Fruit Yield, Polyphenols, and Carotenoids in Long Shelf-Life Tomatoes in Response to Drought Stress and Rewatering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Open-Field Experiment
2.2. Laboratory Analyses
2.2.1. Chemicals
2.2.2. Sample Preparation
2.2.3. HPLC/DAD Quantitative Analyses
2.2.4. HPLC/DAD/ESI-MS Analyses (Identification of Tomato Polyphenols)
2.3. Statistical Analyses
3. Results
3.1. Meteorological Course
3.2. Fruit Yield and Irrigation Water Use Efficiency
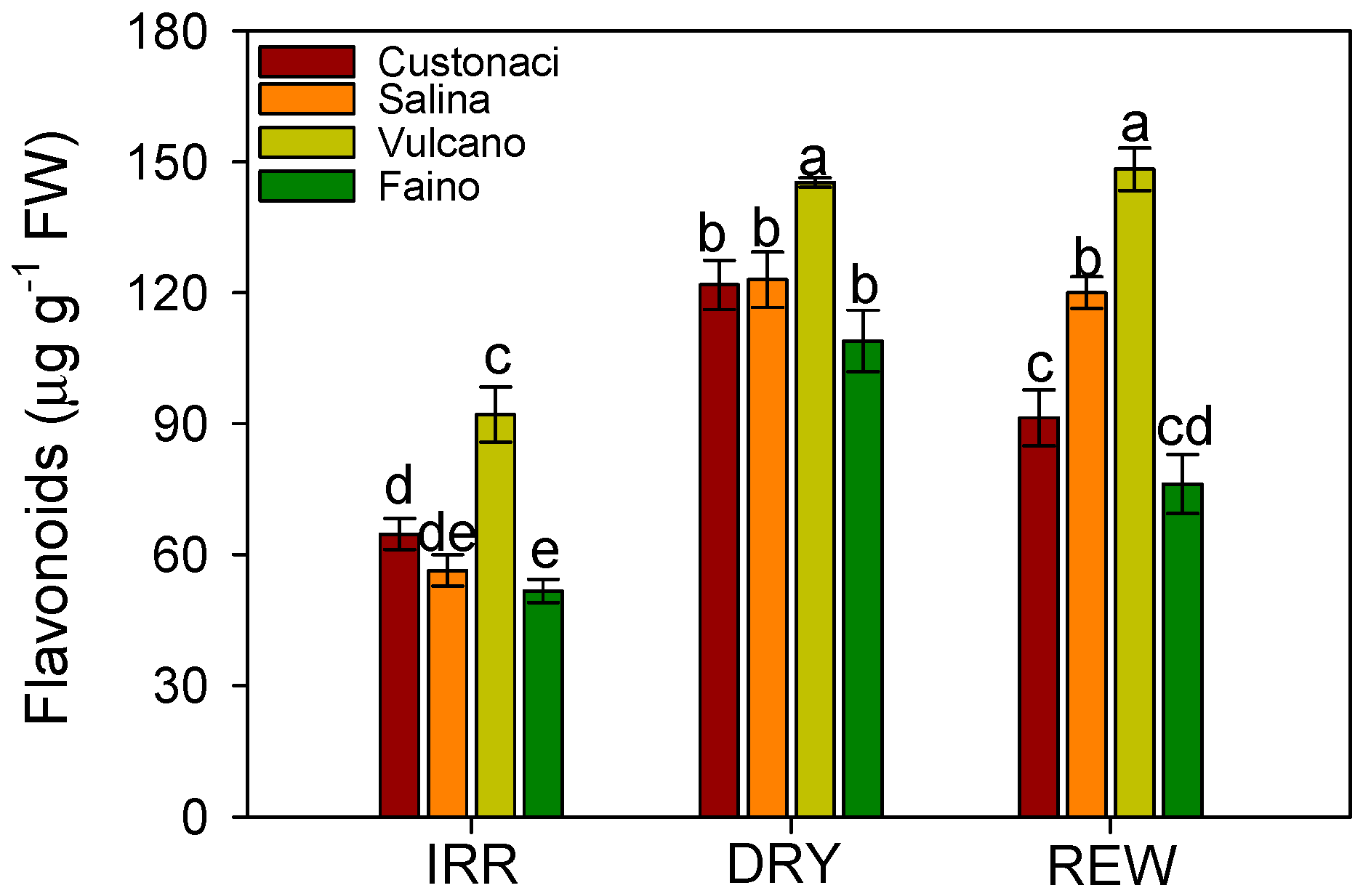
3.3. Total Polyphenols, Flavonoids, and Hydroxycinnamoyl Quinic Acids (HCQA) Content
3.4. Carotenoids Content
3.5. Relationships of Fruit Yield vs. Polyphenols and Carotenoids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W. The tomato as a functional food. J. Nutr. 2005, 134, 1226–1230. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Pernice, R.; Parisi, M.; Giordano, I.; Pentangelo, A.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Antioxidants profile of small tomato fruits: Effect of irrigation and industrial process. Sci. Hortic. 2010, 126, 156–163. [Google Scholar] [CrossRef]
- Barbagallo, R.N.; Di Silvestro, I.; Patanè, C. Yield, physicochemical traits, antioxidant pattern, polyphenol oxidase activity and total visual quality of field-grown processing tomato cv. Brigade as affected by water stress in Mediterranean climate. J. Sci. Food Agric. 2013, 93, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Klunklin, W.; Savage, G. Effect on quality characteristics of tomatoes grown under well-watered and drought stress conditions. Foods 2017, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Dew, T.P.; Orfila, C.; Urwin, P.E. Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). J. Agric. Food Chem. 2011, 59, 9673–9682. [Google Scholar] [CrossRef]
- Hidaka, K.; Yasutake, D.; Kitano, M.; Takahashi, T.; Sago, Y.; Ishikawa, K.; Kawano, T. Production of high quality vegetables by applying low temperature stress to roots. Acta Hortic. 2008, 801, 1431–1436. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; Saita, A.; Siracusa, L.; Ruberto, G.; Barbagallo, R. Mediterranean long storage tomato as a source of novel products for the agrifood industry: Nutritional and technological traits. LWT 2017, 85, 445–448. [Google Scholar] [CrossRef]
- Patanè, C.; Malvuccio, A.; Saita, A.; Rizzarelli, P.; Siracusa, L.; Rizzo, V.; Muratore, G. Nutritional changes during storage in fresh-cut long storage tomato as affected by biocompostable polylactide and cellulose based packaging. LWT 2019, 101, 618–624. [Google Scholar] [CrossRef]
