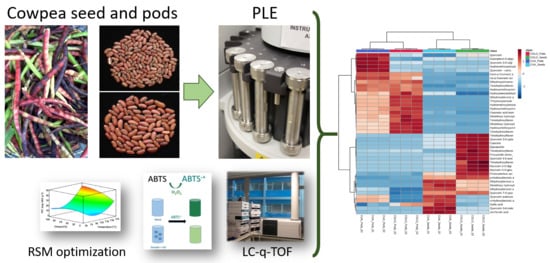
Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Maceration Extraction (ME)
2.3. Pressurized Liquid Extraction (PLE)
2.4. Experimental Design
2.5. In Vitro Experiments
2.5.1. Total Phenolic Content (TPC)
2.5.2. Trolox Equivalents of Antioxidant Capacity (TEAC)
2.5.3. DPPH Radical Scavenging Assay
2.5.4. In Vitro AChE Inhibition Assay
2.6. Liquid Chromatography-Tandem Mass Spectrometry (UHPLC-ESI-q-TOF-MS/MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Maceration Extraction (ME)
3.2. Pressurized Liquid Extraction (PLE)
3.3. Further Characterization of Optimum Extracts
3.3.1. Neuroprotective Activity
3.3.2. Phenolic Profiling Analysis by UHPLC-ESI-qTOF-MS/MS
3.3.3. Linking Phytochemical Composition and Functional Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deshpande, S.S. Food legumes in human nutrition: A personal perspective. Rev. Food Sci. Nutr. 1992, 32, 333–363. [Google Scholar] [CrossRef] [PubMed]
- Espin, J.C.; Garcia-Conesa, M.T.; Tomas-Barberán, F.A. Nutraceuticals: Facts and fiction. Phytochemistry 2007, 68, 2986–3008. [Google Scholar] [CrossRef] [PubMed]
- Avanza, M.V.; Acevedo, B.; Chaves, M.G.; Añón, M.C. Nutritional and anti nutritional components of four cowpea varieties under thermal treatments: Principal component analysis”. LWT Food Sci. Technol. 2013, 51, 148–157. [Google Scholar] [CrossRef]
- Awika, J.M.; Duodu, K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods 2017, 38, 686–697. [Google Scholar] [CrossRef]
- Onyelucheya, C.M.; Nwabanne, T.J. Onyelucheya, O.E.; Onuoha, O.E. Dilute acid hydrolysis of cowpea hulls: A kinetic study. Int. J. Adv. Sci. Eng. Inf. Technol. 2016, 6, 451–455. [Google Scholar] [CrossRef]
- Chikagwa-Malunga, S.K.; Adesogan, A.T.; Szabo, N.J.; Littell, R.C.; Phatak, S.C.; Kim, S.C.; Krueger, N.A. Nutritional characterization of Mucuna pruriens: 3. Effect of replacing soybean meal with Mucuna on intake, digestibility, N balance and microbial protein synthesis in sheep. Anim. Feed Sci. Technol. 2009, 148, 107–123. [Google Scholar] [CrossRef]
- Nguyen, V.T. (Ed.) Recovering Bioactive Compounds from Agricultural Wastes; Wiley-Blackwell, Ltd: Hoboken, NJ, USA, 2017; ISBN 978-1119168829. [Google Scholar]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef]
- Mendis, S.; Fukino, K.; Cameron, A.; Laing, R.; Filipe, A., Jr.; Khatib, O.; Leowski, J.; Ewen, M. The availability and affordability of selected essential drugs for chronic diseases in six low and middle-income countries. Bull. World Health Org. 2007, 85, 279–288. [Google Scholar] [CrossRef]
