Role of Biocontrol Agents in Management of Corm Rot of Saffron Caused by Fusarium oxysporum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Multiplication of Pathogen
2.2. Multiplication of Biocontrol Agents
2.3. In Vitro Evaluation of Biocontrol Agents
2.4. Management of Corm Rot of Saffron under Field Conditions
2.5. Dynamics of Fusarium oxysporum Propagules
2.6. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Evaluation
3.2. Disease Incidences of Corm Rot of Saffron
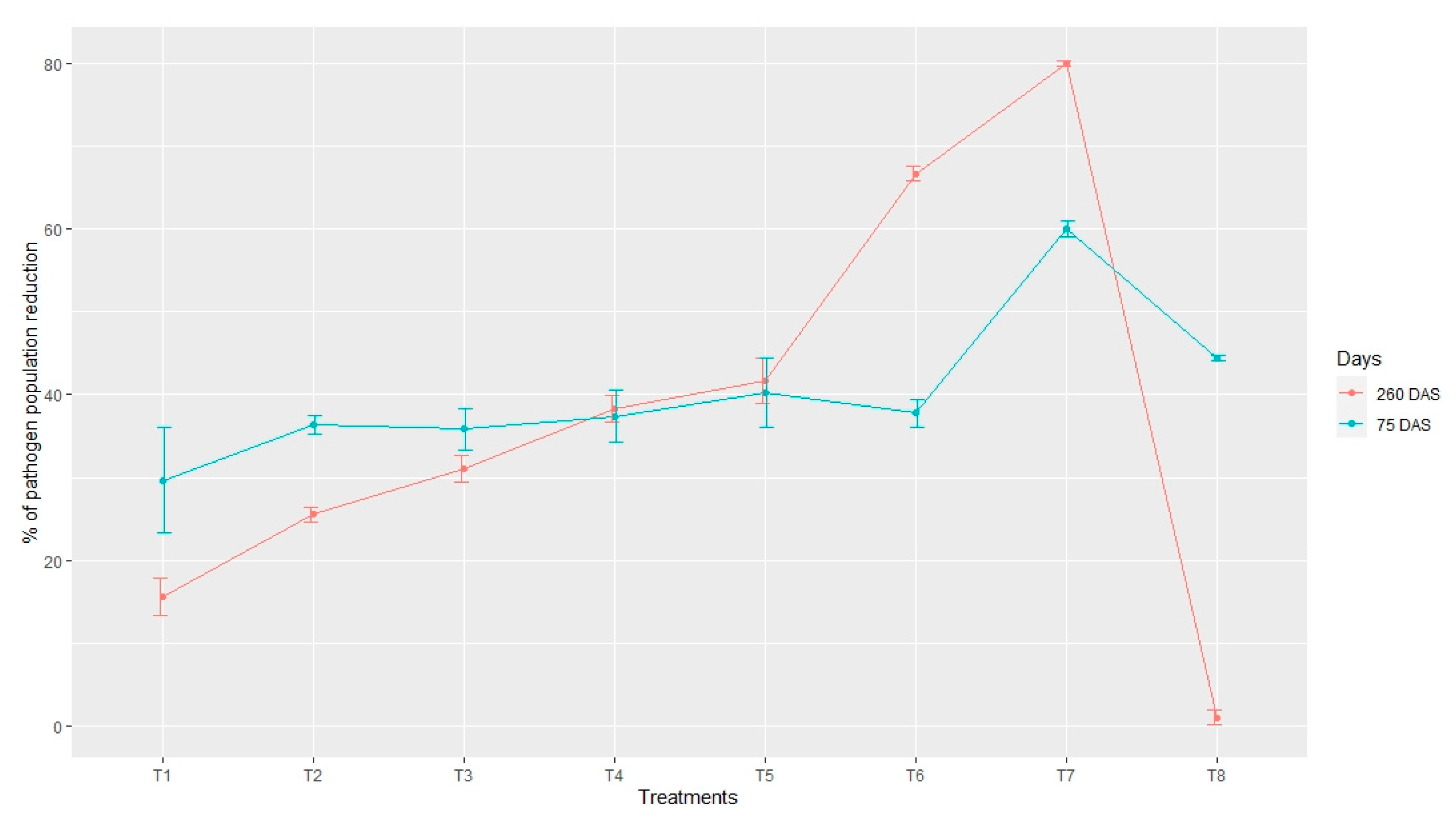
3.3. Dynamics of F. oxysporum Propagules
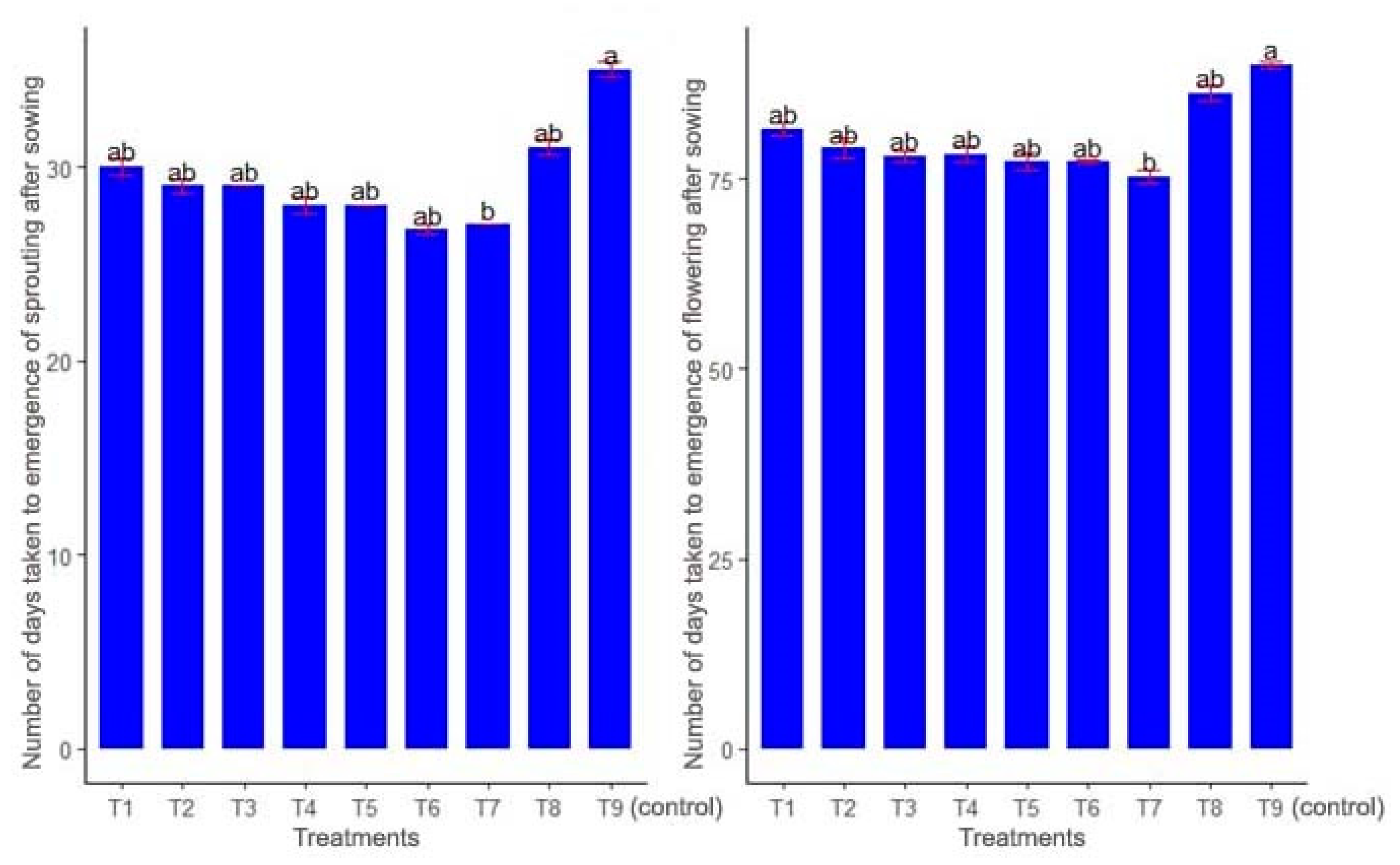
3.4. Growth Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dass, A.A.; Deshpande, S.V. Production and Productivity of Saffron in Jammu and Kashmir. Int. J. Trend Sci. Res. Dev. 2017, 2, 1205–1209. [Google Scholar] [CrossRef] [Green Version]
- Koocheki, A.; Khajeh-Hosseini, M. Saffron: Science, Technology and Health; Woodhead Publ.: Duxford, UK, 2020; p. 569. [Google Scholar]
- Caballero-Ortega, H.; Pereda-Miranda, R.; Abdullaev, F.I. HPLC quantification of major active components from 11 different saffron (Crocus sativus L.). Food Chem. 2007, 100, 1126–1131. [Google Scholar] [CrossRef]
- Razak, M.S.; Anwar, H.; Yee, F.C.; Kadir, M.R.A.; Nayan, N.H.M. A review on medicinal properties of saffron toward major diseases. J. Herbs Spices Med. Plants 2017, 23, 98–116. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Gholamnezhad, Z.; Khazdair, M.R.; Tavakol-Afshari, J. Antiinflammatory and immunomodulatory effects of saffron and its derivatives. In Saffron: Science, Technology and Health; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publ.: Duxford, UK, 2020; pp. 405–421. [Google Scholar]
- Dass, A.A.; Deshpande, S.V. Saffron Cultivation: Area, Production and Productivity in District Pulwama (J&K). New Man Inter. J. Multidiscip. Stud. 2018, 5, 45–48. [Google Scholar]