- Siracusa, L.; Patanè, C.; Rizzo, V.; Cosentino, S.L.; Ruberto, G. Targeted secondary metabolic and physico-chemical traits analysis to assess genetic variability within a germplasm collection of “long storage” tomatoes. Food Chem. 2018, 244, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Patanè, C.; Scordia, D.; Testa, G.; Cosentino, S.L. Physiological screening for drought tolerance in Mediterranean long-storage tomato. Plant Sci. 2016, 249, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Conesa, M.A.; Fullana-Pericàs, M.; Granell, A.; Galmés, J. Mediterranean long shelf-life landraces: An untapped genetic resource for tomato improvement. Front. Plant Sci. 2020, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patanè, C.; Tringali, S.; Sortino, O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Sci. Hortic. 2011, 129, 590–596. [Google Scholar] [CrossRef]
- Patanè, C.; Saita, A. Biomass, fruit yield, water productivity and quality response of processing tomato to plant density and deficit irrigation under a semi-arid Mediterranean climate. Crop Pasture Sci. 2015, 66, 224–234. [Google Scholar] [CrossRef]
- Sharma, S.K.; Le Maguer, M. Kinetics of lycopene degradation in tomato pulp solids under different processing and storage conditions. Food Res. Int. 1996, 29, 309–315. [Google Scholar] [CrossRef]
- Guida, G.; Houssemeddine Sellami, M.H.; Mistretta, C.; Oliva, M.; Buonomo, R.; De Mascellis, R.; Patanè, C.; Rouphael, Y.; Albrizio, R.; Giorio, P. Agronomical, physiological and fruit quality responses of two Italian long-storage tomato landraces under rain-fed and full irrigation conditions. Agric. Water Manag. 2017, 180, 126–135. [Google Scholar] [CrossRef]
- Siracusa, L.; Avola, G.; Patanè, C.; Riggi, E.; Ruberto, G. Re-evaluation of traditional Mediterranean foods. The local landraces of ‘Cipolla di Giarratana’ (Allium cepa L.) and long-storage tomato (Lycopersicon esculentum L.): Quality traits and polyphenol content. J. Sci. Food Agric. 2013, 93, 3512–3519. [Google Scholar] [CrossRef]
- Favati, A.; Lovelli, S.; Galgano, F.; Miccolis, V.; Di Tommaso, T.; Candido, V. Processing tomato quality as affected by irrigation scheduling. Sci. Hortic. 2009, 122, 562–571. [Google Scholar] [CrossRef]
- Zhang, F.; Guo, J.K.; Yang, Y.L.; He, W.L.; Zhang, L.X. Changes in the pattern of antioxidant enzymes in wheat exposed to water deficit and rewatering. Acta Physiol. Plant. 2004, 26, 345–352. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.K.; Dutta, B.K. Variation of physiological and antioxidative responses in tea cultivars subjected to elevated water stress followed by rehydration recovery. Acta Physiol. Plant. 2008, 30, 457–468. [Google Scholar] [CrossRef]
