Herbicide Resistance of Centaurea cyanus L. in Poland in the Context of Its Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Surveys and C. cyanus Seeds
2.2. Biological Tests in Glasshouses
2.2.1. Discriminate Dose Experiments
2.2.2. Whole-Plant Dose-Response Bioassays
3. Results
3.1. Survey-Based Short-Time Herbicide Treatment History of Herbicide-Resistant and Susceptible C. cyanus in Poland
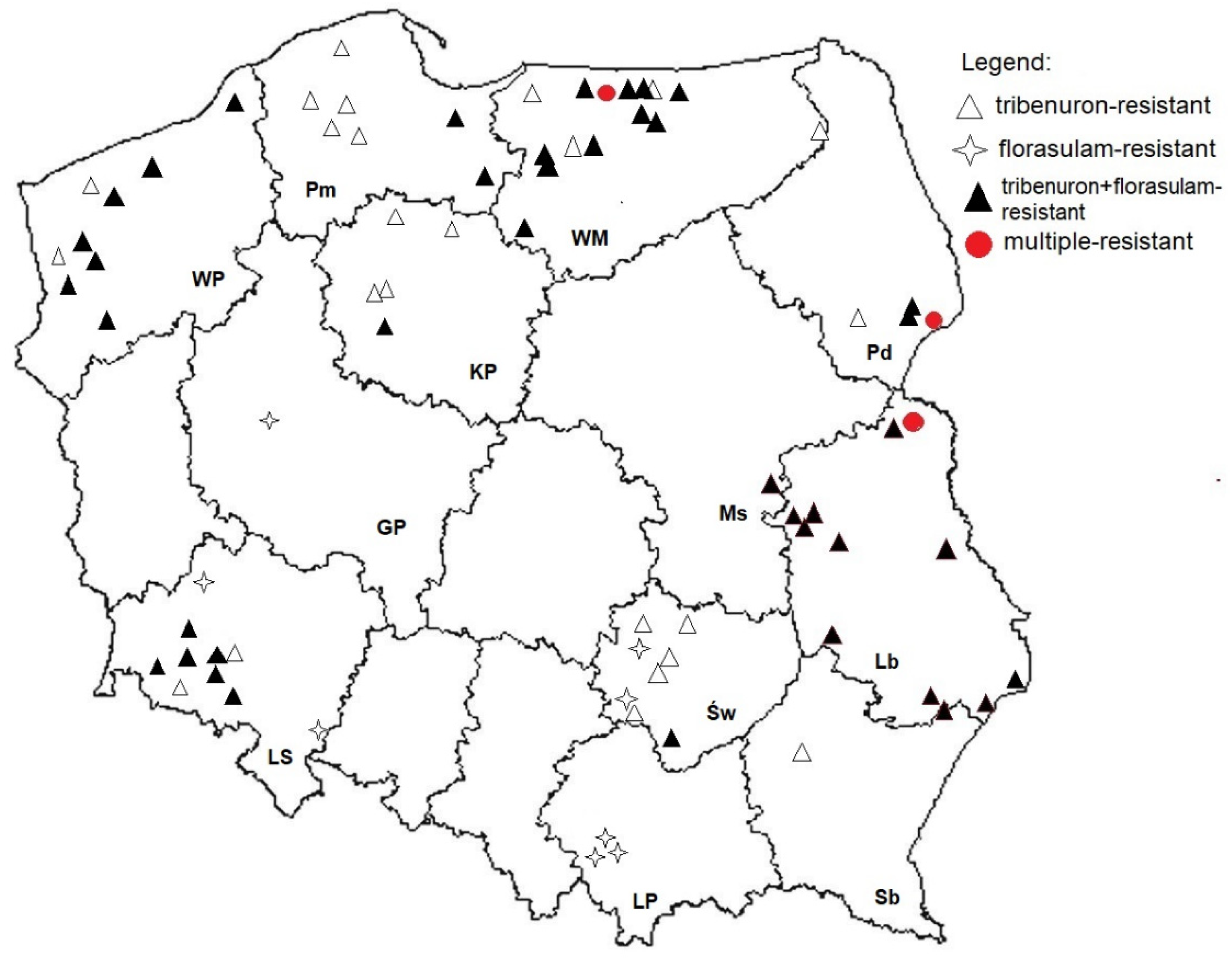
3.2. Distribution and Resistance Index of Herbicide-Resistant Cornflower Populations in Poland
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghanizadeh, H.; Harrington, K.C. Herbicide resistant weeds in New Zealand: State of knowledge. N. Z. J. Agric. Res. 2019, 1–12. [Google Scholar] [CrossRef]
- Ulber, L.; Rissel, D. Farmers’ perspective on herbicide-resistant weeds and application of resistance management strategies: Results from a German survey. Pest Manag. Sci. 2018, 74, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Z.F. Decrease in biodiversity in wheat fields due to changing agricultural practices in five decades. Biodivers. Conserv. 2018, 27, 3267–3286. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 30 July 2021).
