Exogenous Application of Nitric Oxide Mitigates Water Stress and Reduces Natural Viral Disease Incidence of Tomato Plants Subjected to Deficit Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design
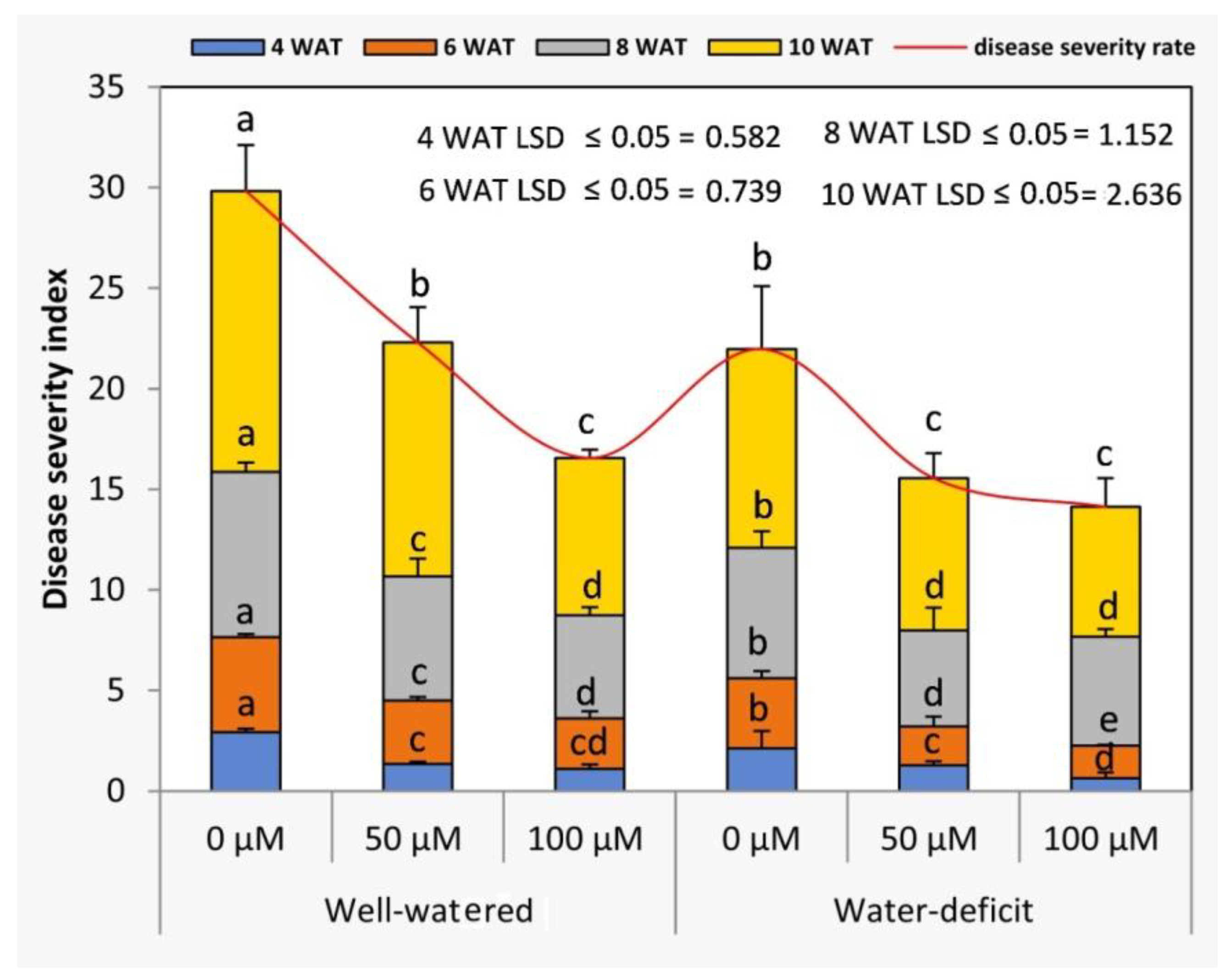
2.3. Data Recorded
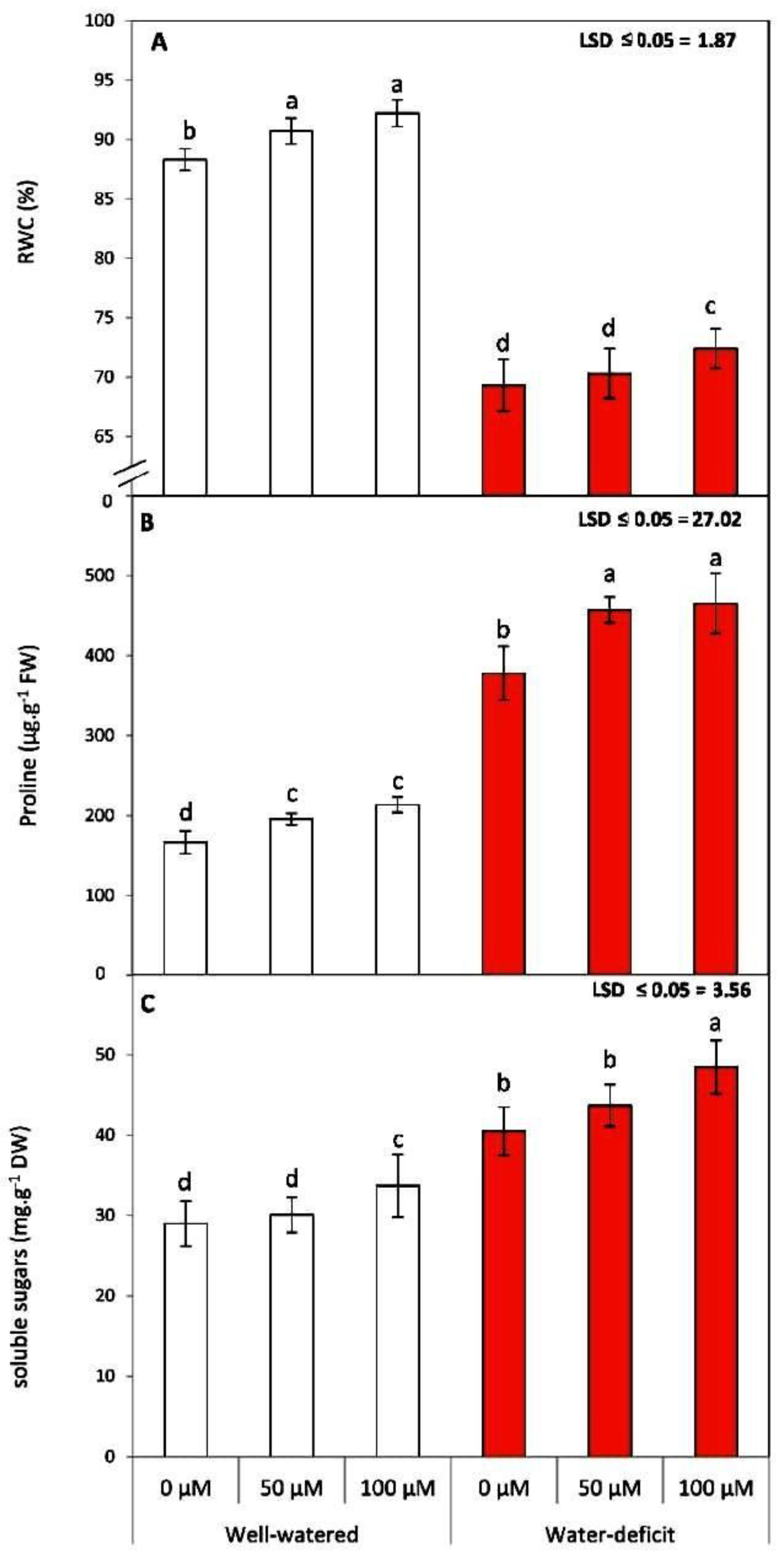