- Maia Júnior, S.O.; Andrade, J.R.; Santos, C.M.; Silva, A.L.J.; Endres, L.; Silva, J.V.; Silva, L.K.S. Osmoregulators’ accumulation minimizes the effects of drought stress in sugarcane and contributes to the recovery of photochemical efficiency in photosystem II after rewatering. Acta Physiol. Plant. 2020, 42, 62. [Google Scholar] [CrossRef]
- Torrecillas, S.; Guillaume, C.; Alarcón, J.J.; Ruiz-Sánchez, M.C. 1995: Water relations of two tomato species under water stress and recovery. Plant Sci. 1995, 105, 169–176. [Google Scholar] [CrossRef]
- Giorio, P.; Guida, G.; Mistretta, C.; Sellami, M.H.; Oliva, M.; Punzo, P.; Iovieno, P.; Arena, C.; De Maio, A.; Grillo., S.; et al. Physiological, biochemical and molecular responses to water stress and rehydration in Mediterranean adapted tomato landraces. Plant Biol. 2018, 20, 995–1004. [Google Scholar] [CrossRef]
- Marín, A.; Rubio, J.S.; Martínez, V.; Gil, M.I. Antioxidant compounds in green and red peppers as affected by irrigation frequency, salinity and nutrient solution composition. J. Agric. Food Chem. 2009, 89, 1352–1359. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [Green Version]
- Koyama, R.; Itoh, H.; Kimura, S.; Morioka, A.; Uno, Y. Augmentation of antioxidant constituents by drought stress to roots in leafy vegetables. HortTechnology 2012, 22, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Nangare, D.D.; Singh, Y.; Kumar, P.S.; Minhas, P.S. Growth, fruit yield and quality of tomato (Lycopersicon esculentum Mill.) as affected by deficit irrigation regulated on phenological basis. Agric. Water Manag. 2016, 171, 73–79. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Riggi, E.; Patanè, C.; Ruberto, G. Carotenoid content at different ripening stages in processing tomato in relation to soil water availability. Aust. J. Agric. Res. 2008, 59, 348–353. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Cammareri, M.; Caretto, S.; Cavallaro, V.; Cominelli, E.; De Palma, M.; Docimo, T.; Giovinazzo, G.; Grandillo, S.; et al. Strategies to modulate specialized metabolism in Mediterranean crops: From molecular aspects to field. Int. J. Mol. Sci. 2021, 22, 2887. [Google Scholar] [CrossRef] [PubMed]






| Source | df | Total Yield | Market. Yield | Yield Losses | IWUE |
|---|---|---|---|---|---|
| (t ha−1) | (t ha−1) | (t ha−1) | (kg m−3) | ||
| p | |||||
| Irrigation (I) | 2 | <0.001 | <0.001 | <0.001 | <0.001 |
| Cultivar (C) | 3 | <0.001 | <0.001 | <0.001 | <0.001 |
| I × C | 6 | <0.001 | <0.001 | 0.012 | <0.001 |
| Treatment | Total Fruit Yield (t ha−1) | Market. Yield (t ha−1) | Yield Losses (t ha−1) | Yield Losses (%) | IWUE (kg m−3) | |
|---|---|---|---|---|---|---|
| Custonaci | IRR | 81.57 ± 1.6 | 74.53 ± 0.9 | 7.03 ± 0.3 | 8.46 ± 0.6 | 17.32 ± 1.2 |