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivraindand, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Torra, J.; Rojano-Delgado, A.M.; Rey-Caballero, J.; Royo-Esnal, A.; Salas, M.L.; De Prado, R. Enhanced 2,4-D metabolism in two resistant Papaver rhoeas populations from Spain. Front. Plant Sci. 2017, 8, 1584. [Google Scholar] [CrossRef] [Green Version]
- Stankiewicz-Kosyl, M.; Synowiec, A.; Haliniarz, M.; Wenda-Piesik, A.; Domaradzki, K.; Parylak, D.; Wrochna, M.; Pytlarz, E.; Gala-Czekaj, D.; Marczewska-Kolasa, K.; et al. Herbicide Resistance and Management Options of Papaver rhoeas L. and Centaurea cyanus L. in Europe: A Review. Agronomy 2020, 10, 874. [Google Scholar] [CrossRef]
- Adamczewski, K.; Kierzek, R. Weeds resistance problem in Poland. Prog. Plant Prot./Post. Ochr. Roślin 2011, 51, 1665–1674. (In Polish) [Google Scholar]
- Rola, H.; Rola, J. Amaranthus retroflexus, Chenopodium album, Echinochloa crus-galli—Biotypes resistant to triazine herbicides in corn in south-western Poland. Pam. Puł. 2002, 129, 11–24. (In Polish) [Google Scholar]
- Ciarka, D.; Gawroński, S.W. Wierzbownica gruczołowata-pierwszy w Polsce chwast wieloletni odporny na herbicydy triazynowe. Ochr. Rośl. 1997, 41, 2. (In Polish) [Google Scholar]
- Jursik, M.; Holec, J.; Andr, J. Biology and control of another important weeds of the Czech Republic: Cornflower (Centaurea cyanus L.). Listy Cukrov. Reparske 2009, 125, 90–93. [Google Scholar]
- Petit, C.; Arnal, H.; Darmency, H. Effect of fragmentation and population size on the genetic diversity of Centaurea cyanus L. (Asteraceae) population. Plant. Ecol. Evolut. 2015, 148, 191–198. [Google Scholar] [CrossRef]
- Sutcliffe, O.L.; Kay, Q.O.N. Changes in the arable flora of central southern England since the 1960s. Biol. Conserv. 2000, 93, 1–8. [Google Scholar] [CrossRef]
- Baessler, C.; Klotz, S. Effects of changes in agricultural land-use on landscape structure and arable weed vegetation over the last 50 years. Agric. Ecosyst. Environ. 2006, 115, 43–50. [Google Scholar] [CrossRef]
- Kolárová, M.; Tyšer, L.; Soukup, J. Impact of site conditions and farming practices on the occurrence of rare and endangered weeds on arable land in the Czech Republic. Weed Res. 2013, 53, 489–498. [Google Scholar] [CrossRef]
- Dąbkowska, T.; Grabowska-Orządała, M.; Łabza, T. The study of the transformation of segetal flora richness and diversity in selected habitats of southern Poland over a 20-year interval. Acta Agrobot. 2017, 70, 1712. [Google Scholar] [CrossRef]
- Hofmeijer, M.A.; Gerowitt, B. The regional weed vegetation in organic spring-sown cereals is shaped by local management, crop diversity and site. Jul. Kühn Arch. 2018, 458, 288–294. [Google Scholar]