- Nothlings, U.; Schulze, M.B.; Wiekert, C. Intake of vegetable legumes and fruit and risk for all-cause cardiovascular and cancer mortality in a European diabetic population. J. Nutr. 2008, 138, 775–781. [Google Scholar] [CrossRef] [Green Version]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing. Age Ageing. 2006, 35, 336–338. [Google Scholar] [CrossRef] [Green Version]
- Senol, F.S.; Orhan, I.; Yilmaz, G.; Cicek, M.; Sener, B.; Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria, L. taxa from Turkey. Food Chem. Toxicol. 2010, 48, 781–788. [CrossRef] [PubMed]
- Karakaya, S.; Yılmaz, S.V.; Koca, M.; Demirci, B.; Sytar, O. Screening of non-alkaloid acetylcholinesterase inhibitors from extracts and essential oils of Anthriscus nemorosa (M.Bieb.) Spreng. (Apiaceae). S. Afr. J. Bot. 2019, 125, 261–269. [Google Scholar] [CrossRef]
- Zou, Y.-P.; Lu, Y.-H.; Wei, D.-Z. Protective effects of a flavonoidsrich extract of Hypericum perforatum L. against hydrogen peroxide-induced apoptosis in PC12 cells. Phytother. Res. 2010, 24, S6–S10. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Sánchez-Camargo, A.P.; Cifuentes, A.; Ibáñez, E. Plants, seaweeds, microalgae and food by-products as natural sources of functional ingredients obtained using pressurized liquid extraction and supercritical fluid extraction. TrAC Trends Anal. Chem. 2015, 71, 26–38. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Yang, L.; Dykes, L.; Awika, J. Proanthocyanidin profile of cowpea (Vigna unguiculata) reveals catechin-O-glucoside as the dominant compound. Food Chem. 2013, 139, 35–43. [Google Scholar] [CrossRef]
- Ojwang, L.O.; Banerjee, N.; Noratto, G.D.; Angel-Morales, G.; Hachibamb, T.; Awika, J.M.; Mertens-Talcott, S.U. Polyphenolic extracts from cowpea (Vigna unguiculata) protect colonic myofibroblasts (CCD18Co cells) from lipopolysaccharide (LPS)-induced inflammation-modulation of microRNA 126. Food Funct. 2015, 6, 146–154. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yanga, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorizationassay, Free Rad. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method toevaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, D.; Andies, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Shahwar, D.; Rehman, S.U.; Raza, M.A. Acetyl cholinesterase inhibition potential and antioxidant activities of ferulic acid isolated from Impatiens bicolor Linn. J. Med. Plants Res. 2010, 4, 260–266. [Google Scholar]
- Vijayalaxmi, S.S.; Jayalakshmi, K.; Sreeramulu, K. Polyphenols from different agricultural residues: Extraction, identification and their antioxidant properties. Food Sci. Technol. 2015, 52, 2761–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zia-Ul-Haq, M.; Ahmad, S.; Amarowicz, R.; De Feo, V. Antioxidant Activity of the Extracts of Some Cowpea (Vigna unguiculata (L) Walp.) Cultivars Commonly Consumed in Pakistan. Molecules 2013, 18, 2005–2017. [Google Scholar] [CrossRef] [PubMed]
