Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Soil Analysis
2.3. Plant Analysis
2.4. Statistical Analyses
3. Results
3.1. Total and Soil Pore Water Metal Concentrations
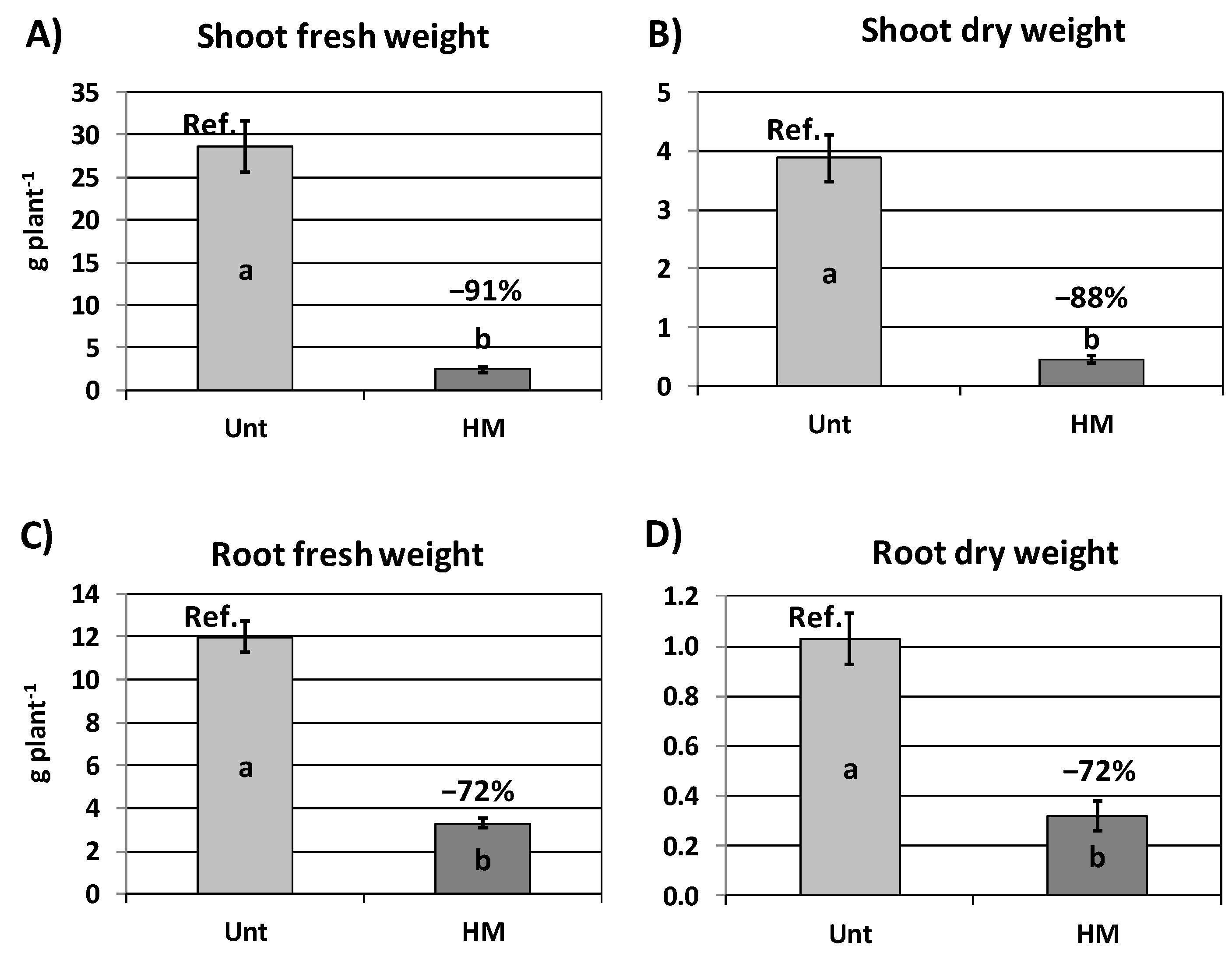
3.2. Shoot Metal Accumulation and Plant Biomass
3.3. Root Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garty, J.; Garty-Spitz, R.L. Lichens and particulate matter: Inter-relations and biomonitoring with Lichens. In Recent Advances in Lichenology; Upreti, D.K., Divakar, P.K., Shukla, V., Bajpai, R., Eds.; Springer: New Delhi, India, 2015; pp. 47–85. [Google Scholar]
- Ouabo, R.E.; Ogundiran, M.B.; Sangodoyin, A.Y.; Babalola, B.A. Ecological risk and human health implications of heavy metals contamination of surface soil in e-waste recycling sites in Douala, Cameroun. J. Health Pollut. 2019, 9, 190310. [Google Scholar] [CrossRef] [PubMed]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Sharifi Rad, J.; Sharifi Rad, M.; Teixeira da Silva, J.A. Effects of exogenous silicon on cadmium accumulation and biological responses of Nigella sativa L. (Black Cumin). Commun. Soil Sci. Plant Anal. 2014, 45, 1918–1933. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Jung, M.C.; Kim, K.H. Remediation of soils contaminated with heavy metals with an emphasis on immobilization technology. Environ. Geochem. Health 2018, 40, 927–953. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.S.; Adriano, D.C.; Naidu, R. Role of phosphorus in (im) mobilization and bioavailability of heavy metals in the soil-plant system. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2003; pp. 1–44. [Google Scholar]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, Z.; Liu, X.; Imran, S.; Peng, L.; Dai, R.; Deng, Y. Environmental materials for remediation of soils contaminated with lead and cadmium using maize (Zea mays L.) growth as a bioindicator. Environ. Sci. Pollut. Res. 2016, 23, 6168–6178. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils. Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef] [Green Version]
- Balkhair, K.S.; Ashraf, M.A. Field accumulation risks of heavy metals in soil and vegetable crop irrigated with sewage water in western region of Saudi Arabia. Saudi J. Biol. Sci. 2016, 23, S32–S44. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar]
- Bortey-Sam, N.; Nakayama, S.M.M.; Ikenaka, Y.; Akoto, O.; Yohannes, Y.B.; Baidoo, E.; Mizukawa, H.; Ishizuka, M. Human health risks from metals and change metalloid via consumption of food animals near Gold Mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. Environ. Saf. 2015, 111, 160–167. [Google Scholar] [CrossRef] [Green Version]