- Husaini, A.M. Challenges of climate change: Omics-based biology of saffron plants and organic agricultural biotechnology for sustainable saffron production. GM Crops Food 2014, 5, 97–105. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.; Kalha, C.S.; Razdan, V.K.; Dolly. Etiology and management of corm rot of saffron in Kishtwar district of Jammu and Kashmir, India. J. Mycol. Plant Pathol. 2011, 41, 361–366. [Google Scholar]
- Ahrazem, O.; Rubio-Moraga, A.; Castillo-Lopez, R.; Trapero-Mozos, A.; Gómez-Gómez, L. Crocus sativus pathogens and defence responses. In Functional Plant Science and Biotechnology; Special Issue: Saffron; Husaini, A.M., Ed.; Global Science Books, Ltd.: London, UK, 2010; pp. 81–90. [Google Scholar]
- Fiori, M. Identification and characterization of Burkholderia isolates obtained from bacterial rot of saffron (Crocus sativus L.) grown in Italy. Phytopathol. Mediterr. 2011, 50, 450–461. [Google Scholar]
- Yamamoto, W.; Omatsu, T.; Takami, K. Studies on the corm rots of Crocus sativus L. I. on saprophytic propagation of Sclerotinia gladioli and Fusarium oxysporum f. sp. gladioli on various plants and soils. Sci. Rep. Hyogo Univ. Agric. 1954, 1, 64–70. [Google Scholar]
- Zhang, T.; Huang, C.; Deng, C.; Zhang, Y.; Feng, Y.; Hu, J.; Wang, R.; Zhao, L.; Wang, Y.; Kai, G. First report of corm rot on saffron caused by Penicillium solitum in China. Plant Dis. 2019, 104, 579. [Google Scholar] [CrossRef]
- Shah, A.; Srivastava, K.K. Control of corm rot of saffron. Prog. Horti. 1984, 16, 141–143. [Google Scholar]
- Kalha, C.S.; Gupta, V.; Gupta, D.; Satya, P. First Report of Sclerotial Rot of Saffron caused by Sclerotium rolfsii in India. Plant Dis. 2007, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, C. Occurrence of Fusarium oxysporum f. sp. gladioli on saffron in Italy. Phyto. Medi. 1994, 33, 93–94. [Google Scholar]
- García-Jiménez, J.; Alfaro-García, A. Fusarium oxysporum Schlecht. as causal agent of a seed-borne disease of saffron (Crocus sativus L.). In Proceedings of the 7th Congress of the Mediterranean Phytopathological Union, Granada, Spain, 20–26 September 1987; p. 156. [Google Scholar]
- Aymani, I.E.; Qostal, S.; Mouden, N.; Selmaoui, K.; Touhami, A.O.; Benkirane, R.; Douira, A. Fungi associated with saffron (Crocus sativus) in Morocco. Plant Cell Biotechnol. Mol. Biol. 2019, 20, 1180–1188. [Google Scholar]