- Zia-Ul-Haq, M.; Ahmad, S.; Iqbal, S.; Luthria, D.L.; Amarowicz, R. Antioxidant potential of lentil cultivars commonly consumed in Pakistan. Oxid. Comm. 2011, 34, 819–831. [Google Scholar]
- Zia-Ul-Haq, M.; Iqbal, S.; Ahmad, S.; Bhanger, M.I.; Wiczkowski, W.; Amarowicz, R. Antioxidant potential of desi chickpea varieties commonly consumed in Pakistan. J. Food Lipids 2008, 15, 326–342. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Zevallos, L.C. Screening methods to measure antioxidant activity of sorghum (Sorghum bicolor) and sorghum products. J. Agric. Food Chem. 2003, 51, 6657–6662. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszynska, A.; Barylko-Pikielna, N.; Shahidi, F. Extracts of polyphenolics from legume seeds—correlation between their total antioxidant activity, total phenolics content, tannins content and astringency. J. Food Lipids 2004, 11, 278–286. [Google Scholar] [CrossRef]
- Brazdauskas, T.; Montero, L.; Venskutonis, P.R.; Ibañez, E.; Herrero, M. Downstream valorization and comprehensive two-dimensional liquid chromatography-based chemical characterization of bioactives from black chokeberries (Aronia melanocarpa) pomace. J. Chromatogr. A 2016, 1468, 126–135. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; García-Cañas, V.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Comparative Study of Green Sub- and Supercritical Processes to Obtain Carnosic Acid and Carnosol-Enriched Rosemary Extracts with in Vitro Anti-Proliferative Activity on Colon Cancer Cells. Int. J. Mol. Sci. 2016, 17, 2046. [Google Scholar] [CrossRef] [Green Version]
- Song, J.E.; Song, J.H.; Cho, S.M.; Min, G.H.; Lee, J.S. Nutritional characteristics and physiological functionality of antidementia acetylcholinesterase inhibitor-containing methanol extract from Sorghum bicolor. Korean J. Food Nutr. 2010, 23, 226–232. [Google Scholar]
- Farhana, M.; Raisa, K.; Muhammad, A.; Muhammad, J. Potent AChE enzyme inhibition activity of Zizyphus oxyphylla: A new source of antioxidant compounds. Pak. J. Pharm. Sci. 2015, 28, 2053–2059. [Google Scholar]
- Ademiluyi, A.O.; Oboh, G.; Boligon, A.A.; Athayde, M.L. Dietary supplementation with fermented legumes modulate hyperglycemia and acetylcholinesterase activities in Streptozotocin-induced diabetes. Pathophysiology 2015, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, E.A.; Steenkamp, V. In vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from southern Africa. Asian Pac. J. Trop. Med. 2011, 4, 829–835. [Google Scholar] [CrossRef] [Green Version]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Rosmarinus officinalis L. essential oils from Spain: Composition, antioxidant capacity, lipoxygenase and acetylcholinesterase inhibitory capacities, and antimicrobial activities. Plant. Biosyst. 2018, 152, 1282–1292. [Google Scholar] [CrossRef]