- Staniak, M.; Haliniarz, M.; Kwiecińska-Poppe, E.; Harasim, E.; Wesołowski, M. Diversity of agrocoenoses in the Lublin region, Poland. Acta Agrobot. 2017, 70, 1722. [Google Scholar] [CrossRef] [Green Version]
- Randall, J.M.; Marinelli, J. Invasive Plants: Weeds of the Global Garden; Science Press: New York, NY, USA, 1996; p. 112. [Google Scholar]
- Marczewska, K.; Rola, H. Identification of resistant to chlorsulfuron of Apera spica-venti and Centaurea cyanus biotypes and chemical methods their control in winter wheat. Prog. Plant Prot. 2006, 46, 215–222. [Google Scholar]
- Adamczewski, K.; Kierzek, R. Cornflower (Centaurea cyanus L) cross resistance on ALS inhibitors. Prog. Plant Prot./Post. Ochr. Roślin 2010, 50, 287–290. (In Polish) [Google Scholar]
- Fuerst, E.P.; Sterling, T.M.; Norman, M.A.; Prather, T.S.; Irzyk, G.P.; Wu, Y.; Lownds, N.K.; Callihan, R.H. Physiological characterization of picloram resistance in yellow starthistle. Pestic. Biochem. Physiol. 1996, 56, 149–161. [Google Scholar] [CrossRef]
- Miller, T.W.; Shinn, S.L.; Thill, D.C. Cross-Resistance in and Chemical Control of Auxinic Herbicide-Resistant Yellow Starthistle (Centaurea solstitialis). Weed Technol. 2001, 15, 293–299. [Google Scholar] [CrossRef]
- Mangin, A.R.; Hall, L.M. First report: Spotted knapweed (Centaurea stoebe) resistance to auxinic herbicides. Can. J. Plant Sci. 2016, 96, 928–931. [Google Scholar] [CrossRef] [Green Version]
- Adamczewski, K.; Matysiak, K.; Kierzek, R.; Kaczmarek, S. Significant increase of weed resistance to herbicides in Poland. J. Plant. Prot. Res. 2019, 59, 139–150. [Google Scholar]
- Madej, A. Processes of concentration in cereal production in Poland. Pol. J. Agron. 2018, 35, 23–31. (In Polish) [Google Scholar]
- Sokólski, M.; Jankowski, K.J.; Załuski, D.; Szatkowski, A. Productivity, Energy and Economic Balance in the Production of Different Cultivars of Winter Oilseed Rape. A Case Study in North-Eastern Poland. Agronomy 2020, 10, 508. [Google Scholar] [CrossRef] [Green Version]
- Burgos, N.L.; Tranel, P.J.; Streibig, J.C.; Davis, V.M.; Shanes, D.; Norsworthy, J.K.; Ritz, C. Review: Confirmation of Resistance to Herbicides and Evaluation of Resistance Levels. Weed Sci. 2013, 61, 4–20. [Google Scholar] [CrossRef]
- Panozzo, S.; Scarabel, L.; Collavo, A.; Sattin, M. Protocols for Robust Herbicide Resistance Testing in Different Weed Species. J. Vis. Exp. 2015, 101, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Burgos, N.L. Whole-Plant and Seed Bioassays for Resistance Confirmation. Weed Sci. 2015, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R software package for dose-response studies: The concept and data analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 8 June 2021).