2.3.1. Vegetative Growth, Chlorophyll Content, and RWC
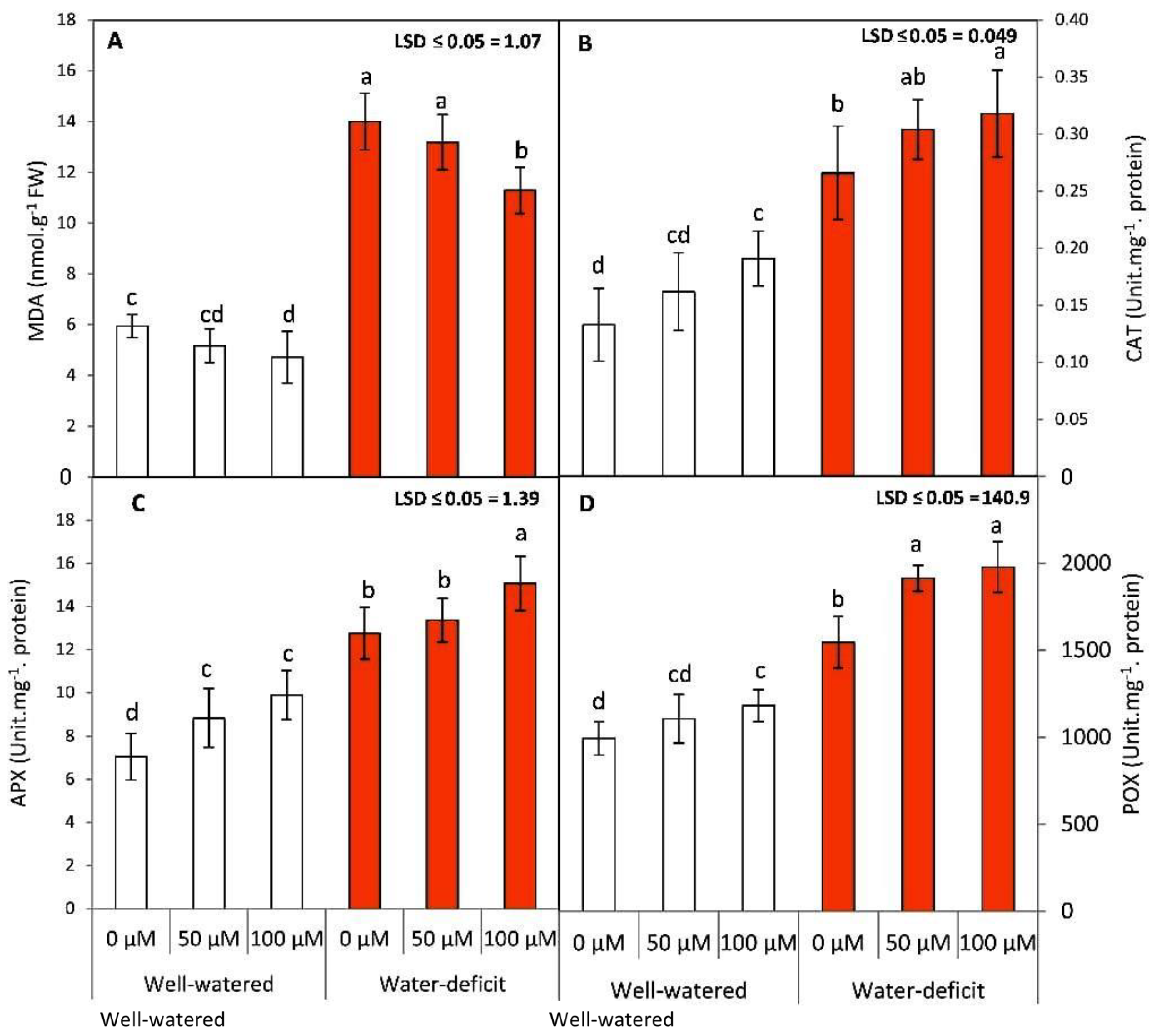
2.3.2. Proline, Soluble Sugars, and Lipid Peroxidation
2.3.3. Enzyme Assays (Ascorbate Peroxidase (APX), Catalase (CAT), and Peroxidase (POX) Activities)
2.3.4. Disease Incidence and Severity (TMV and TYLCV)
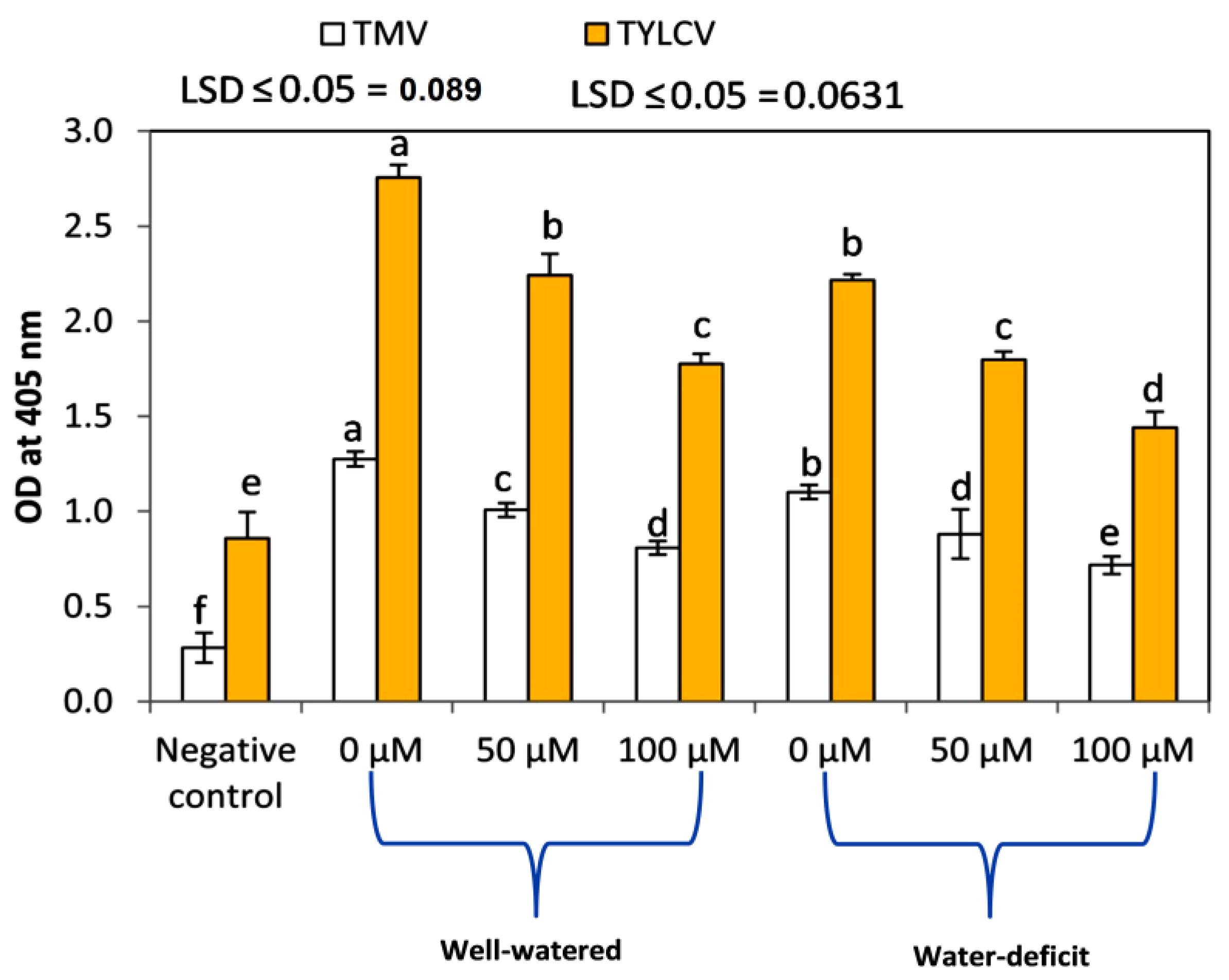
Relative Concentrations of TMV and TYLCV in the Infected Plants
- Serologically: Enzyme-linked immunosorbent assay [32] was used to determine TSWV levels in sap of young leaves of infected tomato plants 30 days postinoculation (dpi). Absorbance values were determined using an ELISA reader (BIE & BERNTSEN AS) at 405 nm for 1 h.
