Abstract
The fermentation quality of alfalfa silage is poor but can be improved with additives. This study investigates the effects of biochar on the fermentation quality and bacterial diversity of high-moisture alfalfa silage. Alfalfa was treated with: (i) control without additive (CK); (ii) 1% biochar (1% carbon C); (iii) 2% biochar (2% C), and fermented for 15 or 30 d. Mixing alfalfa with biochar significantly decreased (p < 0.05) pH, the number of coliform bacteria, nonprotein nitrogen content, and ammonia–nitrogen content, and significantly increased the contents of dry matter, lactic acid, and true protein. The addition of biochar also influenced bacterial community distribution. The relative abundance of Lactobacillus and Enterococcus increased while the abundance of Pantoea decreased with biochar treatment. In conclusion, alfalfa silage’s fermentation quality and microbial community structure are improved by adding biochar from the pyrolysis of waste furniture.
1. Introduction
“Ensiling” is a common preservation method of animal forage in which lactic acid bacteria (LAB) use carbohydrates as substrates to produce organic acids under anaerobic conditions [1]. Alfalfa (Medicago sativa L.) is a widely cultivated and important forage worldwide because of its high economic value and yield [2]. The low water-soluble carbohydrate (WSC) content and high buffering capacity of alfalfa hamper the creation of high-quality alfalfa silage [3]. Ni et al. [4] reported that alfalfa silage has poor fermentation quality, which is usually attributed to the growth of undesirable bacteria from genera such as Clostridium and Enterobacter. Thus, silage additives are necessary to obtain well-fermented alfalfa silage.
“Biochar” is a charcoal-rich product produced by heating wood, fertilizer, and organic waste as the starting material of biomass in a closed container [5]. It is used mainly as a promising multifunctional soil conditioner. Biochar application in combination with high-P fertilizer input may increase alfalfa yields and lower Cd concentrations [6]. Another study showed that soil microbial diversity changed in cornfields after the addition of biochar [7]. Biochar has a porous structure, a large surface area, and a high capacity for gas absorption, so it was used as a carrier to enhance crop growth effectively and to vehicle active degrader strains in soil [8,9]. Biochar application leads to an increased abundance of Nitrosomonas and Nitrospira and promotes fungal growth such as Zygomycota, Glomeromycota, and Neocallimastigomycota. It was reported that biochar provides mineral nutrients and an increased surface area, promotes biofilm habitats suitable for bacterial community proliferation in the animal rumen, and improves ruminal feed digestion [10]. Moreover, it is also possible to reduce ruminal enteric methane emissions by reducing rumen methanogens and increasing methanotrophs [11,12]. Additionally, the cost of biochar is low, and it can also be used as a new silage additive with ecological and economic benefits. Biochar is added to silage, which absorbs nutrients from ruminant intestines and then enters the soil with feces, effectively improving soil fertility and grassland productivity and creating an ideal environment for incorporating biochar into agricultural systems [5,13]. According to previous studies, sugarcane bagasse fermentation could stimulate bacterial growth consequent to biochar treatment [14]. However, few studies have focused on the effects of biochar on the fermentation characteristics and bacterial community in silage thus far.
The primary purpose of the present study is to evaluate the effect of introducing biochar on silage quality and microbial community structure for ensiling alfalfa (Medicago sativa L.).
2. Materials and Methods
2.1. Silage Preparation
Alfalfa was manually collected from three experimental plots (each 10 m2) at South China Agricultural University (23.24° N, 113.64° E, Guangzhou, China) in July 2020. No herbicide or fertilizer was used during planting. Fresh alfalfa was mixed and chopped by hand to 1–2 cm with a paper cutter. Following the method described in an editorial note by Robinson et al. [15], the chopped fresh material was randomly divided into 18 sub-samples (each about 100 g), and each treatment was set up in triplicate. Additives were administered and mixed homogenously with alfalfa and divided into equal portions for the three treatments: no additive (CK); 1% biochar based on fresh matter (FM) (1% carbon C); 2% biochar based on FM (2% C). Next, the alfalfa was packed and compressed manually into plastic-film bags, sealed with a vacuum-packaging machine, and then prepared and stored indoors at 21–30 °C. Three bags for each treatment were opened randomly to analyze fermentation quality and bacterial community after 15 and 30 d of ensiling. Following a method described by Zhang et al. [16], biochar was produced by drying mixed wood waste from a furniture factory and pyrolyzing it for 6 h in a carbonized furnace at 450 °C. It was found to contain many mineral nutrient elements.
