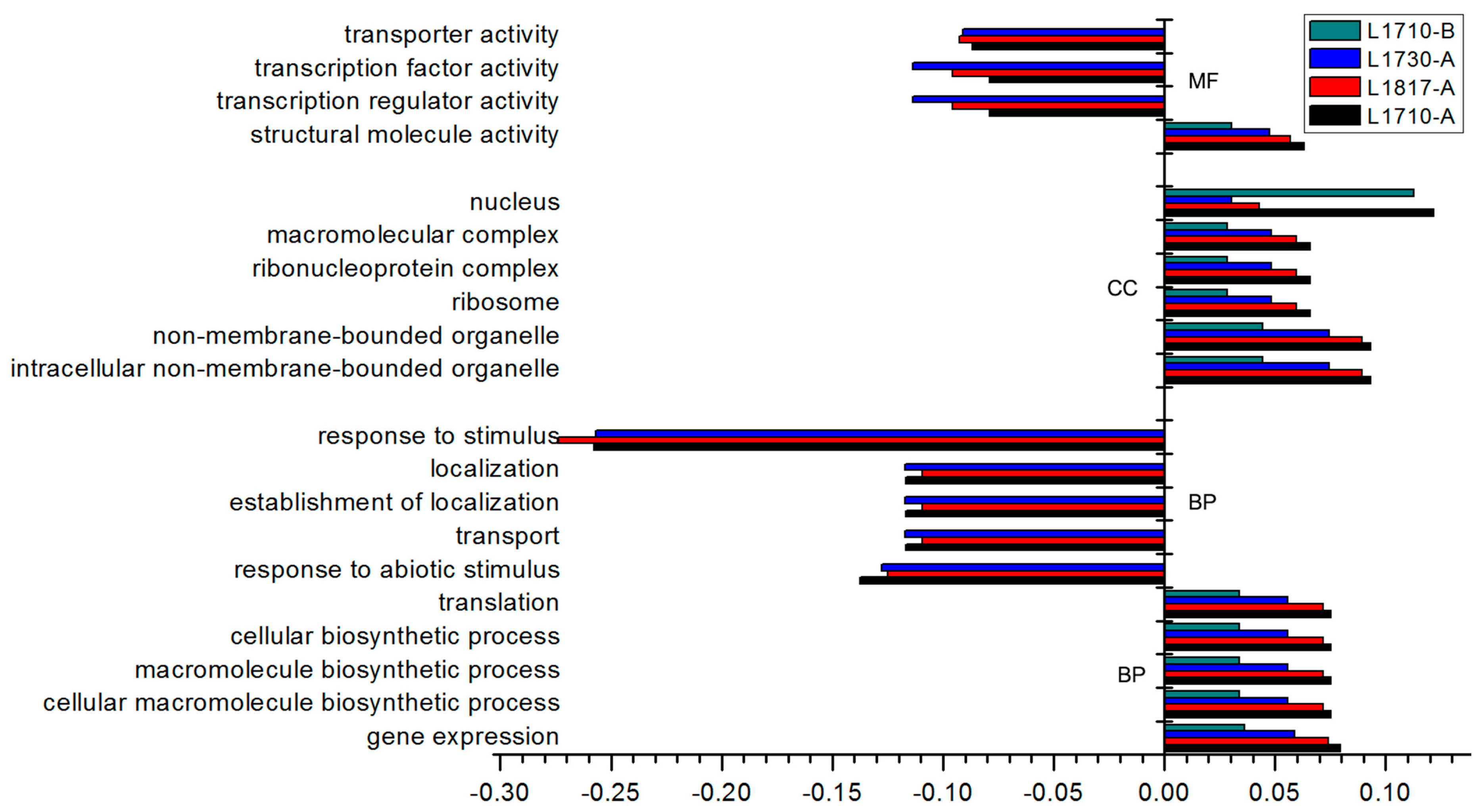
3.3. More GO Terms Were Enriched in DEGs in the Three Progeny/O. sativa Comparisons
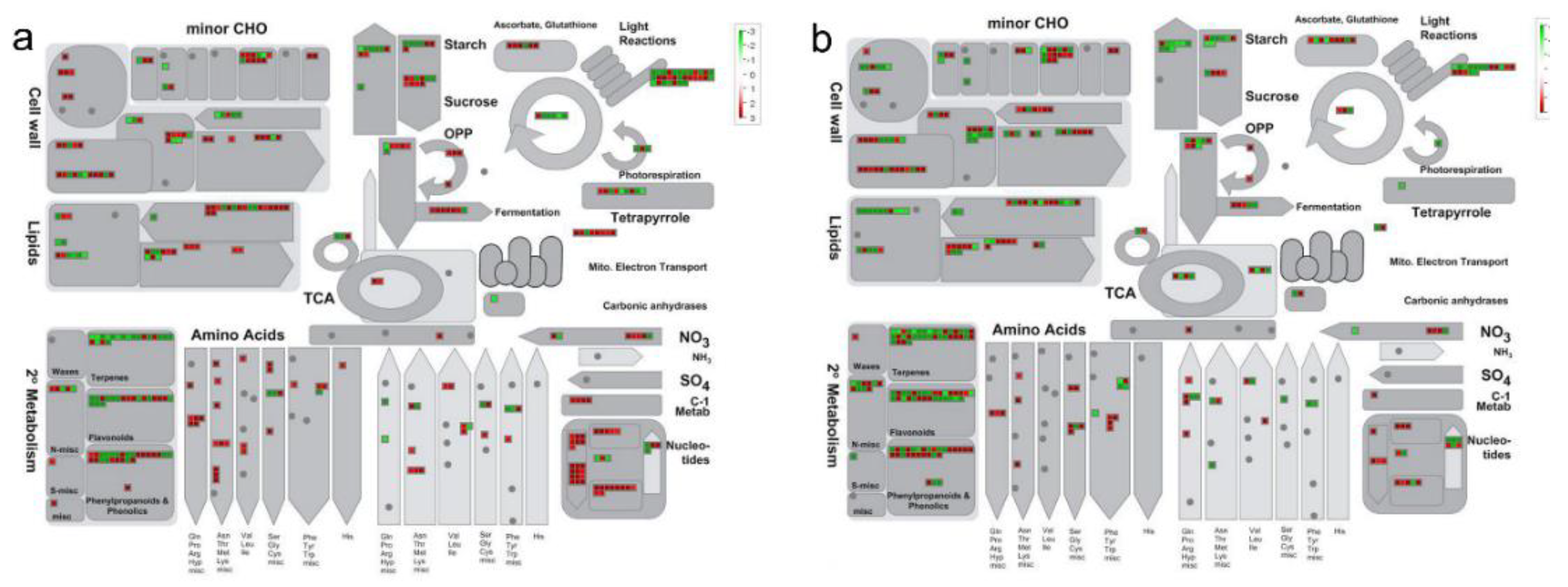
3.4. KEGG and MapMan Pathway Analysis of DEGs in the Three Progenies Compared with Their Parents
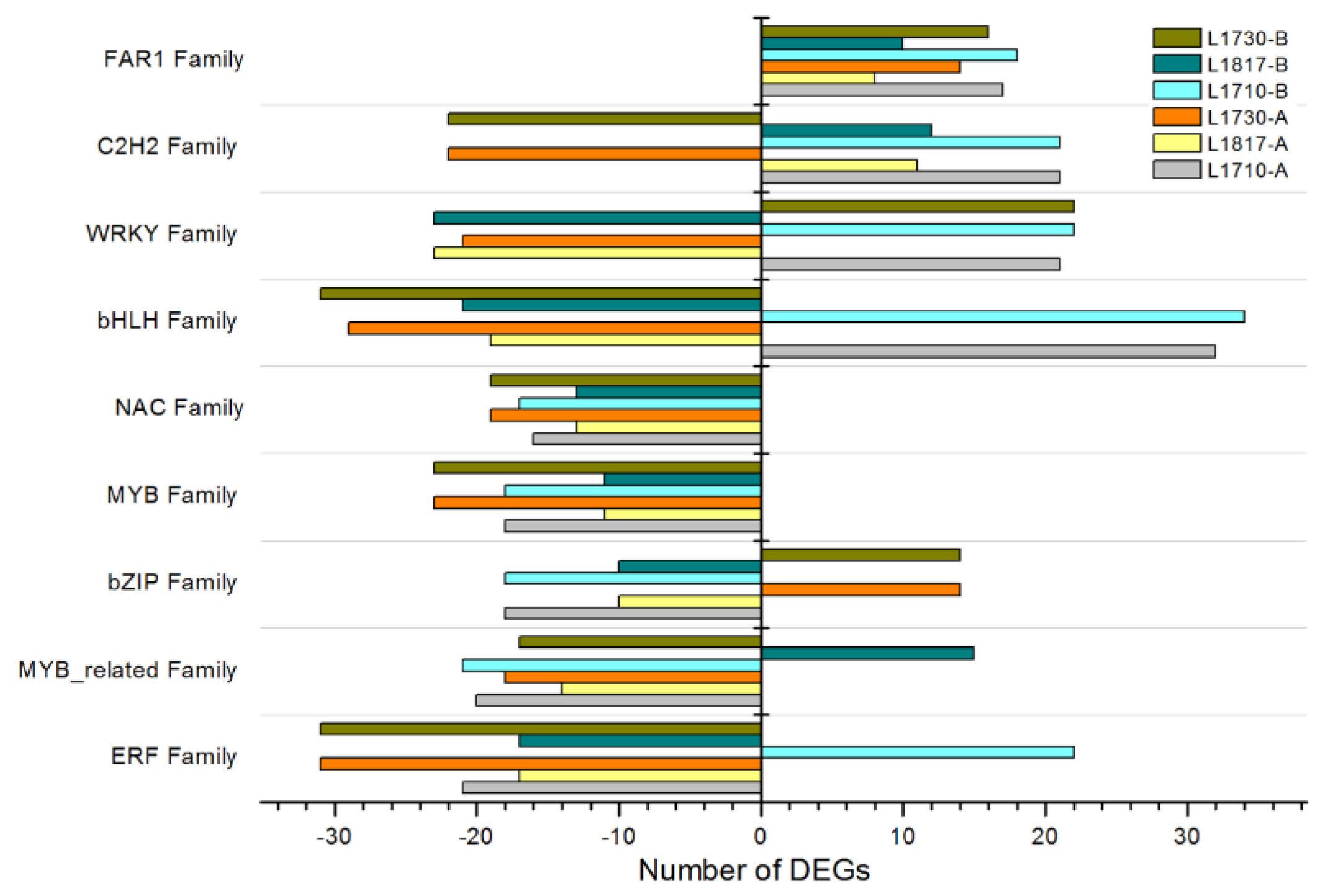
3.5. DEGs in Progeny/Parent Comparisons Involve Many Transcription Factor (TF) Gene Families
3.6. Most miRNAs Were Co-Expressed in the Three Progenies and Their Parents
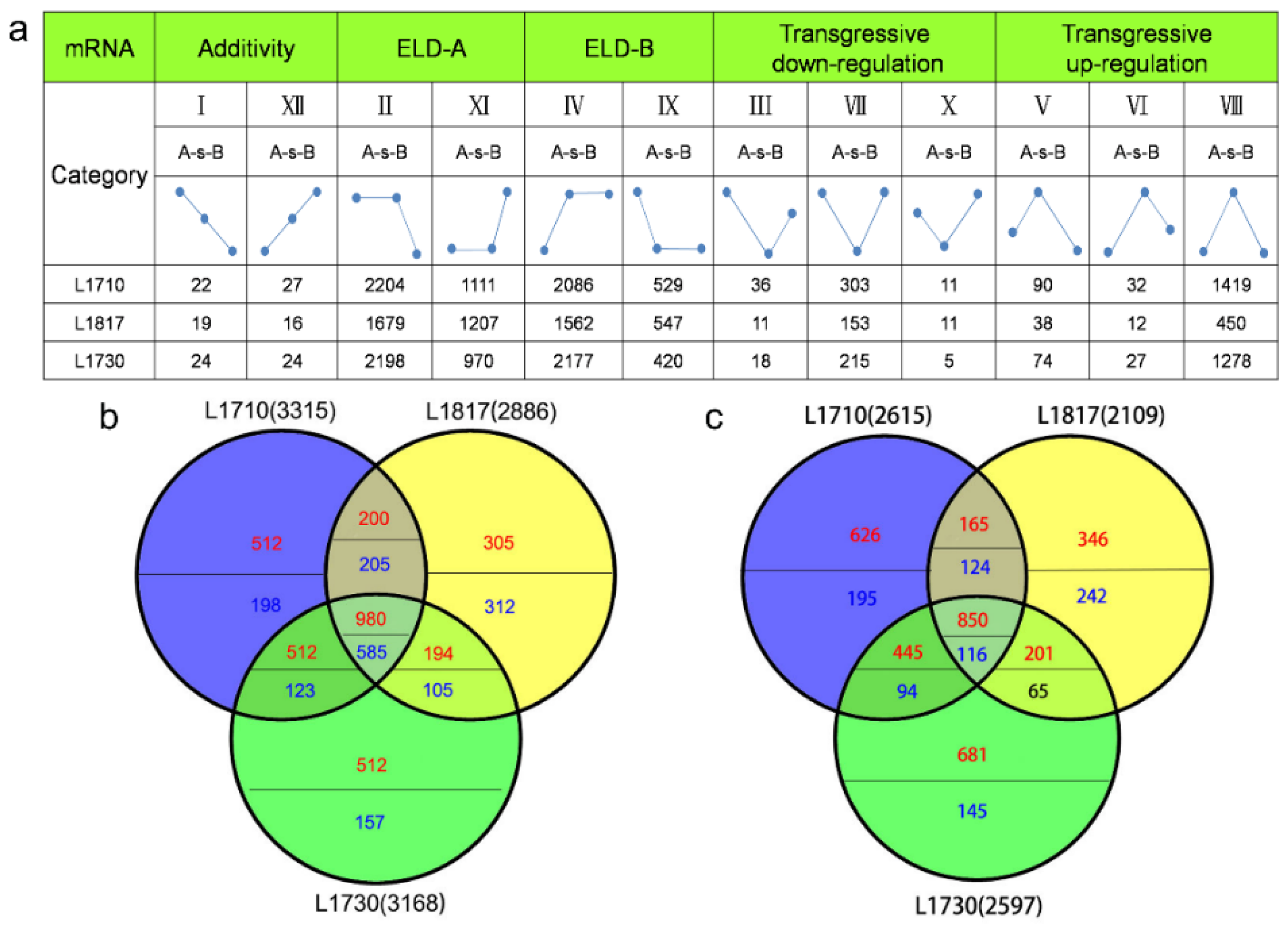
3.7. Expression of miRNA Showed ELD in Three Progenies
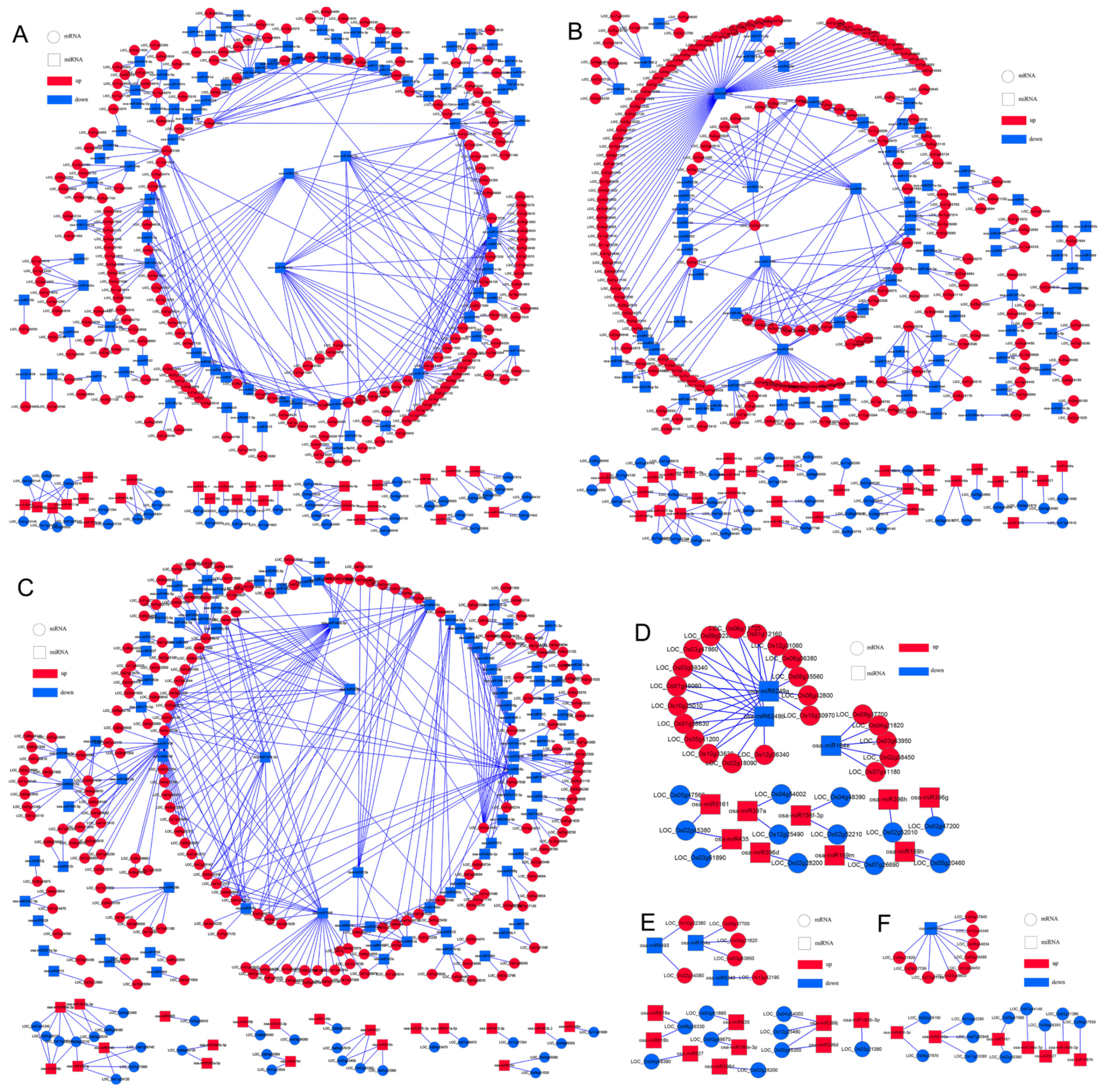
3.8. miRNAs Exhibit Biological Functions through Target Genes
4. Discussion
4.1. miRNA Expression Was More Susceptible than Gene Expression to the Effect of Genomic Composition in Backcrossed Progenies
4.2. Regulation via miRNA Expression Likely Increases Abiotic Stress Responses and Adaptation in Backcrossed Progenies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ge, S.; Sang, T.; Lu, B.R.; Hong, D.Y. Phylogeny of rice genomes with emphasis on origins of allotetraploid species. Proc. Natl. Acad. Sci. USA 1999, 96, 14400–14405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhang, S.; Liu, H.; Fu, B.; Li, L.; Xie, M.; Song, Y.; Li, X.; Cai, J.; Wan, W.; et al. Genome and comparative transcriptomics of African wild rice Oryza longistaminata provide insights into molecular mechanism of rhizomatousness and self-incompatibility. Mol. Plant 2015, 8, 1683–1686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, F.Y.; Tao, D.Y.; Sacks, E.; Fu, B.Y.; Xu, P.; Li, J.; Yang, Y.; Mcnally, K.; Khush, G.S.; Paterson, A.H.; et al. Convergent evolution of perenniality in rice and sorghum. Proc. Natl. Acad. Sci. USA 2003, 100, 4050–4054. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.W.; Hu, F.Y.; Xu, P.; Li, J.; Deng, X.N.; Zhou, J.W.; Li, F.; Chen, S.N.; Tao, D.Y. QTL analysis for hybrid sterility and plant height in interspecific populations derived from a wild rice relative, Oryza longistaminata. Breed. Sci. 2009, 59, 441–445. [Google Scholar] [CrossRef] [Green Version]
- He, R.; Salvato, F.; Park, J.J.; Kim, M.J.; Nelson, W.; Balbuena, T.S.; Willer, M.; Crow, J.A.; May, G.D.; Soderlund, C.A.; et al. A systems-wide comparison of red rice (Oryza longistaminata) tissues identifies rhizome specific genes and proteins that are targets for cultivated rice improvement. BMC Plant Biol. 2014, 14, 46. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Dong, L.Y.; Zhou, J.W.; Li, J.; Zhang, Y.; Hu, F.Y.; Liu, S.F.; Wang, Q.; Deng, W.; Deng, X.N.; et al. Identification and mapping of a novel blast resistance gene Pi57 (t) in Oryza longistaminata. Euphytica 2015, 205, 95–102. [Google Scholar] [CrossRef]
- Moumeni, A.; Satoh, K.; Venuprasad, R.; Serraj, R.; Kumar, A.; Leung, H.; Kikuchi, S. Transcriptional profiling of the leaves of near-isogenic rice lines with contrasting drought tolerance at the reproductive stage in response to water deficit. BMC Genom. 2015, 16, 1110. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Zheng, T.Q.; Hu, X.; Cheng, L.R.; Xu, J.L.; Shi, Y.M.; Li, Z.Q. Examining two sets of introgression lines in rice (Oryza sativa L.) reveals favorable alleles that improve grain Zn and Fe concentrations. PLoS ONE 2015, 10, e0131846. [Google Scholar] [CrossRef]