| DRY | 24.22 ± 2.0 | 22.31 ± 1.8 | 1.91 ± 0.3 | 7.77 ± 0.4 | 53.82 ± 4.5 | |
| REW | 40.96 ± 0.8 | 35.90 ± 0.2 | 5.05 ± 0.6 | 12.27 ± 1.2 | 22.15 ± 0.4 | |
| Salina | IRR | 68.39 ± 0.6 | 60.18 ± 2.0 | 8.21 ± 1.5 | 12.07 ± 2.2 | 14.52 ± 0.1 |
| DRY | 30.54 ± 1.1 | 27.07 ± 0.8 | 3.46 ± 0.2 | 11.31 ± 0.3 | 67.86 ± 2.4 | |
| REW | 46.68 ± 1.9 | 40.70 ± 1.9 | 5.99 ± 0.01 | 12.90 ± 0.6 | 23.25 ± 1.0 | |
| Vulcano | IRR | 60.65 ± 3.6 | 51.54 ± 3.8 | 9.11 ± 0.2 | 15.25 ± 1.3 | 12.88 ± 0.8 |
| DRY | 18.65 ± 2.1 | 17.14 ± 2.2 | 1.52 ± 0.2 | 8.74 ± 1.8 | 41.45 ± 4.6 | |
| REW | 46.04 ± 9.6 | 40.45 ± 8.3 | 5.60 ± 1.4 | 11.85 ± 0.5 | 24.90 ± 5.2 | |
| Faino Hy | IRR | 114.87 ± 3.4 | 91.46 ± 2.8 | 23.41 ± 6.2 | 19.95 ± 4.8 | 24.39 ± 0.7 |
| DRY | 37.72 ± 3.5 | 33.54 ± 3.0 | 4.18 ± 0.5 | 11.01 ± 0.3 | 83.83 ± 7.9 | |
| REW | 56.67 ± 3.9 | 47.80 ± 1.8 | 8.87 ± 2.0 | 15.13 ± 2.5 | 30.65 ± 2.1 | |
| IRR | 81.37 a | 69.43 a | 11.94 a | 11.93 | 17.28 c | |
| DRY | 27.78 c | 25.01 c | 2.77 c | 9.97 | 61.74 a | |
| REW | 47.59 b | 41.21 b | 6.38 b | 13.41 | 25.74 b | |
| Custonaci | 48.92 b | 44.25 b | 4.66 b | 9.50 | 31.10 bc | |
| Salina | 48.54 b | 42.65 b | 5.89 b | 12.13 | 35.87 b | |
| Vulcano | 41.78 c | 36.37 c | 5.41 b | 12.95 | 26.41 c | |
| Faino Hy | 69.75 a | 57.60 a | 12.15 a | 17.42 | 46.29 a | |
| LSDI×C | 10.62 | 9.24 | 5.80 | 10.22 |
| Source | df | Polyphenols | Flavonoids | HCQA |
|---|---|---|---|---|
| (μg g−1) | (μg g−1) | (μg g−1) | ||
| p | ||||
| Irrigation (I) | 2 | <0.001 | <0.001 | <0.001 |
| Cultivar (C) | 3 | <0.001 | <0.001 | <0.001 |
| I × C | 6 | 0.0011 | 0.0054 | 0.0033 |
| Source | df | Lycopene | β-carotene |
|---|---|---|---|
| (μg g−1) | (μg g−1) | ||
| p | |||
| Irrigation (I) | 2 | <0.001 | <0.001 |
| Cultivar (C) | 3 | <0.001 | 0.0073 |
| I × C | 6 | 0.1522 ns | 0.0177 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patanè, C.; Siah, S.; Pellegrino, A.; Cosentino, S.L.; Siracusa, L. Fruit Yield, Polyphenols, and Carotenoids in Long Shelf-Life Tomatoes in Response to Drought Stress and Rewatering. Agronomy 2021, 11, 1943. https://doi.org/10.3390/agronomy11101943
Patanè C, Siah S, Pellegrino A, Cosentino SL, Siracusa L. Fruit Yield, Polyphenols, and Carotenoids in Long Shelf-Life Tomatoes in Response to Drought Stress and Rewatering. Agronomy. 2021; 11(10):1943. https://doi.org/10.3390/agronomy11101943
Chicago/Turabian StylePatanè, Cristina, Sarah Siah, Alessandra Pellegrino, Salvatore L. Cosentino, and Laura Siracusa. 2021. "Fruit Yield, Polyphenols, and Carotenoids in Long Shelf-Life Tomatoes in Response to Drought Stress and Rewatering" Agronomy 11, no. 10: 1943. https://doi.org/10.3390/agronomy11101943