2.2. Analyses of Bacterial Populations, Organic Acids, and Chemical Composition
A total of 20 g (including raw material and silage) of alfalfa was taken randomly, soaked in 180 mL of sterile 0.9% saline for about 15 min, and serially diluted from 10−1 to 10−6 on a clean bench. LAB and coliform bacteria were cultured, and their abundance was estimated using de Man–Rogosa–Sharpe agar and violet red bile agar at 30 °C for 2 d [17]. Samples of yeast and mold were cultured and determined on Rose Bengal agar for 2 d at 28°C [18]. A total of 20 g of each silage sample was homogenized with 180 mL of distilled water for 18 h at 4 °C and then filtered through four layers of cheesecloth and filter paper. A glass-electrode pH meter was immediately employed to measure the pH of this filtrate. Ammonia nitrogen (NH3-N) content was measured using the phenol–hypochlorite assay [19]. The contents of organic acids were determined by high-performance liquid chromatography (HPLC) according to the conditions and procedure described by Wang et al. [17]. Silage samples (~100 g) were dried at 65 °C for 48 h to determine the dry matter (DM) contents. A laboratory knife mill with a 1 mm screen was used to grind the dried samples. The WSC content was analyzed by colorimetry after reaction with an anthrone reagent [18]. The crude protein (CP) content was analyzed following the method described by the Association of Official Analytical Chemists [20]. The contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF) were determined according to the protocols described by Van Soest et al. [21]. Nonprotein nitrogen (NPN) and true protein (TP) contents were measured according to the method detailed by Licitra et al. [22].
2.3. Analyses of Bacterial Communities
2.3.1. Bacterial DNA Isolation and 16S Amplicon Sequencing
To analyze the bacterial community composition of alfalfa fermented with biochar, all samples were collected and then stored at −20 °C before DNA extraction. DNA was extracted using a DNA isolation kit (Omega Biotek, Norcross, GA, USA) according to manufacturer protocols. Polymerase chain reactions (PCRs) were conducted in a 50 μL system (100 ng of template DNA, 5 μL of 2.5 mM deoxynucleoside triphosphates (dNTP), 1.5 μL of primer (5 μM), 1 μL of KOD polymerase, and 5 μL of 10 × KOD buffer) to amplify 16S rDNA V3–V4 hypervariable regions using 341F (CCTACGGGNGGCWGCAG) and 806R (GGACTACHVGGGTATCTAAT) as primers according to the method of Wang et al. [23]. An Illumina platform (Guangzhou Gene Denovo, Guangzhou, China) was used to sequence the PCR products after purification.
2.3.2. Illumina Hiseq2500 Sequencing
Purified amplicon sequencing was conducted using the Illumina platform according to the standard protocols. Raw tags obtained in this study were filtered using the QIIME (V1.9.1) pipeline, and the UCHIME algorithm was applied to identify and remove chimeric sequences.
2.4. Statistical Analyses
The effects of the number of ensiling days, the addition of biochar, and their interactions were compared by a two-way analysis of variance in SPSS 21 (IBM, Armonk, NY, USA). Duncan’s multiple range test was used to analyze the differences among the mean values of each sample. All figures were created using Adobe Illustrator CS 4.0 (San Jose, CA, USA). We then analyzed the DNA-sequencing data using a platform of OmicShare tools (http://www.omicshare.com/tools accessed on 11 October 2020) according to the method of Wang et al. [17].