- Chen, C.; He, W.; Nassirou, T.Y.; Zhou, W.; Yin, Y.; Dong, X.; Rao, Q.; Shi, H.; Zhao, W.; Efisue, A.; et al. Genetic diversity and phenotypic variation in an introgression line population derived from an interspecific cross between Oryza glaberrima and Oryza sativa. PLoS ONE 2016, 11, e0161746. [Google Scholar] [CrossRef]
- Ma, X.; Fu, Y.; Zhao, X.; Jiang, L.; Zhu, Z.; Gu, P.; Xu, W.Y.; Su, Z.; Sun, C.Q.; Tan, L.B. Genomic structure analysis of a set of Oryza nivara introgression lines and identification of yield-associated QTLs using whole-genome resequencing. Sci. Rep. 2016, 6, 27425. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Shi, H.R.; Yu, S.F.; Zhou, W.L.; Li, J.; Liu, S.H.; Deng, M.; Ma, J.; Wei, Y.M.; Zheng, Y.L.; et al. Comprehensive transcriptomics, proteomics, and metabolomics analyses of the mechanisms regulating tiller production in low-tillering wheat. Theor. Appl. Genet. 2019, 132, 2181–2193. [Google Scholar] [CrossRef] [PubMed]
- Kimbembe, R.E.R.; Li, G.Y.; Fu, G.F.; Feng, B.H.; Fu, W.M.; Tao, L.X.; Chen, T.T. Proteomic analysis of salicylic acid regulation of grain filling of two near-isogenic rice (Oryza sativa L.) varieties under soil drying condition. Plant Physiol. Biochem. 2020, 151, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Xia, X.Z.; Zeng, Y.; Nong, B.X.; Zhang, Z.Q.; Wu, Y.Y.; Tian, Q.L.; Zeng, W.Y.; Gao, J.; Zhou, W.Y.; et al. Genome-wide identification of the peptide transporter family in rice and analysis of the PTR expression modulation in two near-isogenic lines with different nitrogen use efficiency. BMC Plant Biol. 2020, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Moumeni, A.; Satoh, K.; Kondoh, H.; Asano, T.; Hosaka, A.; Venuprasad, R.; Serraj, R.; Kumar, A.; Leung, H.; Kikuchi, S. Comparative