- Khadri, A.; Neffati, M.; Smiti, S.; Falé, P.; Lino, R.L.; Serralheiro, M.L.M. Antioxidant, antiacetylcholinesterase and antimicrobialactivities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT Food Sci. Technol. 2010, 43, 331–336. [Google Scholar] [CrossRef]
- Oksana, S. Phenolic acids in the inflorescences of different varieties of buckwheat and their antioxidant activity. J. King Saud Univ. Sci. 2015, 27, 136–142. [Google Scholar]
- Araújo, T.A.S.; Alencar, N.L.; de Amorim, E.L.C.; de Albuquerque, U.P. A new approach to study medicinal plants with tannins and flavonoid contents from the local knowledge. J. Ethnopharmacol. 2008, 120, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef] [Green Version]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of Various Phenolic Acids and Flavonoid Derivatives for their Anticholinesterase Potential. Z. Naturfirsch. C J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Ouyang, M.-A.; Liu, X.; Wang, R.-Z. Acetylcholinesterase Inhibitory Activities of Flavonoids from the Leaves of Ginkgo biloba against Brown Planthopper. J. Chem. 2013, 2013, 645086. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, A.; Arumugam, M.; George, S. Combining in silico and in vitro approaches to evaluate the acetylcholinesterase inhibitory profile of some commercially available flavonoids in the management of Alzheimer’s disease. Int. J. Biol. Macromol. 2017, 95, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.d.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.d.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar]





| Samples | Solvent | Yield (%) | TPC (mg GAE g−1) | TEAC (mmol TE g−1) | EC50 (µg/mL) |
|---|---|---|---|---|---|
| COLO seed | ethanol | 1.67 ± 0.16 c | 17.70 ± 0.40 b | 0.198 ± 0.006 c | 93.86 ± 1.63 c |
| water | 5.55 ± 1.41 b | 15.32 ± 0.25 c | 0.460 ± 0.004 b | 268.92 ± 4.01 a | |
| water-2% v/v formic acid | 7.26 ± 0.81 b | 12.88 ± 0.37 d | 0.121 ± 0.002 c | 271.57 ± 3.99 a | |
| water-5% v/v formic acid | 7.36 ± 1.45 b | 17.40 ± 0.24 b | 0.115 ± 0.001 c | 191.53 ± 3.60 b | |
| acetone-1% v/v formic acid | 12.61 ± 1.13 a | 65.68 ± 0.31 a | 3.650 ± 0.267 a | 21.82 ± 0.46 d | |
| COLO pods | ethanol | 1.18 ± 0.12 b | 16.70 ± 0.49 d | 0.442 ± 0.010 c | 479.64 ± 8.34 b |
| water | 4.54 ± 0.95 a | 43.84 ± 0.26 a | 2.283 ± 0.028 a | 326.74 ± 4.87 c | |
| water-2% v/v formic acid | 5.45 ± 1.02 a | 30.20 ± 0.67 c | 0.086 ± 0.001 d | 566.12 ± 8.32 a | |
| water-5% v/v formic acid | 4.63 ± 0.83 a | 31.50 ± 0.41 b | 0.066 ± 0.005 d | 293.50 ± 5.52 d | |