3. Results and Discussion
3.1. Characteristics of Alfalfa Material
Fresh alfalfa contained 30.0% DM (Table 1). This high DM content of alfalfa explains why it could be ensiled directly without wilting. The CP content (14.1% DM) of alfalfa was slightly lower than that reported by Wang et al. [23], and this difference may have been due to a different climate, fertilization, or harvest time. The NDF and ADF contents were 47.1 and 31.5% DM, respectively. The high fiber content may be related to its high dry matter content. It is necessary to reduce the fiber content of alfalfa by silage fermentation. The WSC content is a crucial indicator for assessing fermentation quality, and it was lower than the theoretical requirement (6–7% DM) in this study [24]. Hence, obtaining high-quality alfalfa silage may be difficult because the substrate for LAB fermentation is limited. In general, the initial LAB count of materials is >5.00 log10 cfu/g fresh matter for high-quality silage [25]. In the present study, the LAB count was 6.68 log10 cfu/g FM, which might be conducive to better alfalfa silage. The number of coliform bacteria was 6.25 log10 cfu/g FM. The numbers of yeasts and molds were 5.06 and 4.37 log10 cfu/g FM, respectively. Undesirable microbes could be very disadvantageous to alfalfa silage if they were present. Therefore, the use of effective additives to obtain a higher quality of alfalfa silage by inhibiting these harmful microbes at the early stage of ensiling would be appropriate.

Table 1.
Chemical composition and microbial population of fresh alfalfa prior to ensiling (±SD, n = 3).
3.2. Fermentation Quality of Alfalfa Silage
The DM content increased significantly (p < 0.01) after mixing with biochar (Table 2), which indicated that the water content of alfalfa was reduced after biochar treatment. pH is a crucial parameter to assess the quality of forage silage and is particularly important in high-moisture silage. The pH value of the alfalfa silage treated with 2% biochar decreased significantly (p < 0.05). In the present study, the pH values of all the silages were higher than the standard threshold of well-fermented silage (pH 4.2) [26], which may be detrimental to aerobic stability and long-time preservation. It might be related to the lower organic acids of alfalfa silage and lower WSC content in alfalfa raw materials. At the same time, high-moisture alfalfa might dilute the acid concentration limiting the decrease in pH. In addition, after 30 d of ensiling, the amount of lactic acid increased significantly (p < 0.05) upon treatment with 2% biochar compared with that in the controls. This phenomenon might explain the significant decrease in pH (p < 0.01) in alfalfa silage after mixing in biochar. The addition of biochar would not affect the amount of acetic acid, which ranged across all treatments from 0.78 to 0.84% DM. Acetic acid is an important antifungal agent, and it could improve the aerobic stability of silage, which might explain why molds and yeasts were not detected. The content of propionic acid was below the lower limit of detection in our study. Butyric acid is a primary product of activities by the Clostridia species during ensiling. Clostridial fermentation is undesirable because it leads to nutritional loss and latent health issues [26]. Furthermore, clostridial activity reduces intake of silages by livestock, especially if the level of butyric acid in silage is >0.5% DM [27]. The content of butyric acid was not detected in 2% biochar-treated silages stored for 30 d. This result might be due to a decrease in pH after biochar treatment, thereby leading to inhibition of the activity of Clostridium species. Silage stored for 30 d had a significantly lower LAB count (p < 0.05) than that stored for 15 d. This result could have occurred because the fermentation substrate for LAB was reduced with the prolongation of silage storage time. The number of coliform bacteria significantly decreased (p < 0.01) after biochar treatment and was below the lower detection limit after 30 d of ensiling. This observation indicated that the growth rate of coliform bacteria after the biochar treatment was much lower than in the controls, and that long-term silage and addition of biochar could effectively inhibit their growth.

Table 2.
Effect of mixing biochar on fermentation parameters and microbial populations of alfalfa ensiled for 15 and 30 d.
In general, a higher proportion of true protein indicates a higher nutritional value of the protein, while the nitrogen utilization ratio of true protein is higher than that of nonprotein-N. The content of true protein in the biochar treatment group was significantly higher (p < 0.01) than in the control group (Table 3). At the same time, proteolysis of high moisture protein feed is of particular concern in silage fermentation. Enzymes in silage first hydrolyze proteins into peptides and free amino acids and further degrade them into amides, amines, and ammonia through microbial activities [28]. Nonprotein-N and ammonia–N are important parameters of proteolysis in silages. Accumulation of ammonia–N during ensiling is closely related to the interaction of proteases and bacterial activity [29]. In the present study, more than half of the protein in alfalfa silage (65.1% TN) was degraded after a 30 d ensiling. As in our previous study, 56.04% of the protein in stylo was degraded after 60 d of silage [30]. Therefore, it is necessary to take measures to inhibit protein hydrolysis. The use of additives is an appropriate method to reduce protein degradation due to the serious protein loss of legumes in natural silage. The nonprotein-N content decreased significantly (p < 0.01) upon the biochar treatment, which indicated that the addition of biochar might improve protein preservation in alfalfa silage. The ammonia–N content decreased significantly in alfalfa silage compared with that in controls (p < 0.01), which may be because the biochar could alter the bacterial community and affect microbe activity [31]. The decrease in pH upon biochar treatment in silage leads to the inactivation of plant enzymes and inhibits proteolysis. Similarly, Wang et al. [32] reported that the activity of proteases decreased when formic acid was added to the Neolamarckia cadamba leaves. Microbial and protease activities are inhibited under acidic conditions; thus, proteins are efficiently preserved. Hence, biochar could be used as an additive to reduce protein degradation and improve the fermentation quality of alfalfa.

