Strategic Identification of New Genetic Diversity to Expand Lentil (Lens culinaris Medik.) Production (Using Nepal as an Example)
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experiments and Data Collection
2.2. Data Analysis
3. Results and Discussions
3.1. Variation in Phenological Traits among Genotypes and Experimental Years
3.2. New Genotypic Options for Nepalese Agricultural Systems
3.3. Identifying Genotypes for Testing in Expanded Growing Regions Using a Photothermal Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Available online: http://www.fao.org/faostat/en/?#data/QC (accessed on 27 February 2020).
- Tullu, A.; Diederichsen, A.; Suvorova, G.; Vandenberg, A. Genetic and genomic resources of lentil: Status, use and prospects. Plant Genet. Resour. 2011, 9, 19–29. [Google Scholar] [CrossRef]
- Khazaei, H.; Caron, C.T.; Fedoruk, M.; Diapari, M.; Vandenberg, A.; Coyne, C.J.; McGee, R.; Bett, K.E. Genetic diversity of cultivated lentil (Lens culinaris Medik.) and its relation to the world’s agro-ecological zones. Front. Plant Sci. 2016, 7, 1093. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.M.; Neupane, S.; Heidecker, T.; Haile, T.A.; Chan, C.; Coyne, C.J.; McGee, R.J.; Udupa, S.; Henkrar, F.; Barilli, E.; et al. Understanding photothermal interactions will help expand production range and increase genetic diversity of lentil (Lens culinaris Medik.). Plants People Planet 2020, 3, 171–181. [Google Scholar] [CrossRef]
- FAO. The Second Report on the State of the World’s Plant Genetic Resources for Food and Agriculture. Exp. Agric. 2020, 47, 574. [Google Scholar]
- Summerfield, R.J.; Roberts, E.H.; Erskine, W.; Ellis, R.H. Effects of Temperature and Photoperiod on Flowering in Lentils (Lens culinaris Medic.). Ann. Bot. 1985, 56, 659–671. [Google Scholar] [CrossRef]
- MOALD Statistical Information on Nepalese Agriculture 2075/76 [2018/19]. Available online: https://www.moald.gov.np/publication/AgricultureStatistics (accessed on 16 June 2021).
- USAID Value Chain/Market Analysis of the Lentil Sub-Sector in Nepal. 2011. Available online: https://ansab.org.np/storage/product/nepal-neat-subsector-market-analysis-lentil-aug-2011-1579689501.pdf (accessed on 20 April 2020).
- Pokhrel, A.; Aryal, L.; Poudel, P. A Review on Research Work of Grain Legumes Research Program, NARC; Nepal Agricultural Research Council, Grain Legume Research Program: Banke, Nepal, 2019.
- SQCC Notified and Denotified Varieties till 2076 04 07; Seed Quality Control Centre: Lalitpur, Nepal, 2020.
- Ghimire, K.H.; Joshi, B.K.; Karki, A.; Gauchan, D. Existing Collections and Important Germplasm in Genebank from Earthquake Affected Districts. In Rebuilding Local Seed System of Native Crops in Earthquake Affected Areas of Nepal. Proceedings of a National Sharingshop 18 December 2017, Kathmandu; Joshi, B.K., Gauchan, D., Eds.; NAGRC, Bioversity International, Crop Trust: Kathmandu, Nepal, 2017. [Google Scholar]
- Joshi, B.K.; Gauchan, D.; Sapkota, S.; Dongol, D.M.; Paudyal, K.; Gautam, S.; Khatiwada, S.; Ghimire, K.H. Red Listing of Crop Landraces in Earthquake Affected Areas. In Rebuilding Local Seed System of Native Crops in Earthquake Affected Areas of Nepal. Proceedings of a National Sharingshop 18 December 2017, Kathmandu; Joshi, B.K., Gauchan, D., Eds.; NAGRC, Bioversity International, Crop Trust: Kathmandu, Nepal, 2017. [Google Scholar]