- Ghorbani, R.; Koocheki, A. Sustainable Cultivation of Saffron in Iran. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; pp. 169–202. [Google Scholar] [CrossRef]
- Kalha, C.S.; Gupta, V.; Gupta, D.; Mohiddin, F.A. Saffron cultivation in Kishtwar. In Saffron Production in Jammu and Kashmir; Nehvi, F.A., Wani, S.A., Eds.; Directorate of Extension Education, SKUAST-K: Shalimar, India, 2008; pp. 142–152. [Google Scholar]
- Nemati, Z.; Harpke, D.; Gemicioglu, A.; Kerndorff, H.; Blattner, F.R. Saffron (Crocus sativus) is an autotriploid that evolved in Attica (Greece) from wild Crocus cartwrightianus. Mol. Phylogenet. Evol. 2019, 136, 14–20. [Google Scholar] [CrossRef]
- Wani, M.A. Studies on Corm Rot of Saffron (Crocus sativus L.). Ph.D. Thesis, Sher-e-Kashmir University of Agricultural Sciences & Technology of Kashmir, Shalimar, India, 2004. [Google Scholar]
- Kumar, K. Biological Control of Corm Rot of Saffron. Master’s Thesis, Sher-e-Kashmir University of Agricultural Sciences & Technology of Jammu, Jammu, India, 2018. [Google Scholar]
- Jan, B.; Baba, S.A. Corm rot of saffron: Symptoms and biological management. EC Microbiol. 2018, 14, 1. [Google Scholar]
- Husaini, A.; Hassan, B.; Ghani, M.; Teixeira da Silva, J.; Kirmani, N. Saffron (Crocus sativus Kashmirianus) cultivation in Kashmir: Practices and problem. Funct. Plant Sci. Biotechnol. 2010, 4, 108–115. [Google Scholar]
- Gupta, R.; Vakhlu, J. Native Bacillus amyloliquefaciens W2 as a potential biocontrol for Fusarium oxysporum R1 causing corm rot of Crocus sativus. Eur. J. Plant Pathol. 2015, 143, 123–131. [Google Scholar] [CrossRef]
- Palmero, D.; Rubio-Moraga, A.; Galvez-Paron, L.; Nogueras, J.; Abato, C.; Gomez-Gomez, L.; Ahrazem, O. Pathogenicity and genetic diversity of Fusarium oxysporum isolates from corms of Crocus sativus. Ind. Crop. Prod. 2014, 61, 186–192. [Google Scholar] [CrossRef]
- Orr, R.; Nelson, P.N. Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Appl. Soil Ecol. 2018, 132, 20–33. [Google Scholar] [CrossRef]