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Rola, H.; Marczewska, K. Sulfonylurea herbicide resistant biotype of weeds in Wroclaw Region. Prog. Plant Protect. 2002, 42, 575–577. [Google Scholar]
- Adamczewski, K.; Kierzek, R. Występowanie biotypów miotły zbożowej (Apera spica-venti L.) odpornej na herbicydy sulfonylomocznikowe. Prog. Plant Prot. 2007, 47, 333–340. [Google Scholar]
- Stankiewicz-Kosyl, M.; Wrochna, M.; Salas, M.; Gawroński, S.W. A strategy of chemical control of Apera spica-venti L. resistant to sulfonylureas traced on the molecular level. J. Plant Prot. Res. 2017, 57, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Adamczewski, K.; Kierzek, R.; Matysiak, K. Multiple resistance to acetolactate synthase (ALS)- and acetyl-coenzyme A carboxylase (ACCase)-inhibiting herbicides in blackgrass (Alopecurus myosuroides Huds.) populations from Poland. J. Plant. Prot. Res. 2016, 56, 402–410. [Google Scholar] [CrossRef]
- Varah, A.; Ahodo, K.; Coutts, S.R.; Hicks, H.L.; Comont, D.; Crook, L.; Hull, R.; Neve, P.; Childs, D.Z.; Freckleton, R.P.; et al. The costs of human-induced evolution in an agricultural system. Nat. Sustain. 2020, 3, 63–71. [Google Scholar] [CrossRef]
- Comont, D.; Hicks, H.; Crook, L.; Hull, R.; Cocciantelli, E.; Hadfield, J.; Childs, D.; Freckleton, R.; Neve, P. Evolutionary epidemiology predicts the emergence of glyphosate resistance in a major agricultural weed. New Phytol. 2019, 223, 1584–1594. [Google Scholar] [CrossRef]
- Rozkrut, D. (Ed.) Statistical Yearbook of Agriculture; Statistics Poland: Warsaw, Poland, 2020. [Google Scholar]
- Moss, S.; Ulber, L.; Hoed, I. A herbicide resistance risk matrix. Crop Prot. 2019, 115, 13–19. [Google Scholar] [CrossRef]
- Moss, S.R.; Perryman, S.A.M.; Tatnell, L.V. Managing Herbicide-Resistant Blackgrass (Alopecurus myosuroides): Theory and Practice. Weed Technol. 2007, 21, 300–309. [Google Scholar] [CrossRef]
- Torra, J.; Royo-Esnal, A.; Rey-Caballero, J.; Recasens, J. Management of Herbicide—Resistant Corn Poppy (Papaver rhoeas) under Different Tillage Systems Does Not Change the Frequency of Resistant Plants. Weed Sci. 2018, 66, 764–772. [Google Scholar] [CrossRef] [Green Version]
- Vijayarajan, V.B.A.; Forristal, P.D.; Cook, S.K.; Staples, J.; Schilder, D.; Hennessy, M.; Barth, S. First Report on Assessing the Severity of Herbicide Resistance to ACCase Inhibitors Pinoxaden, Propaquizafop and Cycloxydim in Six Avena fatua Populations in Ireland. Agronomy 2020, 10, 1362. [Google Scholar] [CrossRef]