| acetone-1% v/v formic acid | 5.44 ± 0.75 a | 44.35 ± 0.96 a | 1.327 ± 0.045 b | 142.45 ± 3.02 e |
| Exp. Run | Extraction Conditions | Response Variables | |||||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Ethanol (%) | Cycles | Yield (%) | TPC (mg GAE g−1) a | ABTS (mmol TE g−1) b | DPPH EC50 (µg mL−1) | |
| 1 | 50 | 0 | 2 | 17.70 ± 0.37 | 13.83 ± 0.15 | 0.207 ± 0.017 | 390.08 ±5.61 |
| 2 | 50 | 50 | 1 | 9.15 ± 0.19 | 22.29 ± 0.35 | 0.468 ± 0.015 | 88.26 ± 1.45 |
| 3 | 50 | 50 | 3 | 14.56 ± 0.30 | 24.86 ± 0.10 | 0.568 ± 0.033 | 73.46 ± 1.01 |
| 4 | 50 | 100 | 2 | 1.14 ± 0.02 | 13.26 ± 0.20 | 0.073 ± 0.002 | 429.10 ± 6.14 |
| 5 | 110 | 0 | 1 | 16.24 ± 0.34 | 9.79 ± 0.15 | 0.164 ± 0.012 | 389.43 ± 6.50 |
| 6 | 110 | 0 | 3 | 19.28 ± 0.40 | 12.73 ± 0.23 | 0.275 ± 0.010 | 283.06 ± 2.32 |
| 7 c | 110 | 50 | 2 | 20.70 ± 0.43 | 13.89 ± 0.21 | 0.324 ± 0.009 | 159.24 ± 1.90 |
| 8 c | 110 | 50 | 2 | 21.44 ± 0.44 | 11,33 ± 0.21 | 0.287 ± 0.011 | 160.40 ± 3.29 |
| 9 c | 110 | 50 | 2 | 20.69 ± 0.43 | 11.03 ± 0.25 | 0.282 ± 0.011 | 160.39 ± 3.28 |
| 10 c | 110 | 50 | 2 | 21.46 ± 0.44 | 12.83 ± 0.21 | 0.297 ± 0.012 | 159.74 ± 1.28 |
| 11 | 110 | 100 | 1 | 2.60 ± 0.05 | 12.73 ± 0.06 | 0.158 ± 0.002 | 318.09 ± 5.28 |
| 12 | 110 | 100 | 3 | 3.81 ± 0.08 | 13.09 ± 0.25 | 0.214 ± 0.007 | 307.72 ± 5.11 |
| 13 | 170 | 0 | 2 | 65.09 ± 1.35 | 19.86 ± 0.17 | 0.275 ± 0.010 | 150.55 ± 2.32 |
| 14 | 170 | 50 | 1 | 35.33 ± 0.73 | 42.76 ± 0.78 | 0.926 ± 0.055 | 49.31 ± 1.42 |
| 15 | 170 | 50 | 3 | 44.57 ± 0.92 | 33.63 ± 0.38 | 0.724 ± 0.040 | 64.22 ± 1.88 |
| 16 | 170 | 100 | 2 | 9.30 ± 0.19 | 40.36 ± 0.26 | 0.895 ± 0.031 | 67.01 ± 1.93 |
| Exp. Run | Extraction Conditions | Response Variables | |||||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Ethanol (%) | Cycles | Yield (%) | TPC (mg GAE g−1) a | ABTS (mmol TE g−1) b | DPPH EC50 (µg mL−1) c | |
| 1 | 50 | 0 | 2 | 8.97 ± 0.20 | 49.19 ± 0.42 | 1.126 ± 0.013 | 409.75 ± 7.19 |
| 2 | 50 | 50 | 1 | 5.35 ± 0.12 | 53.29 ± 0.35 | 2.067 ± 0.046 | 212.24 ± 4.54 |
| 3 | 50 | 50 | 3 | 7.99 ± 0.18 | 52.32 ± 0.95 | 1.565 ± 0.051 | 160.56 ± 1.62 |
| 4 | 50 | 100 | 2 | 1.76 ± 0.04 | 9.02 ± 0.32 | 0.209 ± 0.002 | 579.42 ± 7.24 |
| 5 | 110 | 0 | 1 | 13.11 ± 0.30 | 39.09 ± 0.45 | 0.758 ± 0.024 | 375.30 ± 5.93 |
| 6 | 110 | 0 | 3 | 24 ± 0.55 | 33.86 ± 0.26 | 0.588 ± 0.021 | 398.72 ± 5.90 |
| 7 c | 110 | 50 | 2 | 13.26 ± 0.30 | 50.32 ± 1.20 | 0.916 ± 0.032 | 187.62 ± 3.13 |
| 8 c | 110 | 50 | 2 | 13.54 ± 0.31 | 49.02 ± 0.49 | 0.865 ± 0.006 | 188.47 ± 3.37 |
| 9 c | 110 | 50 | 2 | 13.99 ± 0.32 | 46.06 ± 1.22 | 0.898 ± 0.046 | 193.59 ± 3.17 |
| 10 c | 110 | 50 | 2 | 13.46 ± 0.31 | 49.52 ± 0.72 | 0.906 ± 0.038 | 189.60 ± 3.91 |