- Stover, R.H. Fusarial Wilt (Panama Disease) of Bananas and Other Musa Species; Commonwealth Mycological Institute: Wallingford, UK, 1962; p. 117. [Google Scholar]
- Ploetz, R.C. Fusarium wilt of banana. Phytopathology 2015, 105, 1512–1521. [Google Scholar] [CrossRef] [Green Version]
- Cappelli, C.; Di-Minco, G. Results of a triennial study on saffron diseases in Abruzzi. Info. Fito 1999, 49, 27–32. [Google Scholar]
- Zaki, F.A.; Mantoo, M.A. Pests complex of saffron in Kashmir and their management. In Proceedings of the National Seminar Cum Workshop in the Development of Saffron in Kashmir; Zaki, F.A., Ed.; SKUAST: Shalimar, India, 2001; pp. 113–117. [Google Scholar]
- Francesconi, A. The rotting of bulbs of Crocus sativus L. by Penicillium cycloprium. Werstling. Ann. Bot. Univ. 1974, 32, 63–70. [Google Scholar]
- Abe, T. Studies on a new dry rot disease of the bulb of Crocus sativus L. caused by Fusarium bulbigenum Cke. et Mass var. blasticola (Rostr.) Wr. Trans. Tottori Soc. Agric. Sci. 1933, 4, 212–228. [Google Scholar]
- Shah, M.U.D.; Sagar, V.; Padder, B.A. In vitro evaluation of bioagents and fungitoxicants against Fusarium oxysporum and Fusarium solani causing corm rot of saffron (Crocus sativus) in Kashmir. Acta Hortic 2018, 1200, 125–132. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Cook, R.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; American Phytopathological Society: St. Paul, MN, USA, 1983; p. 539. [Google Scholar]
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant Health Instructor. 2006, 2, 1117–1142. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.; Paulitz, T.C. Theoretical basis for microbial interactions leading to biological control of soil-borne plant pathogens. In Principles and Practice of Managing Soil-Borne Plant Pathogens; Hall, R., Ed.; American Phytopathological Society: St. Paul, MN, USA, 1996; pp. 50–79. [Google Scholar]
- Aneja, K.R. Experiments in Microbiology, Plant Pathology and Biotechnology; New Age International: New Delhi, India, 2005; p. 632. [Google Scholar]
