Indigo as a Plant Growth Inhibitory Chemical from the Fruit Pulp of Couroupita guianensis Aubl.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of C. guianensis Samples and Extraction Procedure
2.2. Chemicals and Test Plants for Bioassay
2.3. Inhibitory Effects of C. guianensis Crude Extracts and Test Compounds
2.4. Effects on Plant Growth Inhibition Activity of Candidate Compounds on Test Plants
2.5. HPLC Analysis of C. guianensis Fruit Pulps
2.6. Effect of Incorporating C. guianensis Fruit Pulps in Soil on the Bioassay Species
2.7. Statistical Analysis
3. Results and Discussion
3.1. Inhibitory Effect of Crude Extracts of C. guianensis Fruit Pulp on Lettuce Seedlings
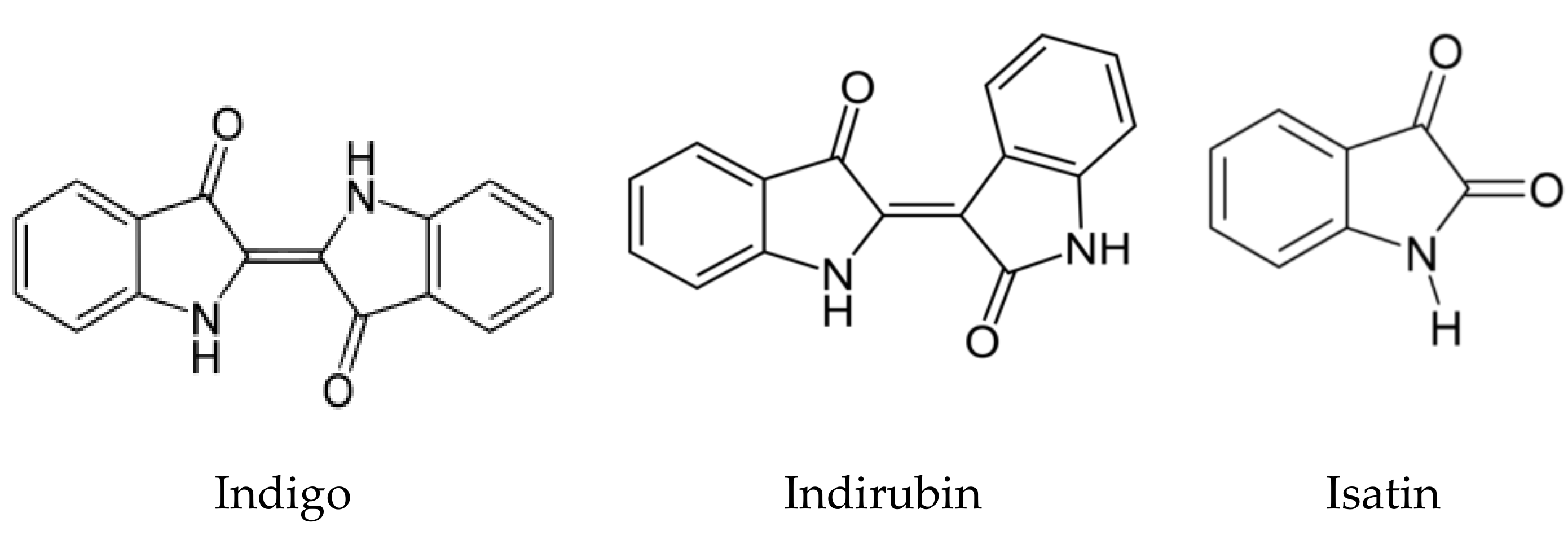
3.2. Plant Growth Inhibitory Effects of Synthetic Compounds Present in Crude Extract of C. guianensis Fruit Pulp
3.3. Estimation of the Contribution of the Candidate Compound on Growth Inhibitory Activity
3.4. Effect of Soil Incorporation of the Crude Extract of C. guianensis Fruit Pulp on Weeds Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WSSA Calculates Billions in Potential Economic Losses from Uncontrolled Weeds; National and Regional Weed Science Societies: Lawrence, KS, USA, 2016.