- Biologically: the number of local lesions appeared after 7 dpi with TMV from the grinded crud sap (1 g collected tomato leaves sample from each treatment with a phosphate buffer (pH: 7.4)) on Nicotiana glutinosa (N. glutinosa) plants.
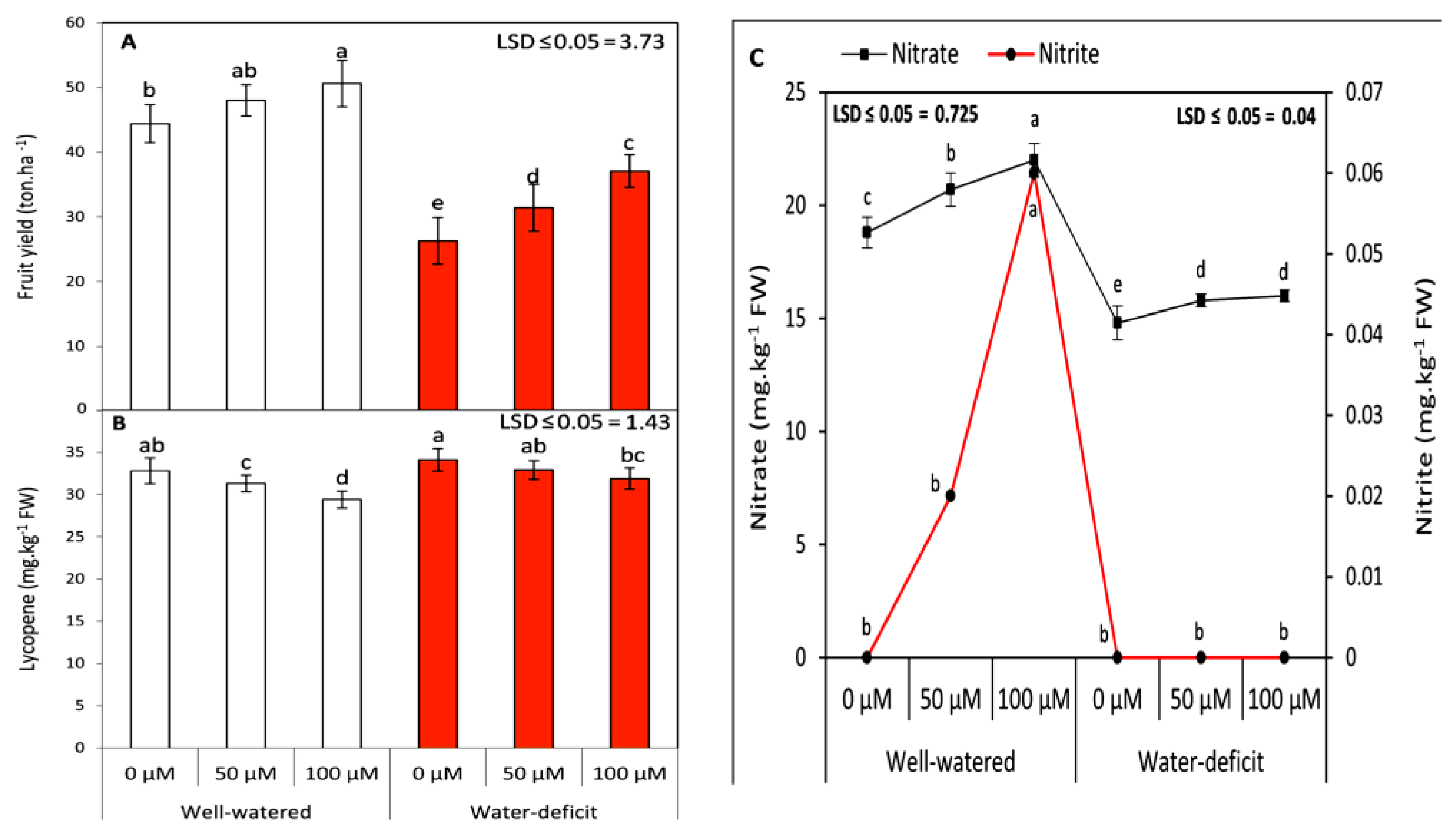
2.3.5. Fruit Yield and Lycopene, Nitrite, and Nitrate Contents
2.4. Statistics
3. Results
3.1. Exogenous SNP Partially Improves Shoot Fresh Weight and Modulates Pigment Content and Fruit Yield Attributes during Water Stress
3.2. SNP Treatment Modulates RWC and Osmolyte Accumulation in Tomato Plants Subjected to Water Stress
3.3. Exogenous SNP Reduces Lipid Peroxidation and Increases Enzymatic Antioxidative Defense in Water-Stressed Tomato Plants
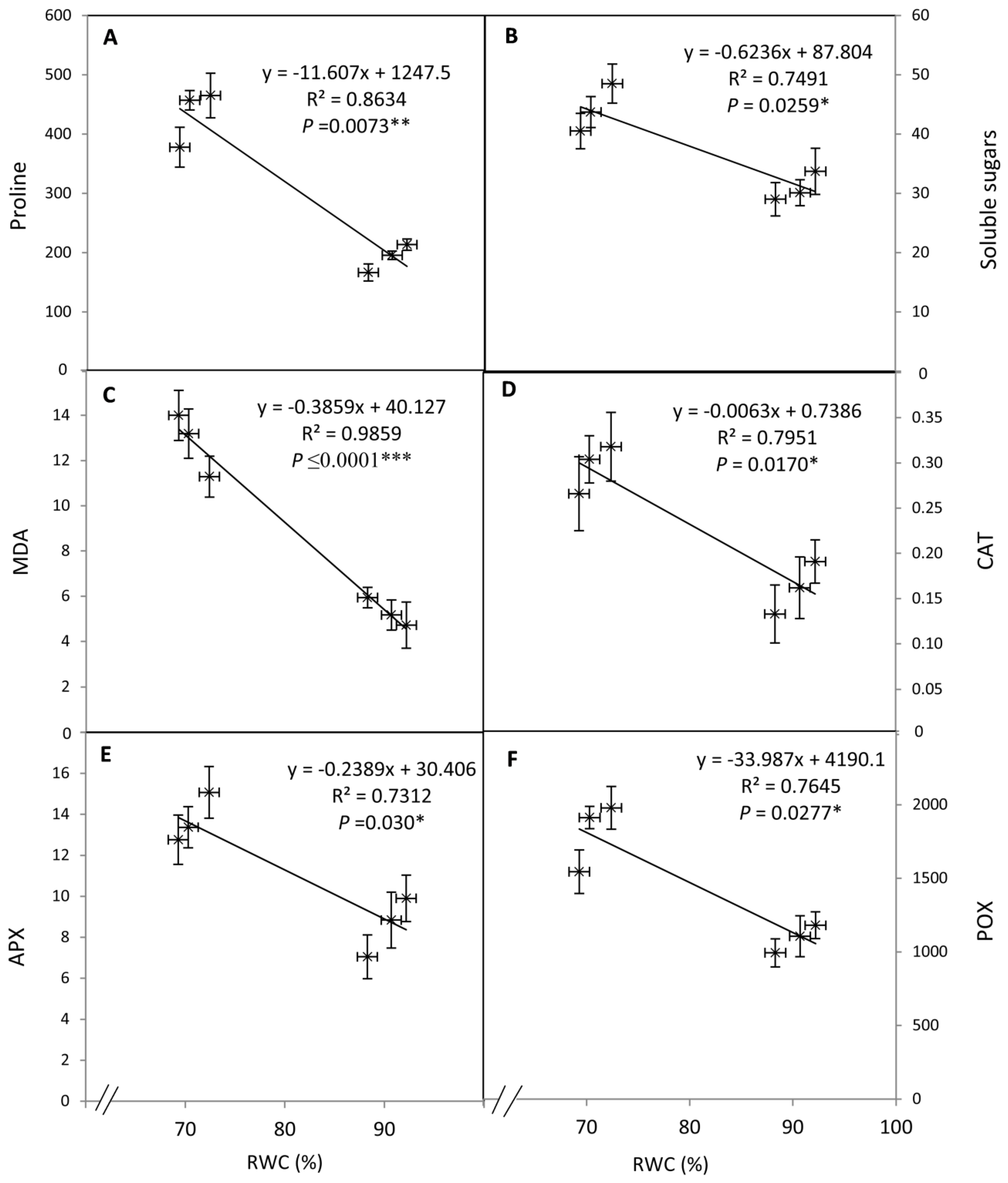
3.4. Correlations Among Osmolytes, Antioxidant Enzyme Activity, and RWC
3.5. Exogenous SNP Modulates Viral Disease Incidence and Severity Index in the Absence and Presence of Water Stress
3.6. Exogenous SNP Application Reduces the Relative Concentrations of TMV and TYLCV
3.7. Effect of Exogenous SNP on the Relative Concentration of TMV with the Biological Method
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habib, N.; Ali, Q.; Ali, S.; Javed, M.T.; Zulqurnain Haider, M.; Perveen, R.; Shahid, M.R.; Rizwan, M.; Abdel-Daim, M.M.; Elkelish, A.; et al. Use of Nitric Oxide and Hydrogen Peroxide for Better Yield of Wheat (Triticum aestivum L.) under Water Deficit Conditions: Growth, Osmoregulation, and Antioxidative Defense Mechanism. Plants 2020, 9, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soliman, M.; Elkelish, A.; Souad, T.; Alhaithloul, H.; Farooq, M. Brassinosteroid Seed Priming with Nitrogen Supplementation Improves Salt Tolerance in Soybean. Physiol. Mol. Biol. Plants 2020. [Google Scholar] [CrossRef] [PubMed]
- Zamin, M.; Fahad, S.; Khattak, A.M.; Adnan, M.; Wahid, F.; Raza, A.; Wang, D.; Saud, S.; Noor, M.; Bakhat, H.F.; et al. Developing the First Halophytic Turfgrasses for the Urban Landscape from Native Arabian Desert Grass. Environ. Sci. Pollut. Res. 2020, 27, 39702–39716. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.H.; Abdulmajeed, A.M.; Alhaithloul, H.; Alharbi, B.M.; El-Esawi, M.A.; Hasanuzzaman, M.; Elkelish, A. Saponin Biopriming Positively Stimulates Antioxidants Defense, Osmolytes Metabolism and Ionic Status to Confer Salt Stress Tolerance in Soybean. Acta Physiol. Plant 2020, 42, 114. [Google Scholar] [CrossRef]
- Ibrahim, M.F.M.; Bondok, A.M.; Al-Senosy, N.K.; Younis, R.A. Stimulation Some of Defense Mechanisms in Tomato Plants under Water Deficit and Tobacco mosaic virus (TMV). World J. Agric. Sci. 2015, 11, 289–302. [Google Scholar]
- Tajul, M.; Naher, K.; Hossain, T.; Siddiqui, Y.; Sariah, M. Tomato yellow leaf curl virus (TYLCV) alters the phytochemical constituents in tomato fruits. Aust. J. Crop Sci. 2011, 5, 575. [Google Scholar]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative Stress Responses of Some Endemic Plants to High Altitudes by Intensifying Antioxidants and Secondary Metabolites Content. Plants 2020, 9, 869. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Inhibitors of Ceramidases. Chem. Phys. Lipids 2016, 197, 60–68. [Google Scholar] [CrossRef]