- Farid, M.; Shakoor, M.B.; Ehsan, S.; Ali, S.; Zubair, M.; Hani, M.S. Morphological, physiological and biochemical responses of different plant species to Cd stress. Int. J. Chem. Biochem. Sci. 2013, 3, 53–60. [Google Scholar]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ullah, E.; Wang, L.; Khan, I.; Shahzad, B. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean Soil Air Water 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Ghani, A. Toxic effects of heavy metals on plant growth and metal accumulation in maize (Zea mays L.). Iran J. Toxicol. 2010, 4, 325–334. [Google Scholar]
- Rizvi, A.; Khan, M.S. Heavy metal induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Lagriffoul, A.; Mocquot, B.; Mench, M.; Vangronsveld, J. Cadmium toxicity effects on growth, mineral and chlorophyll contents, and activities of stress related enzymes in young maize plants (Zea mays L.). Plant Soil 1998, 200, 241–250. [Google Scholar] [CrossRef]
- Ci, D.; Jiang, D.; Dai, T.; Jing, Q.; Cao, W. Effects of cadmium on plant growth and physiological traits in contrast wheat recombinant inbred lines differing in cadmium tolerance. Chemosphere 2009, 77, 1620–1625. [Google Scholar] [CrossRef]
- Chen, L.M.; Lin, C.C.; Kao, C.H. Copper toxicity in rice seedlings: Changes in antioxidative enzyme activities, H2O2 level, and cell wall peroxidase activity in roots. Bot. Bull. Acad. Sin. 2000, 41, 99–103. [Google Scholar]
- Tsonev, T.; Lidon, F.J.C. Zinc in plants—An overview. Emir. J. Food Agric. 2012, 24, 322–333. [Google Scholar]
- USEPA 1995a. EPA method 3051, microwave assisted acid digestion of sediments; sludges; soils; and oils. In Test Methods for Evaluating Solid Waste, 3rd ed.; US Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- USEPA 1995b. EPA Method 3052, microwave assisted acid digestion of siliceous and organically based matrices. In Test Methods for Evaluating Solid Waste, 3rd ed.; US Environmental Protection Agency: Washington, DC, USA, 1995. [Google Scholar]
- do Rosário, G.; Oliveira, M.; Van Noordwijk, M.; Gaze, S.R.; Brouwer, G.; Bona, S.; Mosca, G.; Hairiah, K. Auger sampling, ingrowth cores and pinboard methods. In Root Methods; Springer: Berlin/Heidelberg, Germany, 2000; pp. 175–210. [Google Scholar]
- Chahal, V.; Chand, P.; Nagpal, A.; Kaur, K.J.; Pakad, Y.B. Evaluation of heavy metals contamination and its genotoxicity in agricultural soil of Amritsar, Punjab, India. Int. J. Res. Chem. Environ. 2014, 4, 20–28. [Google Scholar]
- Lone, M.I.; He, Z.; Stoffella, P.J.; Yang, X. Phytoremediation of heavy-metal-polluted soils and water: Progresses and perspectives. J. Zhejiang Univ. Sci. B 2008, 9, 210–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Akhionbare, S.M.O.; Ebe, T.E.; Akhionbare, W.N.; Ac-Chukwuocha, N. Heavy metal uptake by corn (Zea mays) grown on contaminated soil. Res. J. Agric. Biol. Sci. 2010, 6, 993–997. [Google Scholar]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Bindraban, P.S. Synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant 2017, 48, 1895–1920. [Google Scholar] [CrossRef] [Green Version]