- Llewellyn, R.; Ronning, D.; Ouzman, J.; Walker, S.; Mayfield, A.; Clarke, M. Impact of Weeds on Australian Grain Production: The Cost of Weeds to Australian Grain Growers and the Adoption of Weed Management and Tillage Practices; Report for Grains Research & Development Corporation: Canberra, ACT, Australia, 2016. [Google Scholar]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Duke, S.O.; Romagni, J.G.; Dayan, F.E. Natural products as sources for new mechanisms of herbicidal action. Crop Prot. 2000, 19, 583–589. [Google Scholar] [CrossRef]
- Wardle, D.A.; Karban, R.; Callaway, R.M. The ecosystem and evolutionary contexts of allelopathy. Trends Ecol. Evol. 2011, 26, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Weston, L.A.; Duke, S.O. Weed and crop allelopathy. Crit. Rev. Plant Sci. 2003, 22, 367–389. [Google Scholar] [CrossRef]
- International Allelopathy Society. June 2015. Available online: http://allelopathy-society.osupytheas.fr/about/ (accessed on 9 June 2020).
- Fujii, Y. Allelopathy in the natural and agricultural ecosystems and isolation of potent allelochemicals from Velvet bean (Mucuna pruriens) and Hairy vetch (Vicia villosa). Biol. Sci. Space. 2003, 17, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Hiradate, S. Allelopathy: New Concepts & Methodology; Science Publishers, Inc.: Enfield, NH, USA, 2007; ISBN 978-1-57808-446-3. [Google Scholar]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as Bioherbicides—Present and Perspectives. In Herbicides-Current Research and Case Studies in Use; Price, A., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-1112-2. [Google Scholar]
- Kropff, M.J.; Walter, H. EWRS and the challenges for weed research at the start of a new millennium. Weed Res. (Oxford) 2000, 40, 7–10. [Google Scholar] [CrossRef]
- Vyvyan, J.R. Allelochemicals as leads for new herbicides and agrochemicals. Tetrahedron 2002, 58, 1631–1646. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Varela, R.M.; Simonet, A.M.; Carrera, C.; Molinillo, J.M. Allelopathy as a new strategy for sustainable ecosystems development. Biol. Sci. Space 2003, 17, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [Google Scholar] [CrossRef]
- Kong, C.; Liang, W.; Xu, X.; Hu, F.; Wang, P.; Jiang, Y. Release and activity of allelochemicals from allelopathic rice seedlings. J. Agric. Food Chem. 2004, 52, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Shibuya, T.; Yasuda, T. Allelopathy of velvetbean: Its discrimination and identification of L-DOPA as a candidate of allelopathic substances. Jpn. Agric. Res. Q. 1992, 25, 238–247. [Google Scholar]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, B.C.; Sparks, T.C. Natural products for pest control: An analysis of their role, value and future. Pest Manag. Sci. 2014, 70, 1169–1185. [Google Scholar] [CrossRef]
- Heywood, V.H. Popular Encyclopedia of Plants; Vernon, H., Chant, S.R., Eds.; Cambridge University Press: Cambridge, UK, 1982; ISBN 978-0-521-24611-8. [Google Scholar]
- Mori, S.A.; Tsou, C.-H.; Wu, C.-C.; Cronholm, B.; Anderberg, A.A. Evolution of Lecythidaceae with an emphasis on the circumscription of neotropical genera: Information from combined ndhF and trnL-F sequence data. Am. J. Bot. 2007, 94, 289–301. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2012; ISBN 978-90-481-8660-0. [Google Scholar]
- Al-Dhabi, N.A.; Balachandran, C.; Raj, M.K.; Duraipandiyan, V.; Muthukumar, C.; Ignacimuthu, S.; Khan, I.A.; Rajput, V.S. Antimicrobial, antimycobacterial and antibiofilm properties of Couroupita guianensis Aubl. fruit extract. BMC Complement. Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Saluja, A.K.; Shankar, M.B.; Mehta, R.S. Analgesic and anti-inflammatory activity of Couroupita guianensis Aubl. J. Nat. Remedies 2004, 4, 4. [Google Scholar]