analysis of root transcriptome profiles of two pairs of drought-tolerant and susceptible rice near-isogenic lines under different drought stress. BMC Plant Biol. 2011, 11, 174. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Zou, J.; Meng, J.; Mei, S.; Wang, J. Tracing the transcriptomic changes in synthetic trigenomic allohexaploids of Brassica using an RNA-Seq approach. PLoS ONE 2013, 8, e68883. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Feng, Y.; Wang, H.; Zhan, X.; Shen, X.; Wu, W.; Zhang, Y.; Chen, D.; Dai, G.; Yang, Z.; et al. Transcriptome analysis of rice root heterosis by RNA-Seq. BMC Genom. 2013, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zhang, Y.; Ge, L.; Wang, L. Genome-wide transcriptome profiles of rice hybrids and their parents. Int. J. Mol. Sci. 2014, 15, 20833–20845. [Google Scholar]
- Shen, Y.Y.; Zhao, Q.; Zou, J.; Wang, W.L.; Gao, Y.; Meng, J.L.; Wang, J.B. Characterization and expression patterns of small RNAs in synthesized Brassica hexaploids. Plant Mol. Biol. 2014, 85, 287–299. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, Y.; Wang, X.; Lin, X.; Sun, S.; Shen, K.; Wang, J.; Jiang, T.T.; Zhong, S.L.; Xu, C.M.; et al. Transcriptome shock in an interspecific F1 triploid hybrid of Oryza revealed by RNA sequencing. J. Integr. Plant Biol. 2016, 58, 150–164. [Google Scholar] [CrossRef]