- Shrestha, R.; Adhikari, B.; Darai, D.; Gharti, D.; Agrawal, S.; Sarker, A.; Vandengerg, A. Grain Yield and Seed Mineral Contents of Lentil as Influenced by Environments in Nepal. In Proceedings of 30th National Winter Crops Workshop 15–16 February 2017; Bhandari, D., Gautam, A., Shrestha, R., Upreti, H., Ghimire, Y., Mishra, K., Joshi, B., Ansari, A., Tripati, B., Paneru, P., Eds.; Nepal Agricultural Research Council: Kathmandu, Nepal, 2019. [Google Scholar]
- Ghimire, K.H.; Joshi, B.K.; Gurung, R.; Pudasaini, N.; Gauchan, D.; Sthapit, S.; Javris, D. Good Practices for Agrobiodiversity Management A. Introduction 6. Germplasm Rescue and Repatriation. In Good Practices for Agrobiodiversity Management; Joshi, B.K., Javris, D., Eds.; NAGRC, LI-BIRD and Alliance of Bioversity International and CIAT: Kathmandu, Nepal, 2020; pp. 40–47. ISBN 978-92-9255-149-0. [Google Scholar]
- Yadav, N.K.; Ghimire, S.K.; Shrestha, S.M.; Sah, B.P.; Sarker, A.; Sah, S.K. Source of resistant against Fusarium wilt and Stemphylium blight in lentil (Lens culinaris Medikus). Int. J. Appl. Sci. Biotechnol. 2017, 5, 102–107. [Google Scholar] [CrossRef]
- Thapa Magar, D.B.; Darai, R.; Gauchan, D.; Sarker, A. Varietal adoption and marketing of lentilin the mid and far western terairegion of Nepal. Adv. Plants Agric. Res. 2014, I, 164–170. [Google Scholar] [CrossRef][Green Version]
- Ferguson, M.E.; Ford-Lloyd, B.V.; Robertson, L.D.; Maxted, N.; Newbury, H.J. Mapping the geographical distribution of genetic variation in the genus Lens for the enhanced conservation of plant genetic diversity. Mol. Ecol. 1998, 7, 1743–1755. [Google Scholar] [CrossRef]
- Erskine, W.; Sarker, A.; Ashraf, M. Reconstructing an ancient bottleneck of the movement of the lentil (Lens culinaris ssp. culinaris) into South Asia. Genet. Resour. Crop Evol. 2011, 58, 373–381. [Google Scholar] [CrossRef]
- Gharti, D.; Darai, R.; Subedi, S.; Sarker, A.; Kumar, S. Grain Legumes in Nepal: Present Scenario and Future Prospects. World J. Agric. Res. 2014, 2, 216–222. [Google Scholar] [CrossRef]
- Corripio, J.G. Package “Insol”; Version 1.2.1. 2019. Available online: https://cran.r-project.org/web/packages/insol/insol.pdf (accessed on 15 April 2020).
- R Core Team R. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 17 June 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Clarke, E.; Sherrill-Mix, S. Ggbeeswarm Package Usage Example; Version 0.6.0. 2017. Available online: https://cran.r-project.org/web/packages/ggbeeswarm/vignettes/usageExamples.pdf (accessed on 15 April 2020).
- Kassambara, A. Package “ggpubr” Type Package Title “ggplot2” Based Publication Ready Plots; 2017. Available online: https://rpkgs.datanovia.com/ggpubr/index.html (accessed on 15 April 2020).
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Riebl, H.; Singmann, H. Package “Emmeans” Aka Least-Squares Means Version 1.5.2-1. 2020. Available online: https://cran.r-project.org/web/packages/emmeans/emmeans.pdf (accessed on 15 April 2020).
- Sarker, A.; Erskine, W. Recent progress in the ancient lentil. J. Agric. Sci. 2006, 144, 19–29. [Google Scholar] [CrossRef]
- Yadav, S.S.; Rizvi, A.H.; Manohar, M.; Verma, A.K.; Shrestha, R.; Chen, C.; Bejiga, G.; Chen, W.; Yadav, M.; Bahl, P.N. Lentil growers and production systems around the world. In Lentil: An Ancient Crop for Modern Times; Yadav, S.S., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 415–442. [Google Scholar]
- Neupane, R.; Shrestha, R. Report on Lentil Varietal and Agronomical Research; Nepal Agricultural Research Council: Lalitpur, Nepal, 1991.