- Umachigi, S.P.; Jayaveera, K.; Kumar, A.; Kumar, G. Antimicrobial, Wound Healing and Antioxidant potential of Couroupita guianensis in rats. Pharmacol. Online 2007, 3, 269–281. [Google Scholar]
- Patel, S.H.; Suthar, J.V.; Patel, R.K.; Zankharia, U.S.; Jani, V.R.; Gajjar, K.N. Antimicrobial activity investigation of Aegle marmelos, Couroupita guianesis, Manilkarahexandra, cow urine and dung. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 1014–1022. [Google Scholar]
- Shivashankar, M.; Rajeshwari, S.; Nagananda, G.; Rajath, S.; Chandan, N. Comparative antioxidant and antimicrobial studies of cold and hot bark hydromethanolic extract of Couroupita guianensis Aubl. Res. Pharm. 2013, 3, 6–13. [Google Scholar]
- Manimegalai, S.; Sridharan, T.; Rameshpathy, M.; Devi Rajeswari, V. Antioxidant, phytochemical screening and antimicrobial activity of Couroupita guianensis flower extract. Der Pharm. Lett. 2014, 6, 251–256. [Google Scholar]
- Begum, R.; Rahman, M.S.; Chowdhury, A.M.S.; Hasan, C.M.; Rashid, M.A. Secondary metabolites (Triterpenes) from Couroupita guianensis. Orient. Pharm. Exp. Med. 2009, 9, 200–205. [Google Scholar] [CrossRef]
- Gousia, S.K.; Kumar, K.A.; Kumar, T.V.; Latha, J.N.L. Biological Activities and Medicinal Properties of Couroupita guianensis. Int. J. Pharm. Pharm. Sci. Res. 2013, 3, 140–143. [Google Scholar]
- Pandurangan, P.; Sahadeven, M.; Sunkar, S.; Dhana, S.K.N.M. Comparative analysis of biochemical compounds of leaf, flower and fruit of Couroupita guianensis and synthesis of silver nanoparticles. Pharmacogn. J. 2018, 10, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Chanayath, N.; Lhieochaiphant, S.; Phutrakul, S. Pigment Extraction Techniques from the Leaves of Indigofera tinctoria Linn. and Baphicacanthus cusia Brem. and Chemical Structure Analysis of Their Major Components. Chemistry 2005, 1, 149–160. [Google Scholar]
- Simon, J.E.; Chadwick, A.F.; Craker, L.E. Herbs, An Indexed Bibliography, 1971–1980; Elsevier: Amsterdam, The Netherlands, 1984; ISBN 0-444-99626-5. [Google Scholar]
- Lin, Y.-K.; Wong, W.-R.; Chang, Y.-C.; Chang, C.-J.; Tsay, P.-K.; Chang, S.-C.; Pang, J.-H.S. The efficacy and safety of topically applied indigo naturalis ointment in patients with plaque-type psoriasis. Dermatology 2007, 214, 155–161. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, W.; Li, X.; Deng, B.; Xu, W.; Qu, J.; Zhang, G.; Zhang, C.; Sun, L.; Jiang, C.; et al. Evidence-based practice guideline of Chinese herbal medicine for Psoriasis vulgaris (Bai Bi). Eur. J. Integr. Med. 2014, 6, 135–146. [Google Scholar] [CrossRef]
- Qing-hua, L. The Chemical Constituents of Qing Dai. J. Integr. Plant Biol. 1987, 29, 67–72. [Google Scholar]
- Wu, G.Y.; Fang, F.D.; Liu, J.Z.; Chang, A.; Ho, Y.H. Studies on the mechanism of action of indirubin in the treatment of chronic granulocytic leukemia. I. Effects on nucleic acid and protein synthesis in human leukemic cells. Chin. Med. J. 1980, 60, 451–454. [Google Scholar]
- Zhang, S.X. Studies on the chemical constituents of Isatis indigotica root. Chin. Trad Herb Drugs 1983, 14, 247–248. [Google Scholar]
- Zheng, Q.T.; Lu, D.J.; Yang, S.L. Pharmacological studies of indirubin. I. Antitumor effect. Comm. Chin. Herb. Med. 1979, 10, 35–39. [Google Scholar]
- Zheng, Q.T.; Qi, S.B.; Cheng, Z.Y. Pharmacological studies of indirubin. II. Absorption, distribution and excretion of 3H-indirubin. Comm. Chin. Herb Med. 1979, 10, 19–21. [Google Scholar]
- Qi, T.; Li, H.; Li, S. Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget 2017, 8, 36658–36663. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.-P.; Woerdenbag, H.J. Traditional Chinese herbal medicine. Pharm. World Sci. 1995, 17, 103–112. [Google Scholar] [CrossRef]