- Gupta, V. Project completion report of DST funded project. In Diversity Analysis of Pseudomonas Fluorescens and Its Utilization in Disease Suppression and Nutrient Management; Sher-e-Kashmir University of Agricultural Sciences & Technology of Jammu: Jammu, Kashmir, 2016; p. 25. [Google Scholar]
- Gupta, V. Project completion report of ICAR funded project. In Exploration of Plant Growth Promoting Rhizobacteria, Antagonistic and Plant Pathogenic Microbial Resources from High Altitude Agro-Climatic/Cropping Systems of Jammu and Kashmir State for Sustainable Agriculture; Sher-e-Kashmir University of Agricultural Sciences & Technology of Jammu: Jammu, Kashmir, 2018; p. 30. [Google Scholar]
- Jeyaseelan, E.C.; Tharmila, S.; Niranjan, K. Antagonistic activity of Trichoderma spp. and Bacillus spp. against Pythium aphanidermatum isolated from tomato damping off. Arch. Appl. Sci. Res. 2012, 4, 1623–1627. [Google Scholar]
- Grover, R.K.; Moore, J.D. Toxicometric studies of fungicides against brown rot organism, Sclerotinia fruiticola and S. laxa. Phytopathology 1962, 52, 876. [Google Scholar]
- Rodrigues, A.C.; Menezes, M. Identification and pathogenic characterization of endophytic Fusarium species from cowpea seeds. Mycopathologia 2005, 159, 79–85. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Pan, P.; Li, P.; Qi, Z.; Zhang, Q.; Shi, C.; Hao, W.; Zhou, B.; Lin, R. Bacillus altitudinis strain AMCC 101304: A novel potential biocontrol agent for potato common scab. Biocontrol Sci. Technol. 2019, 29, 1009–1022. [Google Scholar] [CrossRef]
- Mc Mullen, M.P.; Stack, R.W. Effects of Isolation techniques and media on the differential isolation of Fusarium species. Phytopathology 1983, 73, 458–462. [Google Scholar] [CrossRef]
- Komada, H. Development of selective media for quantitative isolation of Fusarium oxysporum from natural soil. Rev. Plant Prot. Res. 1975, 8, 114–125. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 10 June 2020).
- Mendiburu, F.D. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-3. 2015. Available online: http://CRAN.R-project.org/package=agricolae (accessed on 10 June 2020).