- Travlos, I.; Prado, R.; Chachalis, D.; Bilalis, D.J. Editorial: Herbicide Resistance in Weeds: Early Detection, Mechanisms, Dispersal, New Insights and Management Issues. Front. Ecol. Evol. 2020, 8, 213. [Google Scholar] [CrossRef]
- Weisberger, D.; Nichols, V.; Liebman, M. Does diversifying crop rotations suppress weeds? A meta-analysis. PLoS ONE 2019, 14, e0219847. [Google Scholar] [CrossRef] [Green Version]
- Massa, D.; Kaiser, Y.I.; Andújar-Sánchez, D.; Carmona-Alférez, R.; Mehrtens, J.; Gerhards, R. Development of a Geo-Referenced Database for Weed Mapping and Analysis of Agronomic Factors Affecting Herbicide Resistance in Apera spica-venti L. Beauv. (Silky Windgrass). Agronomy 2013, 3, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Beckie, H.J.; Harker, K.N. Our top 10 herbicide-resistant weed management practices. Pest Manag. Sci. 2017, 73, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Ngow, Z.; Chynoweth, R.J.; Gunnarsson, M.; Rolston, P.; Buddenhagen, C.E. A herbicide resistance risk assessment for weeds in wheat and barley crops in New Zealand. PLoS ONE 2020, 5, e0234771. [Google Scholar] [CrossRef] [PubMed]
- Thornby, D.; Werth, J.; Hereward, J.; Keenan, M.; Chauhan, B. Herbicide resistance evolution can be tamed by diversity in irrigated Australian cotton: A multi-species, multi-herbicide modelling approach. Pest Manag. Sci. 2018, 74, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Adamczewski, K.; Kierzek, R. Mechanism of resistance on acetylolactate synthase (ALS) of Centaurea cyanus L. biotypes cross-resistant. Prog. Plant Prot./Post. Ochr. Roślin 2011, 51, 317–324. (In Polish) [Google Scholar]
- Gaines, T.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.; Kupper, A.; Dayan, F. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 24, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Hada, Z.; Menchari, Y.; Rojano-Delgado, A.M.; Torra, J.; Menéndez, J.; Palma-Bautista, C.; de Prado, R.; Souissi, T. Point Mutations as Main Resistance Mechanism Together with P450-Based Metabolism Confer Broad Resistance to Different ALS-Inhibiting Herbicides in Glebionis coronaria From Tunisia. Front. Plant Sci. 2021, 12, 626–702. [Google Scholar] [CrossRef] [PubMed]
- Kaloumenos, N.S.; Adamouli, V.N.; Christos, A.; Dordas, C.A.; Eleftherohorinos, I.G. Corn poppy (Papaver rhoeas) cross-resistanceto ALS-inhibiting herbicides. Pest. Manag. Sci. 2011, 67, 574–585. [Google Scholar] [CrossRef]