- Hoessel, R.; Leclerc, S.; Endicott, J.A.; Nobel, M.E.; Lawrie, A.; Tunnah, P.; Leost, M.; Damiens, E.; Marie, D.; Marko, D. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol. 1999, 1, 60–67. [Google Scholar] [CrossRef]
- Sareen, K.; Kohli, R.P.; Amma, M.K.; Gujral, M.L. Anticonvulsant drugs based on the neurochemistry of seizures. Indian J. Physiol. Pharmacol. 1962, 6, 87. [Google Scholar] [PubMed]
- Chocholova, L.; Kolinova, M. Effect of isatin (2, 3-dioxoindoline) on audiogenic seizures in rats and its relationship to electrographic and behavioural phenomena. Physiol. Bohemoslov. 1979, 28, 495. [Google Scholar]
- Glover, V.; Bhattacharya, S.K.; Chakrabarti, A.; Sandler, M. The psychopharmacology of isatin: A brief review. Stress Med. 1998, 14, 225–229. [Google Scholar] [CrossRef]
- Khan, M.S.I.; Kato-Noguchi, H. Assessment of allelopathic potential of Couroupita guianensis Aubl. Plant Omics 2016, 9, 115–120. [Google Scholar] [CrossRef]
- Chandra, S.; Chatterjee, P.; Dey, P.; Bhattacharya, S. Allelopathic effect of Ashwagandha against the germination and radicle growth of Cicer arietinum and Triticum aestivum. Pharmacogn. Res. 2012, 4, 166. [Google Scholar] [CrossRef] [Green Version]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Furubayashi, A.; Fujii, Y. Allelopathic effect of leaf debris, leaf aqueous extract and rhizosphere soil of Ophiopogon japonicus Ker-Gawler on the growth of plants. Weed Biol. Manag. 2004, 4, 43–48. [Google Scholar] [CrossRef]
- Ismail, B.S.; Siddique, M.A.B. The Inhibitory Effect of Grasshopper’s Cyperus (Cyperus iria L.) on the Seedling Growth of Five Malaysian Rice Varieties. Trop. Life Sci. Res. 2011, 22, 81–89. [Google Scholar]
- Magiero, E.C.; Assmann, J.M.; Marchese, J.A.; Capelin, D.; Paladini, M.V.; Trezzi, M.M. Allelopathic effect of Artemisia annua L. on the germination and initial development of lettuce (Lactuca sativa L.) and wild poinsettia (Euphorbia heterophylla L.) seedlings. Rev. Bras. Plantas Med. 2009, 11, 317–324. [Google Scholar] [CrossRef]
- Rizzi, E.S.; Pereira, K.C.L.; de Araujo Abreu, C.A.; de Lima Silva, B.C.F.; Fernandes, R.M.; de Oliveira, A.K.M.; Matias, R. Allelopathic potential and phytochemistry of cambarazinho (Vochysia haenkeana (Spreng.) Mart.) leaves in the germination and development of lettuce and tomato. Biosci. J. 2016, 32, 98–107. [Google Scholar] [CrossRef]
- Qasem, J.R. The allelopathic effect of three Amaranthus spp. (pigweeds) on wheat (Triticum durum). Weed Res. 1995, 35, 41–49. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Yoshimura, H.; Sawai, Y.; Tamotsu, S.; Sakai, A. 1, 8-cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J. Chem. Ecol. 2011, 37, 320–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.J.; Mulla, S.A.; Revdiwala, S.B. Neonatal Sepsis: High Antibiotic Resistance of the Bacterial Pathogens in a Neonatal Intensive Care Unit of a Tertiary Care Hospital. J. Clin. Neonatol. 2012, 1, 72. [Google Scholar] [CrossRef]
- Rashid, M.A.; Alam, S.N.; Rouf, F.M.A.; Talekar, N.S. Socio-Economic Parameters of Eggplant Pest Control in Jessore District of Bangladesh; AVRDC-World Vegetable Center: Tainan, Taiwan, 2003; ISBN 978-92-9058-127-7. [Google Scholar]
- Dasgupta, S.; Meisner, C.; Huq, M. Health Effects And Pesticide Perception As Determinants Of Pesticide Use: Evidence From Bangladesh; Policy Research Working Papers; The World Bank: Washington, DC, USA, 2005. [Google Scholar]
- Mishyna, M.; Laman, N.; Prokhorov, V.; Maninang, J.S.; Fujii, Y. Identification of Octanal as Plant Growth Inhibitory Volatile Compound Released from Heracleum sosnowskyi Fruit. Nat. Prod. Commun. 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Hiradate, S.; Ohse, K.; Furubayashi, A.; Fujii, Y. Quantitative evaluation of allelopathic potentials in soils: Total activity approach. Weed Sci. 2010, 58, 258–264. [Google Scholar] [CrossRef]