- Lombi, E.; Gerzabek, M.H.; Horak, O. Mobility of heavy metals in soil and their uptake by sunflowers grown at different contamination levels. Agronomie 1998, 18, 361–371. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, F.; Fernandes, D.M.; Mota, P.R.D.; Villas Boas, R.L. Electrical conductivity and pH of the substrate solution in gerbera cultivars under fertigation. Hortic. Bras. 2013, 31, 356–360. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Dong, Y.; Li, H.; Wang, Q. Effect of elemental sulphur on solubility of soil heavy metals and their uptake by maize. Environ. Int. 2004, 30, 323–328. [Google Scholar] [CrossRef]
- McIntyre, T. Phytoremediation of heavy metals from soils. Adv. Biochem. Eng. Biotechnol. 2003, 78, 97–123. [Google Scholar]
- Mojiri, A.; Jalalian, A. Relationship between growth of Nitraria schoberi and some soil properties. J. Anim. Plant Sci. 2011, 21, 246–250. [Google Scholar]
- Yaqvob, M.; Golale, A.; Masoud, S.; Hamid, R.G. Influence of different concentration of heavy metals on the seed germination and growth of tomato. Afr. J. Environ. Sci. Technol. 2011, 5, 420–426. [Google Scholar]
- Lu, Y.; Yao, H.; Shan, D.; Jiang, Y.; Zhang, S.; Yang, J. Heavy metal residues in soil and accumulation in maize at long-term wastewater irrigation area in Tongliao, China. J. Chem. 2015, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vamerali, T.; Bandiera, M.; Coletto, L.; Zanetti, F.; Dickinson, M.N.; Mosca, G. Phytoremediation trials on metal- and arsenic-contaminated pyrite wastes (Torviscosa, Italy). Environ. Pollut. 2009, 157, 884–891. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Lucchini, P.; Dickinson, N.M.; Mosca, G. Long-term phytomanagement of metal-contaminated land with field crops: Integrated remediation and biofortification. Eur. J. Agron. 2014, 53, 56–66. [Google Scholar] [CrossRef]
- Mahmood, S.; Hussain, A.; Saeed, Z.; Athar, M. Germination and seedling growth of corn (Zea mays L.) under varying levels of copper and zinc. Int. J. Environ. Sci. Technol. 2005, 2, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Stoyanova, Z.; Doncheva, S. The effect of zinc supply and succinate treatment on plant growth and mineral uptake in pea plants. Braz. J. Plant Physiol. 2002, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Rout, G.R.; Das, P. Effect of metal toxicity on plant growth and metabolism: I. Zinc. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 873–884. [Google Scholar]
- Chang, H.B.; Lin, C.W.; Huang, H.J. Zinc-induced cell death in rice (Oryza sativa L.) roots. Plant Growth Regul. 2005, 46, 261–266. [Google Scholar] [CrossRef]