- Couillerot, O.; Prigent-Combaret, C.; Caballero-Mellado, J.; Moënne-Loccoz, Y. Pseudomonas fluorescens and closely related fluorescent pseudomonads as biocontrol agents of soil-borne phytopathogens. Lett. Appl. Microbiol. 2009, 48, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Elad, Y.; Chet, I.; Katan, J. Trichoderma harzianum: A biocontrol agent effective against Sclerotium rolfsii and Rhizoctonia solani. J. Phytopathol. 1980, 70, 119–121. [Google Scholar] [CrossRef] [Green Version]
- Poddar, R.K.; Singh, D.V.; Dubey, S.C. Integrated application of Trichoderma harzianum mutants and carbendazim to manage chickpea wilt (Fusarium oxysporum f. sp. ciceri). Indian J. Agric. Sci. 2004, 74, 346–348. [Google Scholar]
- Shanmugam, V.; Kanoujia, N.; Singh, M.; Singh, S.; Prasad, R. Biocontrol of vascular wilt and corm rot of gladiolus caused by Fusarium oxysporum f. sp. gladioli using plant growth promoting rhizobacterial mixture. Crop Prot. 2011, 30, 807–813. [Google Scholar] [CrossRef]
- Nene, Y.L.; Thapliya, P.N. Fungicides in Plant Disease Control; Oxford and IBH Publishing CO. Pvt. Ltd.: New Delhi, India, 1973; p. 325. [Google Scholar]
- Davidse, L.C. Benzimidazoles, fungicides mechanism of action and biological impact. Annu. Rev. Phytopathol. 1986, 24, 43–65. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, J.; Zhu, Y.; Duan, Y.; Zhou, M. Mechanism of action of the benzimidazole fungicide on Fusarium graminearum: Interfering with polymerization of monomeric tubulin but not polymerized microtubule. Phytopathology 2016, 106, 807–813. [Google Scholar] [CrossRef] [Green Version]
- Kamili, A.S.; Nehvi, F.A.; Trag, A.R. Saffron—A legendry crop of Kashmir Himalaya. J. Himalyan Ecol. Sustain. Dev. 2007, 2, 1–12. [Google Scholar]
- Ahmad, M.; Sagar, V.; Shah, M.U.D.; Padder, B.A.; Ahanger, F.A.; Sofi, T.A.; Mir, A.A.; Nabi, A.F.; Khan, M.A. Management of corm rot of saffron (Crocus sativus L.) in Kashmir, India. Acta Hortic 2018, 1200, 111–114. [Google Scholar] [CrossRef]
- Bourguet, D.; Guillemaud, T. The hidden and external costs of pesticide use. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Gewerbestr, Switzerland, 2016; pp. 35–120. [Google Scholar]