| 11 | 110 | 100 | 1 | 1.51 ± 0.03 | 44.36 ± 0.53 | 0.996 ± 0.060 | 188.26 ± 3.41 |
| 12 | 110 | 100 | 3 | 3.54 ± 0.08 | 54.99 ± 0.51 | 1.363 ± 0.026 | 168.49 ± 2.33 |
| 13 | 170 | 0 | 2 | 34.01 ± 0.77 | 79.62 ± 0.65 | 0.796 ± 0.019 | 62.03 ± 1.33 |
| 14 | 170 | 50 | 1 | 21.36 ± 0.49 | 124.46 ± 2.52 | 3.037 ± 0.135 | 32.84 ± 0.50 |
| 15 | 170 | 50 | 3 | 29.56 ± 0.67 | 107.42 ± 2.05 | 2.442 ± 0.043 | 35.21 ± 0.58 |
| 16 | 170 | 100 | 2 | 7.86 ± 0.18 | 174.62 ± 1.58 | 4.549 ± 0.179 | 27.08 ± 0.43 |
| Extraction Conditions | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Ethanol (%) | Cycles | Yield (%) | TPC (mg GAE g−1) | ABTS (mmol TE g−1) | DPPH EC50 (µg mL−1) | ORAC (mmol TE g−1) | |
| Predicted | 170 | 50 | 1 | 36.55 | 42.01 | 0.93 | - | |
| Experimental (COLO seeds) | 170 | 50 | 1 | 36.02 ± 1.22 | 40.60 ± 0.27 | 0.805 ± 0.004 | 50.38 ± 2.48 | 82.34 ± 4.15 |
| % RSD | 3.33 | 0.64 | 0.43 | - | ||||
| Experimental (CUA seed) | 170 | 50 | 1 | 32.87 ± 1.23 | 22.69 ± 1.53 | 0.448 ± 0.006 | 86.14 ± 1.75 | 61.78 ± 3.74 |
| Extraction Conditions | Response Variables | |||||||
|---|---|---|---|---|---|---|---|---|
| Temp. (°C) | Ethanol (%) | Cycles | Yield (%) | TPC (mg GAE g−1) | ABTS (mmol TE g−1) | DPPH EC50 (µg mL−1) | ORAC (mmol TE g−1) | |
| Predicted | 170 | 68.93 | 2.99 | 22.86 | 136.36 | 3.505 | - | |
| Experimental (COLO pods) | 170 | 69 | 3 | 23.49 ± 0.93 | 116.97 ± 1.12 | 2.728 ± 0.112 | 33.48 ± 1.08 | 127.65 ± 6.32 |
| % RSD | 4.06 | 0.82 | 3.13 | - | ||||
| Experimental (CUA pods) | 170 | 69 | 3 | 25.38 ± 0.45 | 104.53 ± 1.03 | 2.227 ± 0.101 | 45.41 ± 0.96 | 118.51 ± 5.17 |
| Samples | % Inhibition AChE | mg Galantamine Equivalents Per g Extract * | Extracts Concentration (mg mL−1) |
|---|---|---|---|
| PLE-COLO seed | 34.965 ± 2.530 c | 0.211 ± 0.020 c | 1 |
| PLE-CUA seed | 31.050 ± 2.463 c | 0.180 ± 0.019 c | 1 |
| PLE-COLO pods | 51.630 ± 3.201 a | 0.689 ± 0.050 a | 0.5 |
| PLE-CUA pods | 40.381 ± 2.939 b | 0.473 ± 0.060 b | 0.5 |
| ME-COLO seed | 43.790 ± 0.622 c | 0.281 ± 0.004 c | 1 |
| ME-CUA seed | 40.820 ± 0.622 d | 0.257 ± 0.004 d | 1 |
| ME-COLO pods | 49.820 ± 0.254 a | 0.660 ± 0.004 a | 0.5 |
| ME-CUA pods | 47.435 ± 1.675 b | 0.638 ± 0.049 b | 0.5 |
| Peak No. | RT (min) | Tentative Identification | Molecular Formula | [M-H]- (m/z) (Calculated) | Error (ppm) | Ref.b | Absolute Area Values | |||
|---|---|---|---|---|---|---|---|---|---|---|
| COLO (Seeds) | COLO (Pods) | CUA (Seeds) | CUA (Pods) | |||||||
| P1 | 1.73 | Gallic acid a | C7H6O5 | 169.0142 | −6.2 | [4,16] | 20,381 | 51,733 | 10,471 | 35,212 |
| P2 | 2.03 | Quercetin-O-malonylglucoside | C24H22O15 | 549.0886 | −2.5 | [4] | 13,893 | 9429 | 18,691 | 14,766 |