- Fukao, Y.; Ferjani, A.; Tomioka, R.; Nagasaki, N.; Kurata, R.; Nishimori, Y.; Maeshima, M. iTRAQ analysis reveals mechanisms of growth defects due to excess zinc in Arabidopsis. Plant Physiol. 2011, 155, 1893–1907. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, K.N.; Yet, Z.R.; Som, A.M.; Razali, N.; Rahaizah, N.A.M.; Othman, E.N.; Burok, N.A.; Mohd, Y.; Othman, R.; Fazli, T.; et al. Heavy metal concentration (Pb, Cu, Fe, Zn, Ni) in plant parts of Zea Mays L. cultivated in agricultural area near Alor Gajah, Melaka. Am. J. Environ. Eng. 2015, 5, 8–12. [Google Scholar]
- Hough, R.L.; Hough, R.L.; Young, S.D.; Crout, N.M.J. Modelling of Cd, Cu, Ni, Pb and Zn uptake, by winter wheat and forage maize, from a sewage disposal farm. Soil Use Manag. 2003, 19, 19–27. [Google Scholar] [CrossRef]
- He, S.Y.; Yang, X.E.; He, Z.; Baligar, V.C. Morphological and physiological responses of plants to cadmium toxicity: A review. Pedosphere 2017, 27, 421–438. [Google Scholar] [CrossRef]
- Sandalio, L.M.; Dalurzo, H.C.; Gómez, M.; Romero-Puertas, M.C.; Del Río, L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001, 52, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naz, A.; Asim, M.; Ahmad, S.S.; Youssaf, S.; Muhammad, S. Toxicity and bioaccumulation of heavy metals in spinach seedlings grown on freshly contaminated soil. Pak. J. Bot. 2013, 45, 501–508. [Google Scholar]
- Peralta-Videa, J.R.; Gardea-Torresdey, J.L.; Gomez, E.; Tiermann, K.J.; Parsons, J.G.; Carrillo, G. Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfafa growth and heavy metal uptake. Environ. Pollut. 2002, 119, 291–301. [Google Scholar] [CrossRef]
- Yuan, M.; He, H.; Xiao, L.; Zhong, T.; Liu, H.; Li, S.; Deng, P.; Ye, Z.; Jing, Y. Enhancement of Cd phytoextraction by two Amaranthus species with endophytic Rahnella sp. JN27. Chemosphere 2014, 103, 99–104. [Google Scholar] [CrossRef]
- Alkorta, I.; Hernández-Allica, J.; Becerril, J.M.; Amezaga, I.; Albizu, I.; Garbisu, C. Recent findings on the phytoremediation of soils contaminated with environmentally toxic heavy metals and metalloids such as zinc, cadmium, lead, and arsenic. Rev. Environ. Sci. Biotechnol. 2004, 3, 71–90. [Google Scholar] [CrossRef]
- Vogel-Mikus, K.; Pongrac, P.; Kump, P.; Necemer, M.; Regvar, M. Colonisation of a Zn, Cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous arbuscular mycorrhizal fungal mixture induces changes in heavy metal and nutrient uptake. Environ. Pollut. 2006, 139, 362–371. [Google Scholar] [CrossRef]
- Liu, J.; Qian, M.; Cai, G.; Yang, J.; Zhu, Q. Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J. Hazard. Mater. 2006, 143, 443–447. [Google Scholar] [CrossRef]
- Bandiera, M.; Dal Cortivo, C.; Barion, G.; Mosca, G.; Vamerali, T. Phytoremediation opportunities with alimurgic species in metal-contaminated environments. Sustainability 2016, 8, 357. [Google Scholar] [CrossRef] [Green Version]