- Kati, V.; Scarabel, L.; Thiery-Lanfranchi, D.; Kioleoglou, V.; Liberopoulou, S.; Délye, C. Multiple resistance of Papaver rhoeas L. to 2,4-D and acetolactate synthase inhibitors in four European countries. Weed Res. 2019, 59, 367–376. [Google Scholar] [CrossRef]
- Walsh, M.J.; Owen, M.J.; Powles, S.B. Frequency and distribution of herbicide resistance in Raphanus raphanistrum populations randomly collected across the Western Australian wheatbelt. Weed Res. 2007, 47, 542–550. [Google Scholar] [CrossRef]
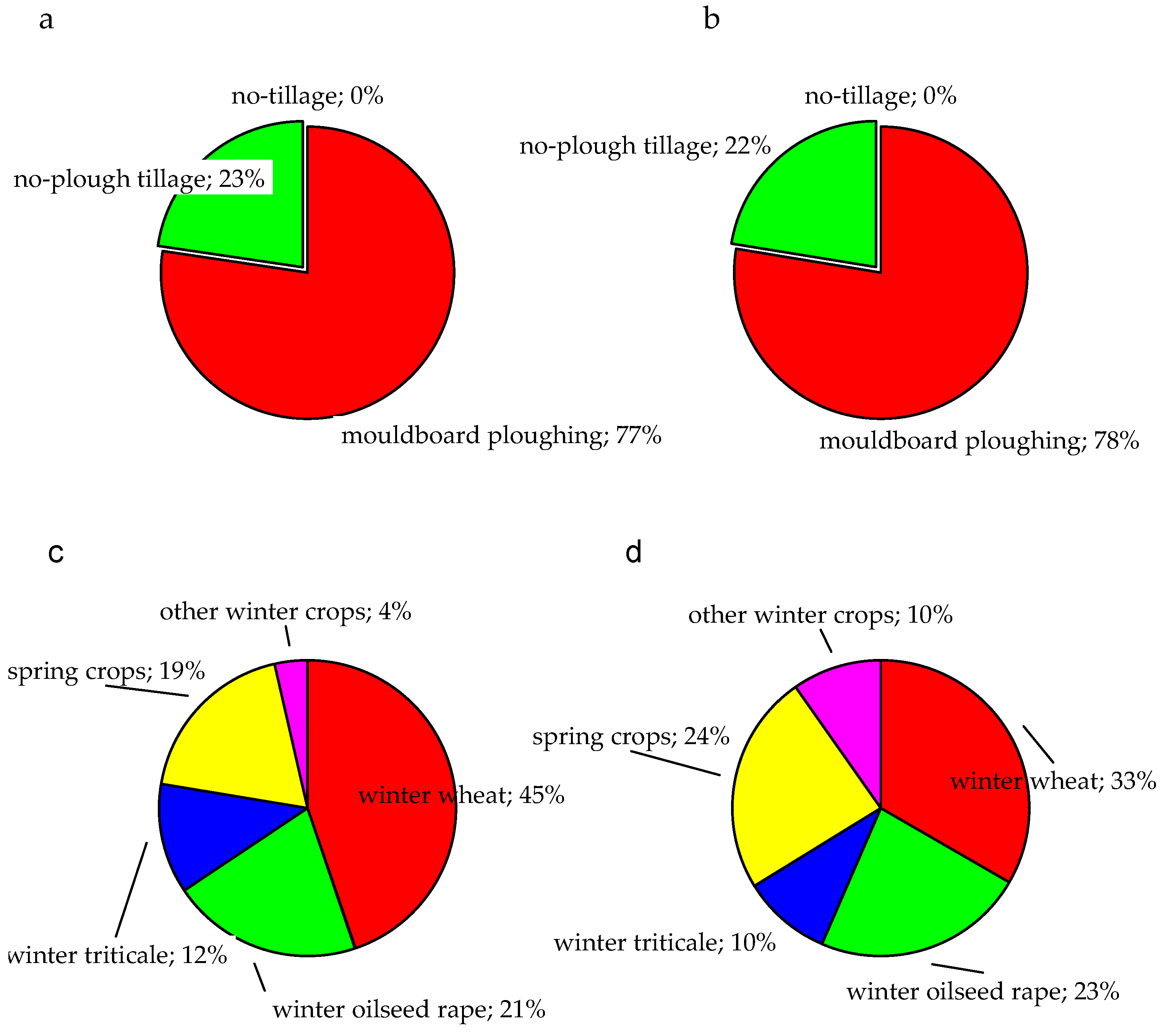


| Active Substance | Commercial Product | Content of Active Substance In Commercial Products | Producer | Field Dose (N1) of Active Substance |
|---|---|---|---|---|
| Tribenuron-methyl | Lumer 500 WG | 500 g kg−1 | ADAMA, PL | 15 g ha−1 |
| Florasulam | Saracen 050 SC | 50 g L−1 | Cheminova, PL | 5 g ha−1 |
| 2,4-D | Aminopielik Standard 600 SL | 600 g L−1 | ADAMA, PL | 750 g ha−1 |
| Dicamba | Dicash | 480 g L−1 | Sharda, PL | 288 g ha−1 |
| HRAC | Active Ingredient | T + F | T | F | S | |
|---|---|---|---|---|---|---|
| 2 | florasulam | 0.70 | 0.57 | 0.25 | 0.45 | |
| tribenuron-methyl | 0.49 | 0.39 | 0.50 | 0.38 | ||
| metsulfuron-methyl | 0.38 | 0.18 | 0.11 | |||
| chlorsulfuron | 0.30 | 0.14 | 0.25 | 0.18 | ||
| iodosulfuron-methyl-Na | 0.32 | 0.18 | 0.13 | 0.16 | ||
| thifensulfuron-methyl | 0.21 | 0.18 | 0.04 | |||
| thiencarbazone-methyl | 0.11 | 0.11 | 0.13 | 0.07 | ||
| sulfosulfuron | 0.04 | |||||
| flupyrsulfuron-methyl | 0.02 | 0.05 | ||||
| triasulfuron | 0.02 | 0.11 | ||||
| Total | 2.59 | 1.86 | 1.26 | 1.44 | ||
| 5 | metribuzin | 0.28 | 0.25 | 0.26 | 0.30 | |
| metamitron | 0.02 | |||||
| terbuthylazine | 0.07 | 0.03 | ||||
| chlorotoluron | 0.26 | 0.29 | 0.12 | 0.24 | ||
| isoproturon | 0.21 | 0.04 | 0.04 | |||
| Total | 0.77 | 0.65 | 0.38 | 0.61 | ||
| 6 | bromoxynil | 0.04 | ||||
| 12 | diflufenican | 0.62 | 0.68 | 0.50 | 0.54 | |
| 9 | glyphosate | 0.02 | 0.05 | |||
| 3 | pendimethalin | 0.19 | 0.07 | 0.12 | ||
| 4 | 2,4 D | 0.51 | 0.21 | 0.12 | 0.32 | |
| aminopyralid | 0.49 | 0.43 | 0.75 | 0.37 | ||
| clopyralid | 0.11 | 0.04 | 0.63 | 0.13 | ||
| dicamba | 0.09 | 0.07 | 0.05 | |||
| fluroxypyr | 0.28 | 0.07 | 0.16 | |||
| MCPA | 0.09 | 0.07 | 0.09 | |||
| MCPB | 0.01 | |||||
| picloram | 0.09 | 0.04 | 0.63 | 0.08 | ||
| Total | 1.66 | 0.93 | 2.13 | 1.21 | ||
| Mean for populations | 5.85 | 4.23 | 4.27 | 3.97 | ||
| Number of populations | 47 | 28 | 8 | 76 | ||
| PT= | 0–0.19 | 0.20–0.39 | 0.40–0.59 | 0.60–0.79 | ||