| P3 | 2.23 | Dihydroxybenzoic acid I | C7H6O4 | 153.0193 | 3.0 | M, H | 686,613 | 158,144 | 356,820 | 202,548 |
| P4 | 2.49 | Protocatechuic acid a | C7H6O4 | 153.0193 | 1.0 | [4] | 108,051 | 52,113 | 20,091 | 55,719 |
| P5 | 2.82 | Dimethoxy hydroxybenzoic acid I | C9H10O5 | 197.0455 | −4.3 | M, H | 6365 | 338,001 | 7059 | 176,840 |
| P6 | 2.91 | Hydroxymethoxycinnamic acid I | C10H10O4 | 193.0506 | −7.9 | M, H | ND | 110,100 | ND | 56,353 |
| P7 | 3.14 | Hydroxymethoxycinnamic acid II | C10H10O4 | 193.0506 | −3.8 | M, H | ND | 21,422 | ND | 14,748 |
| P8 | 3.15 | p-Hydroxybenzoic acid a | C7H6O3 | 137.0244 | 3.5 | [4] | 1,690,090 | 592,038 | 399,005 | 452,670 |
| P9 | 3.19 | Dihydroxybenzoic acid II | C7H6O4 | 153.0193 | −5.4 | M, H | ND | 335,348 | ND | 203,317 |
| P10 | 3.19 | Trihydroxybenzoic acid | C7H6O5 | 169.0142 | −2.0 | M, H | ND | 808,002 | ND | 522,832 |
| P11 | 3.33 | Hydroxymethoxybenzoic acid I | C8H8O4 | 167.0350 | −7.0 | M, H | ND | 272,625 | ND | 180,460 |
| P12 | 3.38 | Tetrahydroxyflavone I | C15H10O6 | 285.0405 | 2.5 | M, H | 39,298 | 620 | 2304 | 3354 |
| P13 | 3.46 | Coumaric acid isomer | C9H8O3 | 163.0401 | −1.6 | M | ND | 136,874 | 2397 | 88,486 |
| P14 | 3.46 | Tetrahydroxyflavonol I | C15H10O7 | 301.0354 | 2.4 | M, H | 12,717 | ND | ND | ND |
| P15 | 3.58 | Catechin a | C15H14O6 | 289.0718 | 1.8 | [4,16] | 316,782 | ND | 20,473 | ND |
| P16 | 3.74 | Hydroxymethoxybenzoic acid II | C8H8O4 | 167.0350 | −7.0 | M, H | ND | ND | ND | 61,579 |
| P17 | 3.91 | Dimethoxy hydroxybenzoic acid II | C9H10O5 | 197.0455 | −6.8 | M, H | ND | 167,147 | ND | 98,377 |
| P18 | 3.99 | Hydroxybenzaldehyde | C7H6O2 | 121.0295 | 3.2 | M, H | 29,494 | 399,547 | 35,866 | 302,155 |
| P19 | 4.27 | Epicatechin a | C15H14O6 | 289.0718 | 3.9 | [4,16] | 148,640 | ND | 8782 | ND |
| P20 | 4.28 | Dihydroxycinnamic acid | C9H8O4 | 179.0350 | −5.4 | M, H | 6182 | 177,996 | 3480 | 165,317 |
| P21 | 4.37 | Dihydroxybenzoic acid III | C7H6O4 | 153.0193 | 5.0 | M, H | 38,756 | ND | 17,376 | 39,222 |
| P22 | 4.46 | Procyanidin dimer (B-type) | C30H26O12 | 577.1352 | 3.2 | [16,17] | 12,617 | ND | ND | ND |
| P23 | 4.46 | Myricetin-O-diglucoside | C27H30O18 | 641.1359 | 1.6 | [4] | 6173 | ND | ND | ND |
| P24 | 4.67 | Quercetin arabinosyldiglucoside | C32H38O21 | 757.1833 | −1.7 | [4] | 10,194 | 6964 | 12,460 | 49,487 |
| P25 | 4.70 | trans-p-Coumaric acid a | C9H8O3 | 163.0401 | −1.6 | [4] | 108,054 | 2,391,370 | 73,608 | 3,001,767 |
| P26 | 4.85 | Dimethoxy hydroxybenzoic acid III | C9H10O5 | 197.0455 | 3.3 | M,H | 52,771 | ND | 30,899 | ND |
| P27 | 4.93 | Quercetin-O-diglucoside | C27H30O17 | 625.1410 | 1.2 | [4,17] | ND | 63,890 | ND | 348,603 |
| P28 | 4.96 | cis-p-Coumaric acid | C9H8O3 | 163.0401 | −1.6 | [4] | ND | 201,089 | ND | 333,810 |