- Saied, E.M.; Arenz, C. Small Molecule Inhibitors of Ceramidases. CPB 2014, 34, 197–212. [Google Scholar] [CrossRef]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef]
- Poad, B.L.J.; Maccarone, A.T.; Yu, H.; Mitchell, T.W.; Saied, E.M.; Arenz, C.; Hornemann, T.; Bull, J.N.; Bieske, E.J.; Blanksby, S.J. Differential-Mobility Spectrometry of 1-Deoxysphingosine Isomers: New Insights into the Gas Phase Structures of Ionized Lipids. Anal. Chem. 2018, 90, 5343–5351. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, P.; Jamsheed, S.; Hameed, A.; Rasool, S.; Sharma, I.; Azooz, M.; Hasanuzzaman, M. Drought stress induced oxidative damage and antioxidants in plants. In Oxidative Damage to Plants; Elsevier: Amsterdam, The Netherlands, 2014; pp. 345–367. [Google Scholar] [CrossRef]
- Corpas, F.J.; Leterrier, M.; Valderrama, R.; Airaki, M.; Chaki, M.; Palma, J.M.; Barroso, J.B. Nitric oxide imbalance provokes a nitrosative response in plants under abiotic stress. Plant Sci. 2011, 181, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Palma, J.M. Assessing nitric oxide (NO) in higher plants: An outline. Nitrogen 2018, 1, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Pagnussat, G.C.; Lanteri, M.L.; Lombardo, M.C.; Lamattina, L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004, 135, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Bodanapu, R.; Gupta, S.K.; Basha, P.O.; Sakthivel, K.; Sreelakshmi, Y.; Sharma, R. Nitric oxide overproduction in tomato shr mutant shifts metabolic profiles and suppresses fruit growth and ripening. Front. Plant Sci. 2016, 7, 1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayat, S.; Yadav, S.; Wani, A.S.; Irfan, M.; Ahmad, A. Nitric oxide effects on photosynthetic rate, growth, and antioxidant activity in tomato. Int. J. Veg. Sci. 2011, 17, 333–348. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Chen, X.; Jin, H.; Cui, X. Exogenous nitric oxide on antioxidative system and ATPase activities from tomato seedlings under copper stress. Sci. Hortic. 2009, 123, 217–223. [Google Scholar] [CrossRef]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [Green Version]
- Jangid, K.K.; Dwivedi, P. Physiological and biochemical changes by nitric oxide and brassinosteroid in tomato (Lycopersicon esculentum Mill.) under drought stress. Acta Physiol. Plant. 2017, 39, 73. [Google Scholar] [CrossRef]
- Hajivar, B.; Zare-Bavani, M.R. Alleviation of salinity stress by hydrogen peroxide and nitric oxide in tomato plants. Adv. Hortic. Sci. 2019, 33. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ünyayar, S.; Keleþ, Y.; Ünal, E. Proline and ABA levels in two sunflower genotypes subjected to water stress. Proc. Bulg. J. Plant Physiol. 2004, 30, 34–47. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Cakmak, I.; Strbac, D.; Marschner, H. Activities of hydrogen peroxide-scavenging enzymes in germinating wheat seeds. J. Exp. Bot. 1993, 44, 127–132. [Google Scholar] [CrossRef]