| Population | Province | Tribenuron-Methyl | Florasulam | ||
|---|---|---|---|---|---|
| RI | ED50 (g ha−1) | RI | ED50 (g ha−1) | ||
| 8818 | GP | S | X | RRR | 27.3 |
| 8551 | KP | RRR | 90.0 | S | X |
| 9263 | KP | RR | 45.0 | S | X |
| 9264 | KP | R | 30.0 | S | X |
| 9334 | KP | R | 35.0 | S | X |
| 9339 | KP | RR | 55.0 | r | 4.5 |
| 9347 | KP | RR | 40.0 | S | X |
| 9586 | LP | S | X | R | 9.0 |
| 9624 | LP | S | X | r | 4.7 |
| 9632 | LP | S | X | R | 9.6 |
| 8920 | LP | S | X | RRR | 60.5 |
| 8924 | LP | RRRR | >480 | RRRR | >160 |
| 8925 | LP | S | X | RRRR | >160 |
| 8948 | LP | RRRR | >480 | RRR | 80.5 |
| 9012 | LP | RRRR | >480 | RRR | 46.9 |
| 9015 | LP | RRRR | >480 | S | X |
| 9017 | LP | RRRR | >480 | RRR | 95.1 |
| 9025 | LP | RRRR | >480 | S | X |
| 9028 | LP | RRRR | >480 | R | 11.4 |
| 9030 | LP | RRRR | >480 | S | X |
| 8413 | Lb | RRR | 80.8 | RRR | 27.7 |
| 8486 | Lb | RRR | 374.1 | S | X |
| 8492 | Lb | RRR | 277.3 | RR | 14.6 |
| 8494 | Lb | RRR | 89.3 | RR | 14.0 |
| 8512 | Lb | RRR | 108.2 | RR | 22.8 |
| 8516 | Lb | RRR | 148.6 | RRR | 60.4 |
| 8524 | Lb | RRR | 119.7 | RRR | 27.2 |
| 8717 | Lb | RRR | 131.7 | RR | 13.7 |
| 8726 | Lb | RRRR | >480 | RRR | 34.3 |
| 8729 | Lb | RRRR | >480 | RRR | 51.8 |
| 8734 | Lb | RRR | 281.7 | RRR | 45.0 |
| 9414 | Lb | RRR | 101.6 | R | 7.2 |
| 10111 | Ms | RR | 43.0 | RR | 17.0 |
| 9302 | Pd | RRR | 158.7 | RRR | 49.4 |
| 9303 | Pd | RRR | 229.5 | RRR | 136.4 |
| 9306 | Pd | RRR | 201.8 | RRR | 99.1 |
| 9307 | Pd | RRR | 151.0 | RRR | 76.6 |
| 9309 | Pd | RR | 50.6 | RRR | 57.7 |
| 9400 | Pd | RRR | 89.0 | R | 7.8 |
| 9402 | Pd | RRR | 281.6 | RRR | 50.4 |
| 10264 | Pd | RRR | 88.8 | S | X |
| 9240 | Pm | RR | 60.0 | S | X |
| 9247 | Pm | RRR | 155.0 | S | X |
| 9249 | Pm | RR | 54.0 | S | X |
| 9251 | Pm | RR | 65.0 | S | X |
| 9256 | Pm | RRR | 90.0 | S | X |
| 9655 | Pm | RRRR | >480 | R | 6.8 |
| 10298 | Pm | RRRR | >480 | RR | 21.0 |
| 10356 | Sb | RRR | 77.3 | S | X |
| 8834 | Św | S | X | R | 7.9 |
| 9597 | Św | R | 30.9 | S | X |
| 9622 | Św | S | X | RRR | 37.9 |
| 10289 | Św | RRR | 75.6 | S | X |
| 10293 | Św | r | 13.5 | S | X |
| 10339 | Św | RRR | 153.6 | S | X |
| 10345 | Św | RRR | 109.3 | S | X |
| 10348 | Św | RRR | 127.9 | S | X |
| 8401 | WM | RRR | 259.7 | RRR | 43.5 |
| 8402 | WM | RRR | 155.4 | RRR | 101.9 |
| 8405 | WM | RRR | 111.1 | RRR | 25.9 |
| 8406 | WM | RRR | 302.0 | RRR | 29.3 |
| 8537 | WM | RRR | 320.0 | R | 10.0 |