- Ghimire, N.H.; Mandal, H.N. Genetic variability, Genetic Advance, Correlation and Heritability of Cold Tolerance Lentil (Lens culinaris Medic.) Genotypes at High Hill of Nepal. Int. J. Adv. Res. Biol. Sci 2019, 6, 105–119. [Google Scholar] [CrossRef]
- Pudasaini, N. Personal communication. 29 April 2021. [Google Scholar]
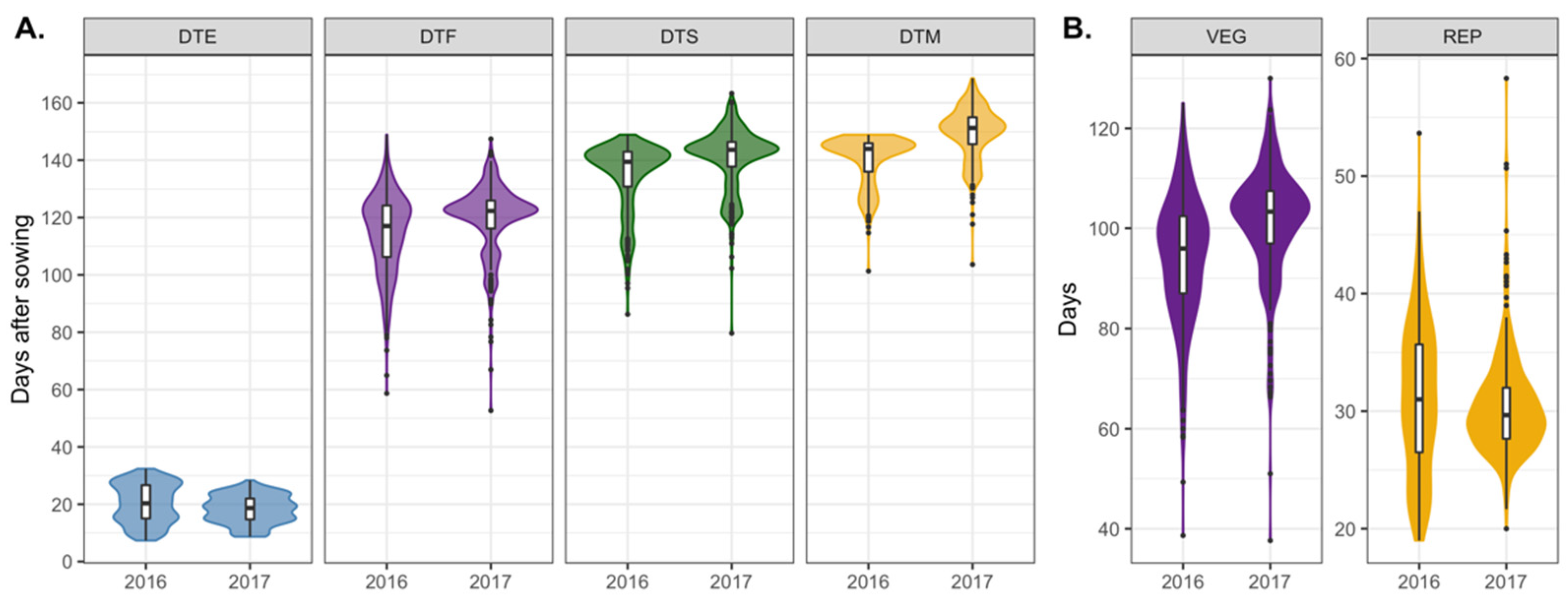

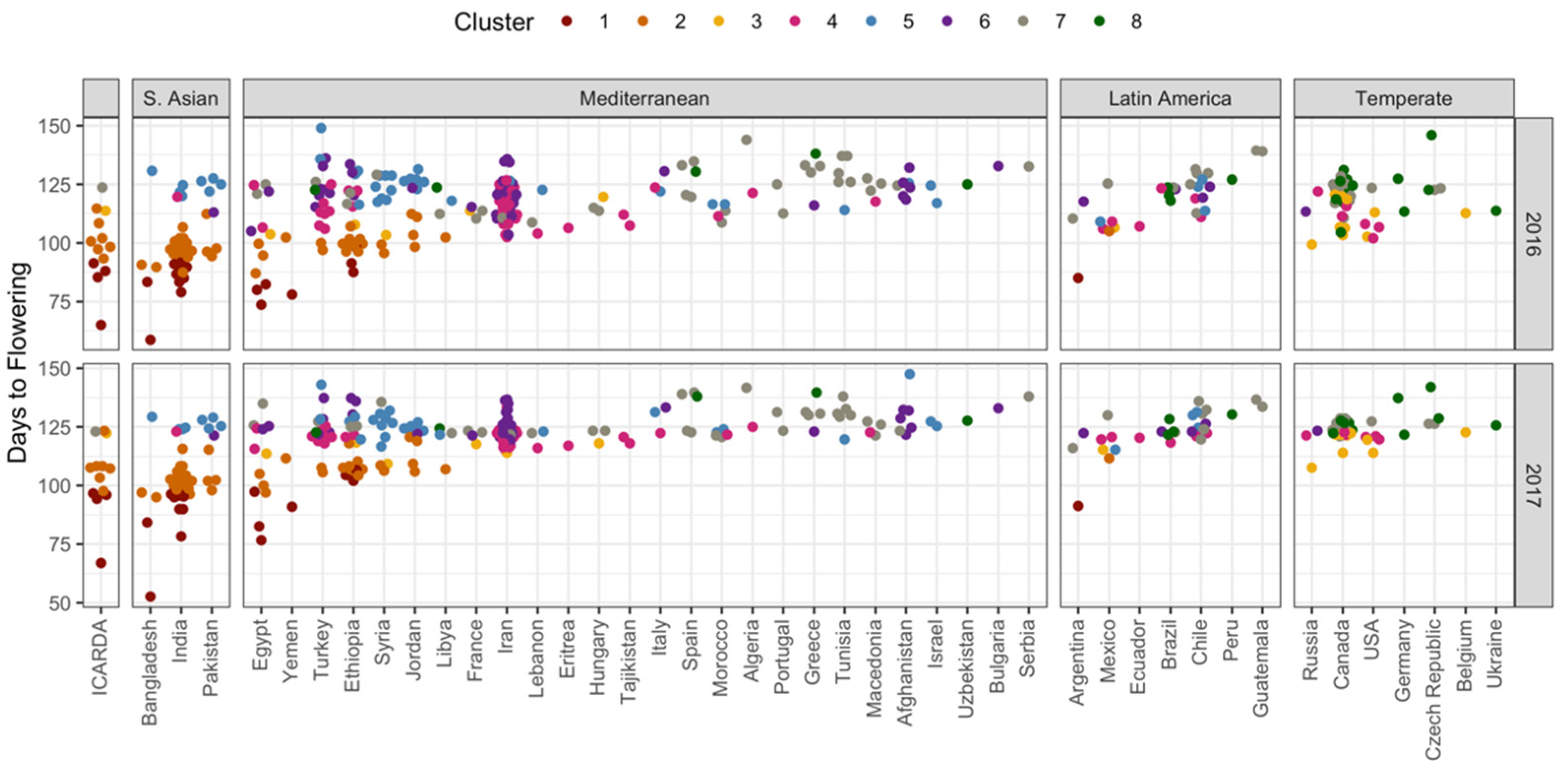
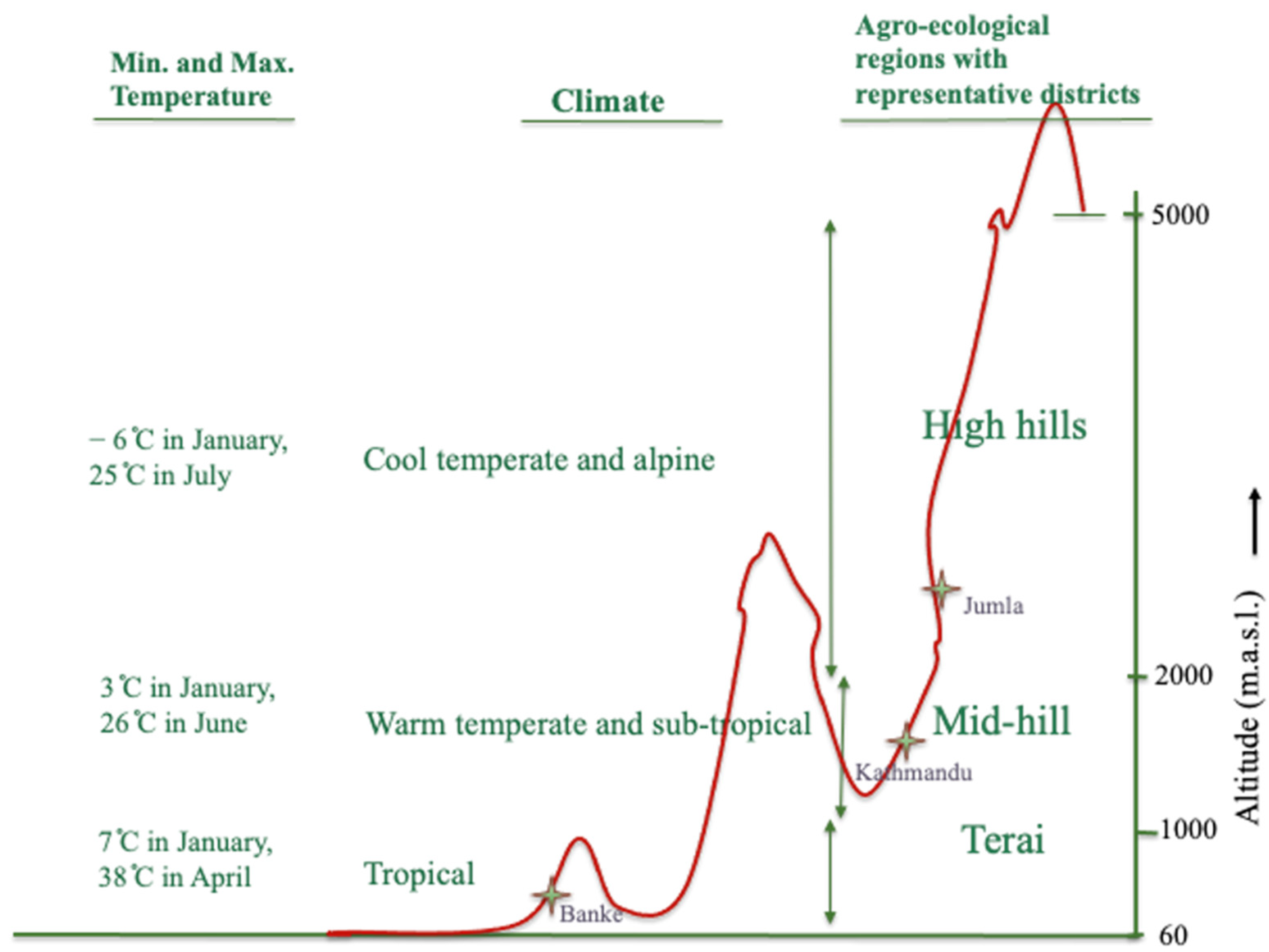
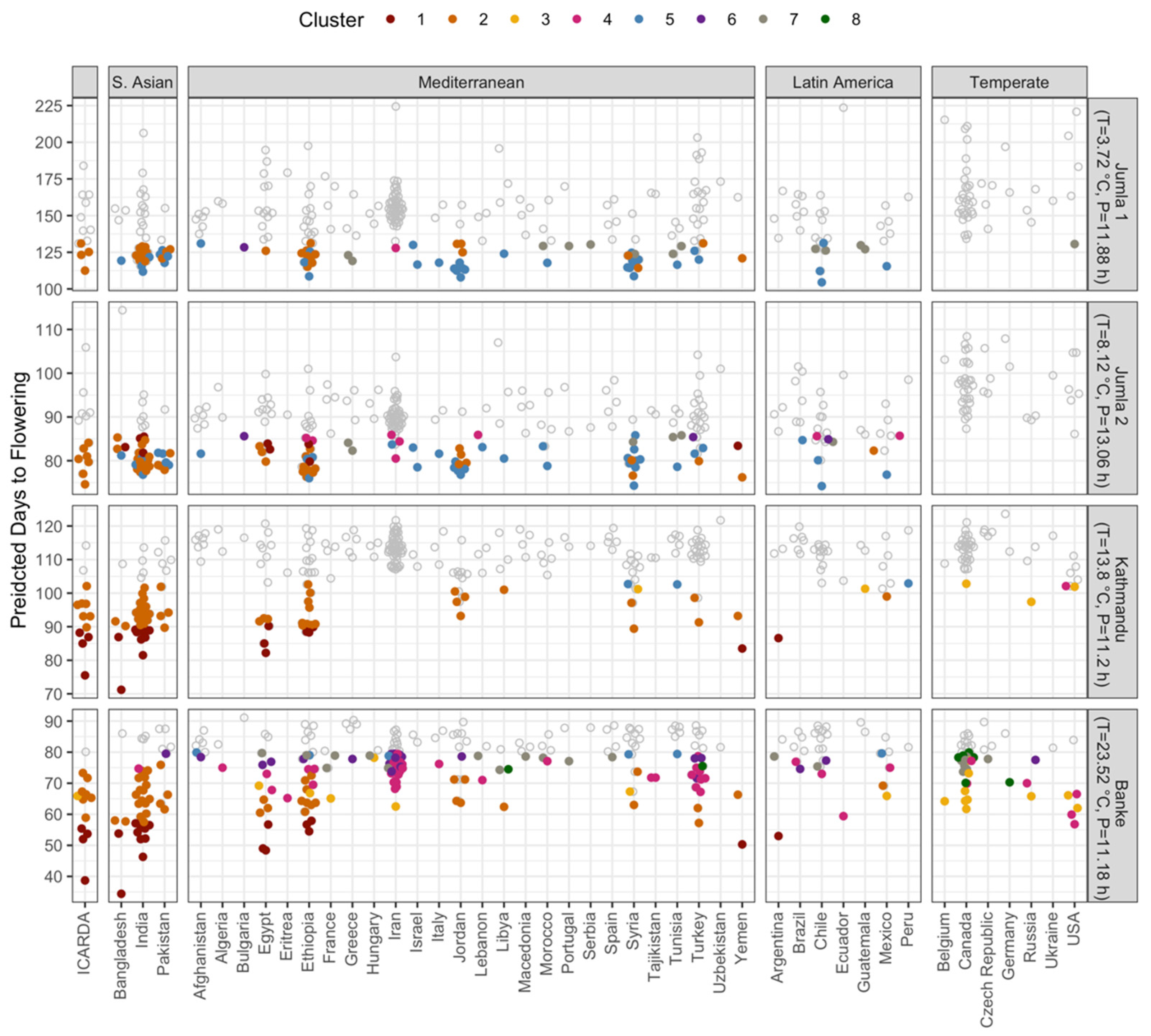