- Hammerschmidt, R.; Nuckles, E.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Nelson, M.R.; Orum, T.V.; Jaime-Garcia, R.; Nadeem, A. Applications of geographic information systems and geostatistics in plant disease epidemiology and management. Plant Dis. 1999, 83, 308–319. [Google Scholar] [CrossRef] [Green Version]
- Clark, M.F.; Adams, A. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fish, W.W.; Perkins-Veazie, P.; Collins, J.K. A quantitative assay for lycopene that utilizes reduced volumes of organic solvents. J. Food Compos. Anal. 2002, 15, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Sen, N.P.; Donaldson, B. Improved colorimetric method for determining nitrate and nitrite in foods. J. Assoc. Off. Anal. Chem. 1978, 61, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- SAS. SAS/STAT User’s Guide: Release 6.03 ed; SAS Institute Inc.: Cary, NC, USA, 1988. [Google Scholar]
- Nasibi, F.; Kalantari, K.M. Influence of nitric oxide in protection of tomato seedling against oxidative stress induced by osmotic stress. Acta Physiol. Plant. 2009, 31, 1037–1044. [Google Scholar] [CrossRef]
- Gan, L.; Wu, X.; Zhong, Y. Exogenously Applied Nitric Oxide Enhances the Drought Tolerance in Hulless Barley. Plant Prod. Sci. 2015, 18, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Faraji, J.; Sepehri, A. Exogenous Nitric Oxide Improves the Protective Effects of TiO2 Nanoparticles on Growth, Antioxidant System, and Photosynthetic Performance of Wheat Seedlings Under Drought Stress. J. Soil Sci. Plant Nutr. 2020, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, X.; Cai, J.; Li, G.; Dong, T.; Li, Z. Basic leucine zipper transcription factor SlbZIP1 mediates salt and drought stress tolerance in tomato. BMC Plant Biol. 2018, 18, 83. [Google Scholar] [CrossRef] [Green Version]
- Chołuj, D.; Karwowska, R.; Ciszewska, A.; Jasińska, M. Influence of long-term drought stress on osmolyte accumulation in sugar beet (Beta vulgaris L.) plants. Acta Physiol. Plant. 2008, 30, 679. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Varela, M.C.; Arslan, I.; Reginato, M.A.; Cenzano, A.M.; Luna, M.V. Phenolic compounds as indicators of drought resistance in shrubs from Patagonian shrublands (Argentina). Plant Physiol. Biochem. 2016, 104, 81–91. [Google Scholar] [CrossRef]
- Weidner, S.; Karolak, M.; Karamac, M.; Kosinska, A.; Amarowicz, R. Phenolic compounds and properties of antioxidants in grapevine roots [Vitis vinifera L.] under drought stress followed by recovery. Acta Soc. Bot. Pol. 2009, 78, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous Nitric Oxide Mitigates Nickel-Induced Oxidative Damage in Eggplant by Upregulating Antioxidants, Osmolyte Metabolism, and Glyoxalase Systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, H.-H.; Shen, W.-B.; Xu, L.-L. Nitric oxide involved in the abscisic acid induced proline accumulation in wheat seedling leaves under salt stress. Acta Bot. Sin. Engl. Ed. 2004, 46, 1307–1315. [Google Scholar]