- MacFarlane, G.R.; Burchett, M.D. Toxicity, growth and accumulation relationships of copper, lead and zinc in the grey mangrove Avicennia marina (Forsk.) Vierh. Mar. Environ. Res. 2002, 54, 65–84. [Google Scholar] [CrossRef]
- Sarwar, N.; Saifullah Malhi, S.S.; Zia, M.H.; Naeem, A.; Bibia, S.; Farida, G. Role of mineral nutrition in minimizing cadmium accumulation by plants. J. Sci. Food Agric. 2010, 90, 925–937. [Google Scholar] [CrossRef]
- Nan, Z.; Li, J.; Zhang, J.; Cheng, G. Cadmium and zinc interactions and their transfer in soil-crop system under actual field conditions. Sci. Total Environ. 2002, 285, 187–195. [Google Scholar] [CrossRef]
- Wang, F.Y.; Lin, X.G.; Yin, R. Effect of arbuscular mycorrhizal fungal inoculation on heavy metal accumulation of maize grown in a naturally contaminated soil. Int. J. Phytoremediat. 2007, 9, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Munjral, N.; Gupta, A.K.; Kaur, N. Cadmium induced changes in carbohydrate status and enzymes of carbohydrate metabolism, glycolysis and pentose phosphate pathway in pea. Environ. Exp. Bot. 2007, 61, 167–174. [Google Scholar] [CrossRef]
- Auda, A.M.; Ali, E.S. Cadmium and Zinc toxicity effects on growth and Mineral nutrients of carrot (Daucus carota). Pak. J. Bot. 2010, 42, 341–351. [Google Scholar]
- Naz, A.; Khan, S.; Qasim, M.; Khalid, S.; Muhammad, S.; Tariq, M. Metals toxicity and its bioaccumulation in purslane seedlings grown in controlled environment. Nat. Sci. 2013, 5, 573–579. [Google Scholar] [CrossRef] [Green Version]
- Bashmakov, D.I.; Lukatkin, A.S.; Revin, V.V.; Duchovskis, P.; Brazaitytë, A.; Baranauskis, K. Growth of maize seedlings affected by different concentrations of heavy metals. Ekologija 2005, 3, 22–27. [Google Scholar]
- Fahr, M.; Laplaze, L.; Bendaou, N.; Hocher, V.; El Mzibri, M.; Didier Bogusz, D.; Smouni, A. Effect of lead on root growth. Front. Plant Sci. 2013, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, B.; Bolan, N.S.; Naidu, R. Review: Rhizosphere-induced heavy metal (loid) transformation in relation to bioavailability and remediation. J. Soil Sci. Plant Nutr. 2015, 15, 524–548. [Google Scholar]
- Redjala, T.; Zelko, I.; Sterckeman, T.; Legué, V.; Lux, A. Relationship between root structure and root cadmium uptake in maize. Environ. Exp. Bot. 2011, 71, 241–248. [Google Scholar] [CrossRef]
- Vaculík, M.; Landberg, T.; Greger, M.; Luxová, M.; Stoláriková, M.; Lux, A. Silicon modifies root anatomy, and uptake and subcellular distribution of cadmium in young maize plants. Ann. Bot. 2012, 110, 433–443. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, T.; Islam, K.R.; Muhammad, S. Toxic effects of heavy metals on early growth and tolerance of cereal crops. Pak. J. Bot. 2007, 39, 451–462. [Google Scholar]