| P29 | 4.97 | Myricetin-O-glucoside | C21H20O13 | 479.0831 | 2.8 | [4] | 13,221 | ND | ND | ND |
| P30 | 5.25 | o-Hydroxybenzoic acid | C7H6O3 | 137.0244 | 4.9 | M, H | 40,566 | 16,008 | 41,841 | 180,368 |
| P31 | 5.26 | Kaempferol-O-diglucoside | C27H30O16 | 609.1461 | −1.9 | [4,17] | 4936 | 5198 | 1948 | 26,837 |
| P32 | 5.28 | cis-Ferulic acid a | C10H10O4 | 193.0506 | −0.1 | [4] | 25,911 | 18,107 | 31,672 | 17,318 |
| P33 | 5.41 | Quercetin rutinoside a | C27H30O16 | 609.1461 | −3.1 | [4] | 1979 | 3812 | 485 | 11,698 |
| P34 | 5.48 | Quercetin-O-glucoside | C21H20O12 | 463.0882 | 2.1 | [4] | 22,274 | 6358 | 6239 | 16,978 |
| P35 | 5.57 | Quercetin-O-galactoside | C21H20O12 | 463.0882 | 1.7 | [4] | 129,183 | 17,698 | 10,282 | 10,786 |
| P36 | 5.86 | Tetrahydroxyflavone II | C15H10O6 | 285.0405 | −4.0 | M | 2247 | 21,751 | ND | 23,389 |
| P37 | 5.91 | Tetrahydroxyflavonol II | C15H10O7 | 301.0354 | 4.7 | M | 39,460 | 14,011 | ND | 10,276 |
| P38 | 6.26 | Quercetin-O-acetylglucoside | C23H22O13 | 505.0988 | 2.0 | [4,17] | 32,405 | ND | 1190 | ND |
| P39 | 7.62 | Quercetin a | C15H10O7 | 301.0354 | 2.0 | [4] | 12,115 | 16,470 | ND | 52,235 |
| P40 | 7.63 | Tetrahydroxyflavone III | C15H10O6 | 285.0405 | −1.9 | M, H | ND | 128,925 | ND | 68,960 |
| P41 | 7.79 | Hydroxymethoxycinnamic acid III | C10H10O4 | 193.0506 | −5.3 | M, H | ND | 20,692 | ND | 21,232 |
| P42 | 8.24 | Tetrahydroxyflavonol III | C15H10O7 | 301.0354 | −3.2 | M, H | ND | 50,728 | ND | 25,997 |
| Sum of total phenolic compounds | 3,631,368 | 6,614,211 | 1,113,439 | 6,873,696 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations All authors have read and agreed to the published version of the manuscript.. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avanza, M.V.; Álvarez-Rivera, G.; Cifuentes, A.; Mendiola, J.A.; Ibáñez, E. Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies. Agronomy 2021, 11, 162. https://doi.org/10.3390/agronomy11010162
Avanza MV, Álvarez-Rivera G, Cifuentes A, Mendiola JA, Ibáñez E. Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies. Agronomy. 2021; 11(1):162. https://doi.org/10.3390/agronomy11010162
Chicago/Turabian StyleAvanza, M. Victoria, Gerardo Álvarez-Rivera, Alejandro Cifuentes, José A. Mendiola, and Elena Ibáñez. 2021. "Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies" Agronomy 11, no. 1: 162. https://doi.org/10.3390/agronomy11010162
APA StyleAvanza, M. V., Álvarez-Rivera, G., Cifuentes, A., Mendiola, J. A., & Ibáñez, E. (2021). Phytochemical and Functional Characterization of Phenolic Compounds from Cowpea (Vigna unguiculata (L.) Walp.) Obtained by Green Extraction Technologies. Agronomy, 11(1), 162. https://doi.org/10.3390/agronomy11010162