- Duan, X.; Su, X.; You, Y.; Qu, H.; Li, Y.; Jiang, Y. Effect of nitric oxide on pericarp browning of harvested longan fruit in relation to phenolic metabolism. Food Chem. 2007, 104, 571–576. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Hou, J.; Fan, Z. Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Pang, C.-H.; Wang, B.-S. Role of ascorbate peroxidase and glutathione reductase in ascorbate–glutathione cycle and stress tolerance in plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: New York, NY, USA, 2010; pp. 91–113. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [Green Version]
- Pandey, H.C.; Baig, M.; Chandra, A.; Bhatt, R. Drought stress induced changes in lipid peroxidation and antioxidant system in genus Avena. J. Environ. Biol. 2010, 31, 435–443. [Google Scholar]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biol. Open 2018, 7, bio035279. [Google Scholar] [CrossRef] [Green Version]
- Leshem, Y.a.Y.; Pinchasov, Y. Non-invasive photoacoustic spectroscopic determination of relative endogenous nitric oxide and ethylene content stoichiometry during the ripening of strawberries Fragaria anannasa (Duch.) and avocados Persea americana (Mill.). J. Exp. Bot. 2000, 51, 1471–1473. [Google Scholar] [CrossRef]
- Manjunatha, G.; Gupta, K.J.; Lokesh, V.; Mur, L.A.; Neelwarne, B. Nitric oxide counters ethylene effects on ripening fruits. Plant Signal. Behav. 2012, 7, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, G.; Lokesh, V.; Neelwarne, B. Nitric oxide in fruit ripening: Trends and opportunities. Biotechnol. Adv. 2010, 28, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Eum, H.L.; Kim, H.B.; Choi, S.B.; Lee, S.K. Regulation of ethylene biosynthesis by nitric oxide in tomato (Solanum lycopersicum L.) fruit harvested at different ripening stages. Eur. Food Res. Technol. 2009, 228, 331. [Google Scholar] [CrossRef]
- Galatro, A.; Puntarulo, S. An update to the understanding of nitric oxide metabolism in plants. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer: New York, NY, USA, 2014; pp. 3–15. [Google Scholar] [CrossRef]
- Bista, D.R.; Heckathorn, S.A.; Jayawardena, D.M.; Mishra, S.; Boldt, J.K. Effects of drought on nutrient uptake and the levels of nutrient-uptake proteins in roots of drought-sensitive and-tolerant grasses. Plants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef]
- Sun, Z.-H.; Zhou, Y.-H.; Shi, K.; Li, X.; Zhang, G.-Q.; Xia, X.-J.; Chen, Z.-X.; Yu, J.-Q. The role of hydrogen peroxide and nitric oxisde in the induction of plant-encoded RNA-dependent RNA polymerase 1 in the basal defense against tobacco mosaic virus. PLoS ONE 2013, 8, e76090. [Google Scholar] [CrossRef]
- Wendehenne, D.; Durner, J.; Klessig, D.F. Nitric oxide: A new player in plant signalling and defence responses. Curr. Opin. Plant Biol. 2004, 7, 449–455. [Google Scholar] [CrossRef]
- Cao, N.; Zhan, B.; Zhou, X. Nitric Oxide as a Downstream Signaling Molecule in Brassinosteroid-Mediated Virus Susceptibility to Maize Chlorotic Mottle Virus in Maize. Viruses 2019, 11, 368. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Liu, Z.; Shao, Y.; Su, J.; Li, X.; Sun, F.; Zhang, Y.; Li, S.; Zhang, Y.; Cui, J. Nitric Oxide Enhances Rice Resistance to Rice Black-Streaked Dwarf Virus Infection. Rice 2020, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, T.S.; Majumdar, U.; Roy, A.; Maiti, D.; Goswamy, A.M.; Bhattacharjee, A.; Ghosh, S.; Ghosh, S.K. Production of nitric oxide in host-virus interaction: A case study with a compatible begomovirus-kenaf host-pathosystem. Plant Signal. Behav. 2010, 5, 668–676. [Google Scholar] [CrossRef] [Green Version]