- Li, A.; Liu, D.; Wu, J.; Zhao, X.; Hao, M.; Geng, S.; Yan, J.; Jiang, X.; Zhang, L.; Wu, J.; et al. mRNA and small RNA transcriptomes reveal insights into dynamic homoeolog regulation of allopolyploid heterosis in nascent hexaploid wheat. Plant Cell 2014, 26, 1878–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, M.; Xie, M.; He, L.; Wang, Y.; Shi, S.; Tang, T. Expression variations of miRNAs and mRNAs in rice (Oryza sativa). Genome Biol. Evol. 2016, 8, 3529–3544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, D.; Maji, R.K.; Dey, S.; Sarkar, A.; Ghosh, Z.; Kundu, P. Integrated miRNA and mRNA expression profiling reveals the response regulators of a susceptible tomato cultivar to early blight disease. DNA Res. 2017, 24, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Jin, J.; Li, S.; Wang, J. Integrated analysis of mRNA and miRNA expression profiling in rice backcrossed progenies (BC2F12) with different plant height. PLoS ONE 2017, 12, e0184106. [Google Scholar] [CrossRef] [Green Version]
- Li, M.D.; Cao, A.Q.; Wang, R.H.; Li, Z.Y.; Li, S.Q.; Wang, J.B. Genome-wide identification and integrated analysis of lncRNAs in rice backcross introgression lines (BC2F12). BMC Plant Biol. 2020, 20, 300. [Google Scholar] [CrossRef]
- Kawahara, Y.; de la Bastide, M.; Hamilton, J.P.; Kanamori, H.; McCombie, W.R.; Ouyang, S.; Schwartz, D.C.; Tanaka, T.; Wu, J.Z.; Zhou, S.G.; et al. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice 2013, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Li, R.Q.; Li, Y.R.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.Y.; Wu, Y.; Wang, W.L.; Mao, B.G.; Zhao, B.R.; Wang, J.B. Genetic subtraction profiling identifies candidate miRNAs involved in rice female gametophyte abortion. G3-Genes Genomes Genet. 2017, 7, 2281–2293. [Google Scholar] [CrossRef] [Green Version]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Wang, L.K.; Feng, Z.X.; Wang, X.; Wang, X.W.; Zhang, X.G. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, R.; Palatnik, J.F.; Riester, M.; Schommer, C.; Schmid, M.; Weigel, D. Specific effects of microRNAs on the plant transcriptome. Dev. Cell 2005, 8, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Liu, Y.; Yan, H.; You, Q.; Yi, X.; Du, Z.; Xu, W.; Su, Z. agriGO v2.0: A GO analysis toolkit for the agricultural community, 2017 update. Nucleic Acids Res. 2017, 45, W122–W129. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhang, H.; Kong, L.; Gao, G.; Luo, J. PlantTFDB 3.0: A portal for the functional and evolutionary study of plant transcription factors. Nucleic Acids Res. 2014, 42, D1182–D1187. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, H.; Shen, Y.Y.; Wang, J.B. Transcriptomic analysis of rice (Oryza sativa) endosperm using the RNA-Seq technique. Plant Mol. Biol. 2013, 81, 363–378. [Google Scholar] [CrossRef]
- Yoo, M.J.; Szadkowski, E.; Wendel, J.F. Homoeolog expression bias and expression level dominance in allopolyploid cotton. Heredity 2012, 110, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Li, Y.; Moose, S.P.; Hudson, M.E. Transposable elements, mRNA expression level and strand-specificity of small RNAs are associated with non-additive inheritance of gene expression in hybrid plants. BMC Plant Biol. 2015, 15, 168. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, C.; Wang, Y.; Niu, M.; Ren, Y.; Zhou, K.; Zhang, H.; Lin, Q.; Wu, F.; Cheng, Z.; et al. WSL3, a component of the plastid-encoded plastid RNA polymerase, is essential for early chloroplast development in rice. Plant Mol. Biol. 2016, 92, 581–595. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, J.; Huang, L.; Leng, Y.; Dai, L.; Rao, Y.; Chen, L.; Wang, Y.; Tu, Z.; Hu, J.; et al. PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J. Exp. Bot. 2016, 67, 1297–1310. [Google Scholar] [CrossRef] [Green Version]
- Hubbart, S.; Ajigboye, O.O.; Horton, P.; Murchie, E.H. The photoprotective protein PsbS exerts control over CO2 assimilation rate in fluctuating light in rice. Plant J. 2012, 71, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.I.; Ryoo, N.; Ko, S.; Lee, S.K.; Lee, J.; Jung, K.H.; Lee, Y.H.; Bhoo, S.H.; Winderickx, J.; An, G.; et al. Structure, expression, and functional analysis of the hexokinase gene family in rice (Oryza sativa L.). Planta 2006, 224, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, D.; Mao, D.; Liu, X.; Ji, C.; Li, X.; Zhao, X.F.; Cheng, Z.K.; Chen, C.Y.; Zhu, L.H. Overexpression of microRNA319 impacts leaf morphogenesis and leads to enhanced cold tolerance in rice (Oryza sativa L.). Plant Cell Environ. 2013, 36, 2207–2218. [Google Scholar] [CrossRef]
- Fang, Y.; Xie, K.; Xiong, L. Conserved miR164-targeted NAC genes negatively regulate drought resistance in rice. J. Exp. Bot. 2014, 65, 2119–2135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Chen, X.; Chai, X.; Qiu, Y.; Gong, C.; Zhang, Z.; Wang, T.; Zhang, Y.; Li, J.; Wang, A. Effects of low temperature on mRNA and small RNA transcriptomes in Solanum lycopersicoides leaf revealed by RNA-Seq. Biochem. Biophys. Res. Commun. 2015, 464, 768–773. [Google Scholar] [CrossRef]
- Sperotto, R.A.; Ricachenevsky, F.K.; Duarte, G.L.; Boff, T.; Lopes, K.L.; Sperb, E.R.; Grusak, M.A.; Fett, J.P. Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta 2009, 230, 985–1002. [Google Scholar] [CrossRef]