- Handelsman, J.; Stabb, E.V. Biocontrol of Soilborne Plant Pathogens. Plant Cell. 1996, 8, 1855–1869. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Varma, A. Microbial-mediated Induced Systemic Resistance in Plants; Springer: Singapore, 2016; p. 226. [Google Scholar]
- Ganeshan, G.; Kumar, A.M. Pseudomonas fluorescens: A potential bacterial antagonist to control plant diseases. J. Plant Interact. 2005, 1, 123–134. [Google Scholar] [CrossRef]
- Gupta, V.; Razdan, V.K.; Fatima, K.; Sharma, S.; Kumar, A. Pseudomonas-A potential biocontrol agent for managing plant diseases. In Microbial Antagonists: Their Role in Biological Control of Plant Diseases; Pandey, R.N., Chakraborty, B.N., Singh, D., Sharma, P., Eds.; Today & Tomorrow’s Printers and Publishers: New Delhi, India, 2017; pp. 399–430. [Google Scholar]
- Khan, M.R.; Ashraf, S.; Rasool, F.; Salati, K.M.; Mohiddin, F.A.; Haque, Z. Field performance of Trichoderma species against wilt disease complex of chickpea caused by Fusarium oxysporum f. sp. ciceri and Rhizoctonia solani. Turk. J. Agric. For. 2014, 38, 447–454. [Google Scholar] [CrossRef]
- Bhagat, S.; Bambawale, O.M.; Tripathi, A.K.; Ahmad, I.; Srivastava, R.C. Biological management of fusarial wilt of tomato by Trichoderma spp. in Andamans. Indian J. Hort. 2013, 70, 397–403. [Google Scholar]
- Nehvi, F.A.; Lone, A.; Khan, M.A.; Maqhdoomi, M.I. Comparative study on effect of nutrient management on growth and yield of saffron under temperate conditions of Kashmir. Acta Hortic. 2010, 850, 165–170. [Google Scholar] [CrossRef]
- Ambardar, S.; Singh, H.R.; Gowda, M.; Vakhlu, J. Comparative metagenomics reveal phylum level temporal and spatial changes in mycobiome of belowground parts of Crocus sativus. PLoS ONE 2016, 11, e0163300. [Google Scholar] [CrossRef] [Green Version]
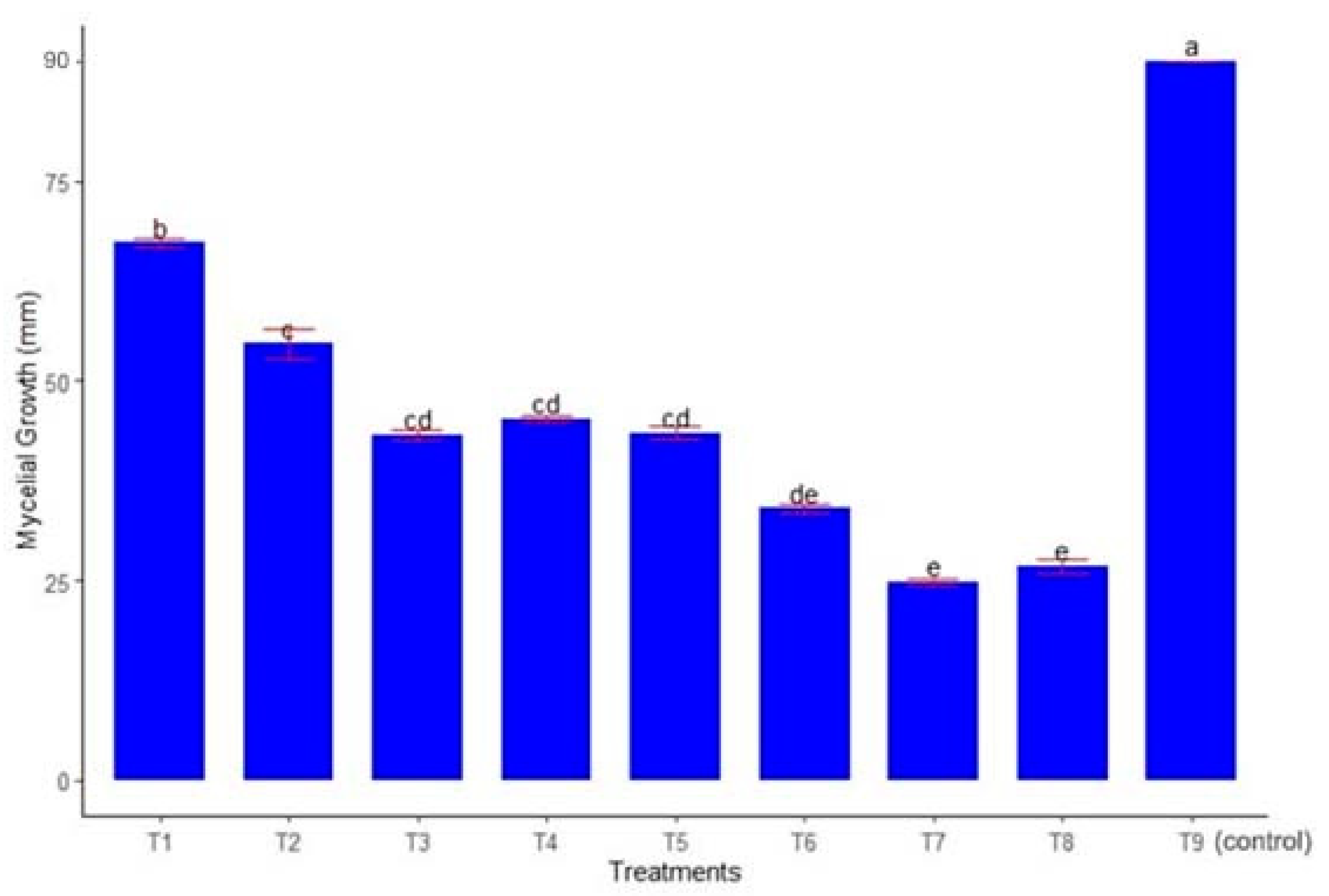
| Treatment | Disease Incidence (%) | Disease Reduction Efficacy (%) | ||
|---|---|---|---|---|
| 1st Year | 2nd Year | 1st Year | 2nd Year | |
| T1 | 40.27 ± 1.39 ab * | 71.22 ± 3.58 ab | 28.63 | 14.52 |
| T2 | 36.10 ± 3.20 ab | 66.11 ± 0.55 abc | 36.02 | 20.65 |
| T3 | 22.22 ± 0.01 ab | 47.49 ± 0.83 bcd | 60.62 | 42.99 |
| T4 | 37.50 ± 2.77 ab | 50.00 ± 2.04 bcd | 33.54 | 39.99 |