| Season | Macroelements (%) | pH | Microelements (ppm) | |||||
|---|---|---|---|---|---|---|---|---|
| N | P | K | Fe | B | Zn | |||
| 2018 | 0.28 | 0.43 | 0.42 | 8.20 | 1.98 | 3.90 | 2.97 | |
| 2019 | 0.20 | 0.31 | 0.42 | 7.88 | 2.54 | 4.05 | 2.76 | |
| CaCO3 (%) | EC (dS m−1) | Soluble anions (meq L−1) | Soluble cations (meq L−1) | |||||
| HCO3− | SO4− | Cl− | Ca++ | Mg++ | Na+ | |||
| 2018 | 1.43 | 0.98 | 2.86 | 2.40 | 3.20 | 5.04 | 3.55 | 2.72 |
| 2019 | 1.28 | 0.84 | 3.20 | 3.28 | 3.66 | 4.83 | 2.70 | 3.23 |
| Soil | Sand (%) | Silte (%) | Clay (%) | Soil texture | ||||
| 22.56 | 41.21 | 36.23 | Clay loam | |||||
| Temperature Average (°C) | Maximum Temperature (°C) | Minimum Temperature (°C) | Relative Humidity (%) | Solar Radiation (MJ m–2 day−1) | Precipitation Sum (mm day−1) | |
|---|---|---|---|---|---|---|
| 2018 | ||||||
| September | 28.1 | 36.3 | 21 | 47.1 | 22.68 | 0 |
| October | 24.1 | 31.7 | 18.1 | 50.8 | 18.17 | 4.82 |
| November | 19.5 | 26.5 | 14.2 | 55.9 | 10.1 | 6.17 |
| December | 14.3 | 20.5 | 9.7 | 63.3 | 10.89 | 9.15 |
| 2019 | ||||||
| September | 27.6 | 36 | 20.7 | 47.56 | 18.86 | 0 |
| October | 24.8 | 32.5 | 18.7 | 53.52 | 17.78 | 16.82 |
| November | 20.7 | 28.5 | 14.9 | 51.79 | 14.39 | 0.11 |
| December | 14.4 | 21 | 9.6 | 63.7 | 11.46 | 25.9 |
| Depth of Soil (cm) | 2018 | 2019 | ||||
|---|---|---|---|---|---|---|
| FC (%) | Wilting Percentage | Available Water (%) | FC (%) | Wilting Percentage | Available Water (%) | |
| 0–20 | 23.42 | 13.67 | 9.75 | 22.82 | 12.93 | 9.89 |
| 20–40 | 22.75 | 12.43 | 10.32 | 22.61 | 12.88 | 9.73 |
| Average | 23.09 | 13.05 | 10.04 | 22.72 | 12.91 | 9.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkelish, A.; Ibrahim, M.F.M.; Ashour, H.; Bondok, A.; Mukherjee, S.; Aftab, T.; Hikal, M.; El-Yazied, A.A.; Azab, E.; Gobouri, A.A.; et al. Exogenous Application of Nitric Oxide Mitigates Water Stress and Reduces Natural Viral Disease Incidence of Tomato Plants Subjected to Deficit Irrigation. Agronomy 2021, 11, 87. https://doi.org/10.3390/agronomy11010087
Elkelish A, Ibrahim MFM, Ashour H, Bondok A, Mukherjee S, Aftab T, Hikal M, El-Yazied AA, Azab E, Gobouri AA, et al. Exogenous Application of Nitric Oxide Mitigates Water Stress and Reduces Natural Viral Disease Incidence of Tomato Plants Subjected to Deficit Irrigation. Agronomy. 2021; 11(1):87. https://doi.org/10.3390/agronomy11010087
Chicago/Turabian StyleElkelish, Amr, Mohamed F. M. Ibrahim, Hatem Ashour, Ahmed Bondok, Soumya Mukherjee, Tariq Aftab, Mohamed Hikal, Ahmed Abou El-Yazied, Ehab Azab, Adil A. Gobouri, and et al. 2021. "Exogenous Application of Nitric Oxide Mitigates Water Stress and Reduces Natural Viral Disease Incidence of Tomato Plants Subjected to Deficit Irrigation" Agronomy 11, no. 1: 87. https://doi.org/10.3390/agronomy11010087