- Huang, D.; Wang, S.; Zhang, B.; Shang-Guan, K.; Shi, Y.; Zhang, D.; Liu, X.; Wu, K.; Xu, Z.; Fu, X.; et al. A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice. Plant Cell 2015, 27, 1681–1696. [Google Scholar] [CrossRef] [Green Version]
- Wakuta, S.; Suzuki, E.; Saburi, W.; Matsuura, H.; Nabeta, K.; Imai, R.; Matsui, H. OsJAR1 and OsJAR2 are jasmonyl-L-isoleucine synthases involved in wound- and pathogen-induced jasmonic acid signalling. Biochem. Biophys. Res. Commun. 2011, 409, 634–639. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Hillwig, M.L.; Wu, Y.; Peters, R.J. CYP701A8: A rice ent-kaurene oxidase paralog diverted to more specialized diterpenoid metabolism. Plant Physiol. 2012, 158, 1418–1425. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, A.; Wang, R.; Wang, J. Gene Expression and miRNA Regulation Changes in Leaves of Rice Backcross Introgression Lines. Agronomy 2020, 10, 1381. https://doi.org/10.3390/agronomy10091381
Cao A, Wang R, Wang J. Gene Expression and miRNA Regulation Changes in Leaves of Rice Backcross Introgression Lines. Agronomy. 2020; 10(9):1381. https://doi.org/10.3390/agronomy10091381
Chicago/Turabian StyleCao, Aqin, Ruihua Wang, and Jianbo Wang. 2020. "Gene Expression and miRNA Regulation Changes in Leaves of Rice Backcross Introgression Lines" Agronomy 10, no. 9: 1381. https://doi.org/10.3390/agronomy10091381
APA StyleCao, A., Wang, R., & Wang, J. (2020). Gene Expression and miRNA Regulation Changes in Leaves of Rice Backcross Introgression Lines. Agronomy, 10(9), 1381. https://doi.org/10.3390/agronomy10091381