- Golisz, A.; Lata, B.; Gawronski, S.W.; Fujii, Y. Specific and total activities of the allelochemicals identified in buckwheat. Weed Biol. Manag. 2007, 7, 164–171. [Google Scholar] [CrossRef]
- Morikawa, C.; Miyaura, R.; Kamo, T.; Hiradate, S.; Pérez, J.; Fujii, Y. Isolation of Umbelliferone as a Principal Allelocheical from The Peruvian Medicinal plant Diplostephium foliosissimum (Asteraceae). Rev. Soc. Quím. Perú. 2011, 77, 285–291. [Google Scholar]
- Fujii, Y.; Hiradate, S. The Regional Institute-A critical survey of allelochemicals in action-The importance of total activity and the weed suppression equation. In Proceedings of the 4th World Congress on Allelopathy, Wagga Wagga, NSW, Australia, 21–26 August 2005; pp. 73–76. [Google Scholar]
- Js, M.; White, R. The Organic Chemistry of Museum Objects; Buttersworth: London, UK, 1994. [Google Scholar]
- Gilbert, K.G.; Maule, H.G.; Rudolph, B.; Lewis, M.; Vandenburg, H.; Sales, E.; Tozzi, S.; Cooke, D.T. Quantitative Analysis of Indigo and Indigo Precursors in Leaves of Isatis spp. and Polygonum tinctorium. Biotechnol. Prog. 2004, 20, 1289–1292. [Google Scholar] [CrossRef]
- Honda, G.; Tosirisuk, V.; Tabata, M. Isolation of an antidermatophytic, tryptanthrin, from indigo plants, Polygonum tinctorium and Isatis tinctoria. Planta Med. 1980, 38, 275–276. [Google Scholar] [CrossRef]
- Epstein, E.; NABORS, M.W.; Stowe, B.B. Origin of indigo of woad. Nature 1967, 216, 547–549. [Google Scholar] [CrossRef]
- Begum, K.; Shammi, M.; Hasan, N.; Asaduzzaman, M.d.; Appiah, K.S.; Fujii, Y. Potential allelopathic candidates for land use and possible sustainable weed management in south asian ecosystem. Sustainability 2019, 11, 2649. [Google Scholar] [CrossRef] [Green Version]
- Begum, K.; Shammi, M.; Hasan, N.; Appiah, K.S.; Fujii, Y. Evaluation of Potential Volatile Allelopathic Plants from Bangladesh, with Sapindus mukorossi as a Candidate Species. Agronomy 2020, 10, 49. [Google Scholar] [CrossRef] [Green Version]
- Baeyer, A. Ueber die reduction aromatischer verbindungen mittelst zinkstaub. Justus Liebigs Ann. Der Chem. 1866, 140, 295–296. [Google Scholar] [CrossRef] [Green Version]
- Fischer, E.; Jourdan, F. Ueber die hydrazine der brenztraubensäure. Ber. Der Dtsch. Chem. Ges. 1883, 16, 2241–2245. [Google Scholar] [CrossRef] [Green Version]
- Erb, M.; Veyrat, N.; Robert, C.A.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Sato, Y.; Ebina, T.; Yokoyama, C.; Takahasi, S.; Mito, Y.; Tanabe, H.; Nishiguchi, N.; Nagaoka, K. Separation of high purity indole from coal tar by high pressure crystallization. Fuel 1991, 70, 565–566. [Google Scholar] [CrossRef]
- Mackie, R.I.; Stroot, P.G.; Varel, V.H. Biochemical identification and biological origin of key odor components in livestock waste. J. Anim. Sci. 1998, 76, 1331–1342. [Google Scholar] [CrossRef]
- Frey, M.; Stettner, C.; Paré, P.W.; Schmelz, E.A.; Tumlinson, J.H.; Gierl, A. An herbivore elicitor activates the gene for indole emission in maize. Proc. Natl. Acad. Sci. USA 2000, 97, 14801–14806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Lee, J. Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 2010, 34, 426–444. [Google Scholar] [CrossRef]
- Fujii, Y. Strange Name of Plants-Does Skunkvine Really Stink? Kagaku Dojin: Kyoto, Japan, 2019. [Google Scholar]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose–response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jeffrey, C. Gynostemma Blume. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=114356 (accessed on 15 September 2019).