- Bandiera, M.; Mosca, G.; Vamerali, T. Phytotoxicity and metal leaching in EDDS-assisted phytoextraction from pyrite wastes with Ethiopian mustard and fodder radish. Plant Biosyst. 2010, 144, 490–498. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochicchio, R.; Sofo, A.; Terzano, R.; Terzano, R.; Gattullo, C.E.; Amato, M.; Scopa, A. Root architecture and morphometric analysis of Arabidopsis thaliana grown in Cd/Cu/Zn-gradient agar dishes: A new screening technique for studying plant response to metals. Plant Physiol. Biochem. 2015, 91, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Vamerali, T.; Bandiera, M.; Mosca, G. In situ phytoremediation of arsenic- and metal-polluted pyrite waste with field crops: Effects of soil management. Chemosphere 2011, 83, 1241–1248. [Google Scholar] [CrossRef]


| Unit | IGV 1 | Unt | HM | % var. | ||
|---|---|---|---|---|---|---|
| Element | Ca | g kg−1 | 62.9 ± 0.94 (a) | 62.9 ± 1.68 (a) | −0.10 | |
| Cd | mg kg−1 | 2 | 0.39 ± 0.017 (b) | 16.8 ± 0.198 (a) | +4197.00 | |
| Co | mg kg−1 | 20 | 10.3 ± 0.217 (b) | 170 ± 1.82 (a) | +1549.00 | |
| Cu | mg kg−1 | 120 | 29.5 ± 0.28 (b) | 799 ± 0.59 (a) | +2605.00 | |
| K | g kg−1 | 7.18 ± 0.23 (a) | 7.06 ± 0.15 (a) | −1.67 | ||
| Mg | g kg−1 | 31.3 ± 0.44 (a) | 30.6 ± 0.76 (a) | −2.00 | ||
| Ni | mg kg−1 | 24.4 ± 0.49 (a) | 26.2 ± 0.03 (a) | +7.00 | ||
| P | mg kg−1 | 799 ± 11.8 (a) | 703 ± 6.60 (a) | −12.00 | ||
| Pb | mg kg−1 | 100 | 19.6 ± 0.12 (b) | 906 ± 6.59 (a) | +4523.00 | |
| S | g kg−1 | 0.24 ± 0.006 (b) | 1.13 ± 0.035 (a) | +371.00 | ||
| Zn | mg kg−1 | 150 | 87.8 ± 0.56 (b) | 1056 ± 36.90 (a) | +1103.00 | |
| Parameter | pH | 8.18 ± 0.08 (a) | 7.19 ± 0.06 (b) | −12.00 | ||
| EC | mS cm−1 | 0.485 ± 0.008 (b) | 2.62 ± 0.017 (a) | +440.00 |
| Element | Metal Concentration in Soil Pore Water (mg L−1) | ||
|---|---|---|---|
| Unt | HM | % var. | |
| Ca | 132.07 ± 21.230 (b) | 542.730 ± 17.59 (a) | +311 |
| Cd | b.d.l. 1 | 0.042 ± 0.003 (a) | — |
| Co | 0.0004 ± 0.00007 (b) | 0.620 ± 0.093 (a) | +154,900 |
| Cu | 0.02 ± 0.002 (b) | 0.131 ± 0.008 (a) | +555 |
| K | 1.72 ± 0.291 (a) | 12.170 ± 1.229 (b) | +606 |
| Mg | 30.25 ± 3.732 (b) | 88.360 ± 8.839 (a) | +192 |
| Ni | 0.005 ± 0.0006 (a) | 0.004 ± 0.0005 (b) | −20 |
| P | 0.032 ± 0.016 (a) | 0.107 ± 0.034 (a) | +234 |
| Pb | b.d.l. | 0.0019 ± 0.001 (a) | — |
| S | 55.03 ± 4.348 (b) | 482.300 ± 21.220 (a) | +776 |
| Zn | 0.022 ± 0.011 (b) | 1.834 ± 0.447 (a) | +8236 |
| Element | Shoot Metal Concentration (mg kg−1) | ||
|---|---|---|---|
| Unt | HM | % var. | |
| Ca | 9601.000 ± 836.000 (b) | 19,182.000 ± 2438.000 (a) | +100 |
| Cd | 0.337 ± 0.169 (b) | 0.897 ± 0.448 (a) | +166 |