| Source of Variation | DTE | DTF | DTS | DTM | VEG | REP |
|---|---|---|---|---|---|---|
| Genotype | *** | *** | *** | *** | *** | *** |
| Year | ns | * | ** | *** | ** | ns |
| Genotype × Year (G × E) | *** | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, S.; Dhakal, R.; Wright, D.M.; Shrestha, D.K.; Dhakal, B.; Bett, K.E. Strategic Identification of New Genetic Diversity to Expand Lentil (Lens culinaris Medik.) Production (Using Nepal as an Example). Agronomy 2021, 11, 1933. https://doi.org/10.3390/agronomy11101933
Neupane S, Dhakal R, Wright DM, Shrestha DK, Dhakal B, Bett KE. Strategic Identification of New Genetic Diversity to Expand Lentil (Lens culinaris Medik.) Production (Using Nepal as an Example). Agronomy. 2021; 11(10):1933. https://doi.org/10.3390/agronomy11101933
Chicago/Turabian StyleNeupane, Sandesh, Rajeev Dhakal, Derek M. Wright, Deny K. Shrestha, Bishnu Dhakal, and Kirstin E. Bett. 2021. "Strategic Identification of New Genetic Diversity to Expand Lentil (Lens culinaris Medik.) Production (Using Nepal as an Example)" Agronomy 11, no. 10: 1933. https://doi.org/10.3390/agronomy11101933
APA StyleNeupane, S., Dhakal, R., Wright, D. M., Shrestha, D. K., Dhakal, B., & Bett, K. E. (2021). Strategic Identification of New Genetic Diversity to Expand Lentil (Lens culinaris Medik.) Production (Using Nepal as an Example). Agronomy, 11(10), 1933. https://doi.org/10.3390/agronomy11101933