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching, and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy; Academic Press: Orlando, FL, USA, 2012; ISBN 978-0-08-092539-4. [Google Scholar]
- Bhadoria, P. Allelopathy: A Natural Way towards Weed Management. Am. J. Exp. Agric. 2011, 1, 7–20. [Google Scholar] [CrossRef]
- Waddington, J. Growth of barley, bromegrass and alfalfa in the greenhouse in soil containing rapeseed and wheat residues. Can. J. Plant Sci. 1978, 58, 241–248. [Google Scholar] [CrossRef]
- Cheema, Z.A.; Farooq, M.; Wahid, A. Allelopathy: Current Trends and Future Applications; Springer Science & Business Media: Berlin, Germany, 2012; ISBN 978-3-642-30595-5. [Google Scholar]
- Pérez-González, S. Relationship between Parental Blossom Season and Speed of Seed Germination in Peach. HortScience 1990, 25, 958–960. [Google Scholar] [CrossRef] [Green Version]
- Novak, N.; Novak, M.; Barić, K.; Šćepanović, M.; Ivić, D. Allelopathic potential of segetal and ruderal invasive alien plants. J. Cent. Eur. Agric. 2018, 19, 408–422. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.-P.; Li, Z.-L.; He, F.-F.; Wang, Y.-H.; Dong, M. Screening Allelochemical-Resistant Species of the Alien Invasive Mikania micrantha for Restoration in South China. PLoS ONE 2015, 10, e132967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putnam, A.R.; Tang, C.S. The Science of Allelopathy; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Leather, G.R.; Einhelling, F.A. Bioassays in the Study of Allelopathy. In The Science of Allelopathy; John Wiley and Sons: New York, NY, USA, 1986; pp. 133–145. [Google Scholar]
- Inderjit, O.M. Bioassays for Rice Allelopathy: Some Concerns. Allelopathy in Rice; Int. Rice Res. Inst. Pres: Manila, Philippines, 1998. [Google Scholar]
- Navarez, D.C.; Olofsdotter, M. Relay Seeding Technique for Screening Allelopathic Rice (Oryza sativa). In Proceedings of the 2nd International Weed Control Congress, Copenhagen, Denmark, 25–28 June 1996; pp. 1285–1290. [Google Scholar]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begum, K.; Motobayashi, T.; Hasan, N.; Appiah, K.S.; Shammi, M.; Fujii, Y. Indigo as a Plant Growth Inhibitory Chemical from the Fruit Pulp of Couroupita guianensis Aubl. Agronomy 2020, 10, 1388. https://doi.org/10.3390/agronomy10091388
Begum K, Motobayashi T, Hasan N, Appiah KS, Shammi M, Fujii Y. Indigo as a Plant Growth Inhibitory Chemical from the Fruit Pulp of Couroupita guianensis Aubl. Agronomy. 2020; 10(9):1388. https://doi.org/10.3390/agronomy10091388
Chicago/Turabian StyleBegum, Kohinoor, Takashi Motobayashi, Nazmul Hasan, Kwame Sarpong Appiah, Mashura Shammi, and Yoshiharu Fujii. 2020. "Indigo as a Plant Growth Inhibitory Chemical from the Fruit Pulp of Couroupita guianensis Aubl." Agronomy 10, no. 9: 1388. https://doi.org/10.3390/agronomy10091388
APA StyleBegum, K., Motobayashi, T., Hasan, N., Appiah, K. S., Shammi, M., & Fujii, Y. (2020). Indigo as a Plant Growth Inhibitory Chemical from the Fruit Pulp of Couroupita guianensis Aubl. Agronomy, 10(9), 1388. https://doi.org/10.3390/agronomy10091388