| Co | 0.036 ± 0.018 (b) | 9.754 ± 4.877 (a) | +26,994 |
| Cu | 0.706 ± 0.353 (b) | 23.700 ± 11.850 (a) | +3257 |
| K | 2514.000 ± 1250.000 (a) | 1600.000 ± 800.100 (b) | −36 |
| Mg | 614.500 ± 307.3 (b) | 2506.000 ± 1253.000 (a) | +308 |
| Ni | 0.991 ± 0.496 (a) | 0.124 ± 0.062 (b) | −87 |
| P | 295.900 ± 148.000 (a) | 331.900 ± 165.900 (a) | +12 |
| Pb | 0.032 ± 0.016 (b) | 1.851 ± 0.925 (a) | +5684 |
| S | 429.500 ± 214.700 (b) | 2929.000 ± 1465.000 (a) | +582 |
| Zn | 2.762 ± 1.380 (b) | 75.800 ± 37.900 (a) | +2644 |
| Ca | Cd | Co | Cu | K | Mg | Ni | P | Pb | S | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca | 0.94 ** | 0.98 ** | 0.97 ** | −0.74 * | 0.97 ** | −0.75 ns | 0.15 ns | 0.82 * | 0.97 ** | 0.92 ** | |
| Cd | 0.94 ** | 0.96 ** | 0.89 ** | −0.80 * | 0.97 ** | −0.62 ns | 0.02 ns | 0.66 ns | 0.92 ** | 0.86 ** | |
| Co | 0.98 ** | 0.96 ** | 0.98 ** | −0.71 * | 0.97 ** | −0.84 * | 0.17 ns | 0.78 * | 0.97 ** | 0.89 ** | |
| Cu | 0.97 ** | 0.89 ** | 0.98 ** | −0.62 ns | 0.91 ** | −0.82 * | 0.21 ns | 0.84 ** | 0.97 ** | 0.90 ** | |
| K | −0.74 * | −0.80 * | −0.71 * | −0.62 ns | −0.83 * | 0.70 ns | 0.48 ns | −0.69 ns | −0.79 * | −0.78 * | |
| Mg | 0.97 ** | 0.97 ** | 0.97 ** | 0.91 ** | −0.83 * | −0.82 * | 0.05 ns | 0.74 * | 0.96 ** | 0.92 ** | |
| Ni | −0.75 ns | −0.62 ns | −0.84 * | −0.82 * | 0.70 ns | −0.82 * | 0.27 ns | −0.70 ns | −0.83 * | −0.83 * | |
| P | 0.15 ns | 0.02 ns | 0.17 ns | 0.21 ns | 0.48 ns | 0.05 ns | 0.27 ns | −0.10 ns | 0.02 ns | −0.08 ns | |
| Pb | 0.82 * | 0.66 ns | 0.78 * | 0.84 ** | −0.69 ns | 0.74 * | −0.70 ns | −0.10 ns | 0.86 ** | 0.79 * | |
| S | 0.97 ** | 0.92 ** | 0.97 ** | 0.97 ** | −0.79 * | 0.96 ** | −0.83 * | 0.02 ns | 0.86 ** | 0.96 ** | |
| Zn | 0.92 ** | 0.86 ** | 0.89 ** | 0.90 ** | −0.78 * | 0.92 ** | −0.83 * | −0.08 ns | 0.79 * | 0.96 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romdhane, L.; Panozzo, A.; Radhouane, L.; Dal Cortivo, C.; Barion, G.; Vamerali, T. Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination. Agronomy 2021, 11, 178. https://doi.org/10.3390/agronomy11010178
Romdhane L, Panozzo A, Radhouane L, Dal Cortivo C, Barion G, Vamerali T. Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination. Agronomy. 2021; 11(1):178. https://doi.org/10.3390/agronomy11010178
Chicago/Turabian StyleRomdhane, Leila, Anna Panozzo, Leila Radhouane, Cristian Dal Cortivo, Giuseppe Barion, and Teofilo Vamerali. 2021. "Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination" Agronomy 11, no. 1: 178. https://doi.org/10.3390/agronomy11010178
APA StyleRomdhane, L., Panozzo, A., Radhouane, L., Dal Cortivo, C., Barion, G., & Vamerali, T. (2021). Root Characteristics and Metal Uptake of Maize (Zea mays L.) under Extreme Soil Contamination. Agronomy, 11(1), 178. https://doi.org/10.3390/agronomy11010178