| 8738 | WM | RRRR | >480 | RRR | 40.3 |
| 8743 | WM | RRRR | >480 | RRR | 51.8 |
| 8748 | WM | RRRR | >480 | RRR | 48.9 |
| 8794 | WM | R | 27.6 | S | X |
| 8798 | WM | R | 39.2 | RRR | 35.0 |
| 8821 | WM | RRRR | >480 | S | X |
| 9258 | WM | RRR | 85.0 | r | 5.0 |
| 9260 | WM | RR | 60.0 | r | 5.0 |
| 9380 | WM | RRR | 117.3 | R | 11.3 |
| 9661 | WM | RRRR | >480 | S | X |
| 10280 | WM | RRRR | >480 | S | X |
| 10370 | WM | RRRR | >480 | RRR | 54.0 |
| 8972 | WP | RRRR | >480 | RR | 17.1 |
| 10301 | WP | RRRR | >480 | R | 10.8 |
| 10302 | WP | R | 29.5 | S | X |
| 10304 | WP | RRRR | >480 | RRR | 48.5 |
| 10309 | WP | RRR | 320.4 | R | 7.1 |
| 10317 | WP | RRRR | >480 | R | 9.6 |
| 10318 | WP | RRR | 149.7 | RRR | 26.7 |
| 10327 | WP | RRR | 118.6 | RRR | 26.5 |
| 10333 | WP | RRRR | >480 | S | X |
| Biotype | Province | Dicamba | 2,4-D | ||
|---|---|---|---|---|---|
| RI | ED50 (g ha−1) | RI | ED50 (g ha−1) | ||
| 8486 | Lb | r | 397.5 | r | 696 |
| 9400 | Pd | r | 334.34 | r | 732 |
| 9380 | WM | R | 583.8 | r | 750 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stankiewicz-Kosyl, M.; Haliniarz, M.; Wrochna, M.; Synowiec, A.; Wenda-Piesik, A.; Tendziagolska, E.; Sobolewska, M.; Domaradzki, K.; Skrzypczak, G.; Łykowski, W.; et al. Herbicide Resistance of Centaurea cyanus L. in Poland in the Context of Its Management. Agronomy 2021, 11, 1954. https://doi.org/10.3390/agronomy11101954
Stankiewicz-Kosyl M, Haliniarz M, Wrochna M, Synowiec A, Wenda-Piesik A, Tendziagolska E, Sobolewska M, Domaradzki K, Skrzypczak G, Łykowski W, et al. Herbicide Resistance of Centaurea cyanus L. in Poland in the Context of Its Management. Agronomy. 2021; 11(10):1954. https://doi.org/10.3390/agronomy11101954
Chicago/Turabian StyleStankiewicz-Kosyl, Marta, Małgorzata Haliniarz, Mariola Wrochna, Agnieszka Synowiec, Anna Wenda-Piesik, Ewa Tendziagolska, Magdalena Sobolewska, Krzysztof Domaradzki, Grzegorz Skrzypczak, Witold Łykowski, and et al. 2021. "Herbicide Resistance of Centaurea cyanus L. in Poland in the Context of Its Management" Agronomy 11, no. 10: 1954. https://doi.org/10.3390/agronomy11101954
APA StyleStankiewicz-Kosyl, M., Haliniarz, M., Wrochna, M., Synowiec, A., Wenda-Piesik, A., Tendziagolska, E., Sobolewska, M., Domaradzki, K., Skrzypczak, G., Łykowski, W., Krysiak, M., Bednarczyk, M., & Marcinkowska, K. (2021). Herbicide Resistance of Centaurea cyanus L. in Poland in the Context of Its Management. Agronomy, 11(10), 1954. https://doi.org/10.3390/agronomy11101954