| T5 | 31.00 ± 5.55 ab | 40.27 ± 1.39 cd | 45.06 | 51.67 |
| T6 | 21.00 ± 2.78 ab | 34.50 ± 0.73 d | 62.78 | 58.88 |
| T7 | 12.50 ± 2.77 b | 26.13 ± 1.34 d | 77.84 | 68.63 |
| T8 | 9.72 ± 2.97 b | 43.12 ± 1.41 cd | 82.77 | 48.24 |
| T9 | 56.43 ± 10.84 a | 83.32 ± 2.26 a | ||
| Fusarium oxysporum (cfu/g) | |||
|---|---|---|---|
| Treatment | Before Sowing (×104) | 75 DAS (×103) | 250 DAS (×102) |
| T1 | 4.50 ± 0.408 a * | 3.16 ± 0.121 b | 3.80 ± 0.058 c |
| T2 | 4.70 ± 0.004 a | 2.99 ± 0.023 b | 3.50 ± 0.020 cd |
| T3 | 4.49 ± 0.298 a | 2.88 ± 0.054 bc | 3.10 ± 0.029 de |
| T4 | 4.70 ± 0.027 a | 2.94 ± 0.066 bc | 2.90 ± 0.027 e |
| T5 | 4.50 ± 0.064 a | 2.80 ± 0.007 bc | 2.80 ± 0.033 e |
| T6 | 4.80 ± 0.057 a | 2.86 ± 0.075 bc | 1.50 ± 0.001 f |
| T7 | 5.00 ± 0.043 a | 2.00 ± 0.013 c | 0.99 ± 0.002 f |
| T8 | 4.50 ± 0.001 a | 2.50 ± 0.009 bc | 4.30 ± 0.064 b |
| T9 | 4.50 ± 0.004 a | 5.49 ± 0.011 a | 5.99 ± 0.004 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, V.; Kumar, K.; Fatima, K.; Razdan, V.K.; Sharma, B.C.; Mahajan, V.; Rai, P.K.; Sharma, A.; Gupta, V.; Hassan, M.G.; et al. Role of Biocontrol Agents in Management of Corm Rot of Saffron Caused by Fusarium oxysporum. Agronomy 2020, 10, 1398. https://doi.org/10.3390/agronomy10091398
Gupta V, Kumar K, Fatima K, Razdan VK, Sharma BC, Mahajan V, Rai PK, Sharma A, Gupta V, Hassan MG, et al. Role of Biocontrol Agents in Management of Corm Rot of Saffron Caused by Fusarium oxysporum. Agronomy. 2020; 10(9):1398. https://doi.org/10.3390/agronomy10091398
Chicago/Turabian StyleGupta, Vishal, Krishna Kumar, Kausar Fatima, Vijay Kumar Razdan, Bhagwati Charan Sharma, Vidushi Mahajan, Pradeep Kumar Rai, Akash Sharma, Vikas Gupta, Mir Gulam Hassan, and et al. 2020. "Role of Biocontrol Agents in Management of Corm Rot of Saffron Caused by Fusarium oxysporum" Agronomy 10, no. 9: 1398. https://doi.org/10.3390/agronomy10091398
APA StyleGupta, V., Kumar, K., Fatima, K., Razdan, V. K., Sharma, B. C., Mahajan, V., Rai, P. K., Sharma, A., Gupta, V., Hassan, M. G., & Hussain, R. (2020). Role of Biocontrol Agents in Management of Corm Rot of Saffron Caused by Fusarium oxysporum. Agronomy, 10(9), 1398. https://doi.org/10.3390/agronomy10091398