Table 3.
Effect of mixing biochar on the protein fractions of alfalfa ensiled for 15 and 30 d.
3.3. Bacterial Community of Alfalfa Silage
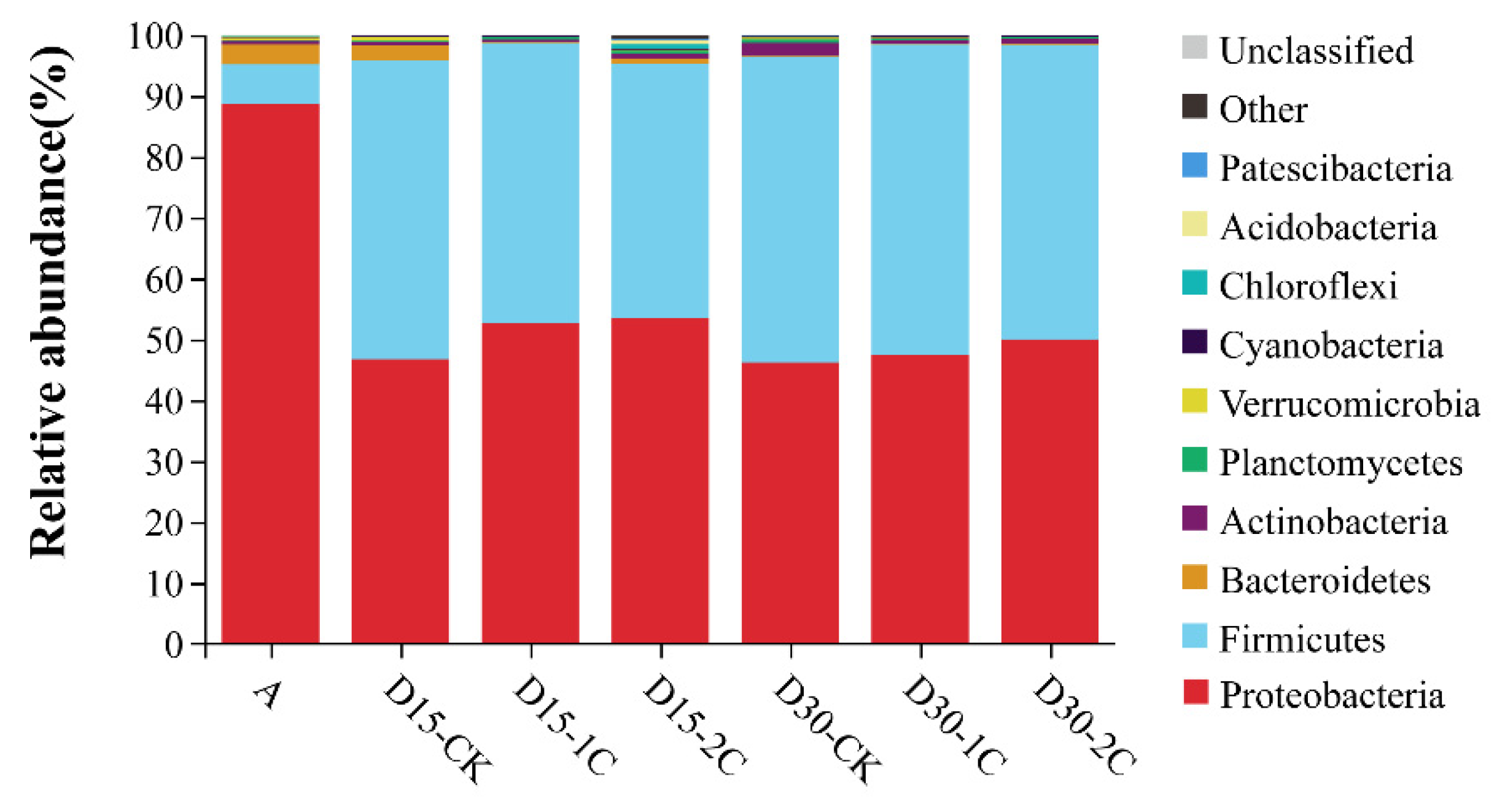
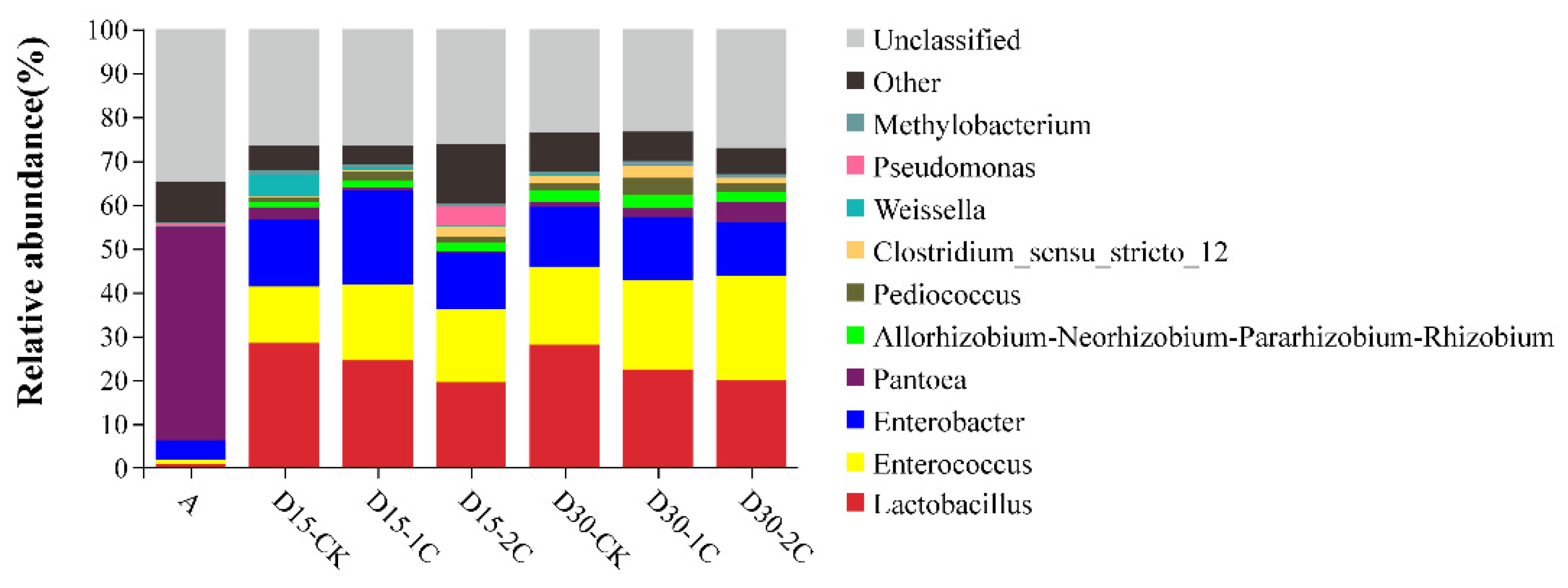
The phyla of bacterial communities and their relative abundance in alfalfa silage are shown in Figure 1. Proteobacteria was the most abundant phylum in raw material (~90%). After fermentation for 15 and 30 d, bacteria of the phylum Proteobacteria decreased from 88.6 to 46.7 and 46.2%, respectively, but remained the most abundant. The relative abundance of bacteria of the phylum Firmicutes increased rapidly compared with their abundance in the raw material. Similar results have been documented by Liu et al. [33], where Firmicutes and Proteobacteria were the most abundant phyla at any time during barley silage, and their relative richness continued to increase to 99% after 60 d of silage. In the present study, the total number of bacteria from the phyla Firmicutes and Proteobacteria increased to ~99% in the latter stage of ensiling, and they were the most abundant phyla of all treatments of raw material during ensiling. The dominance of these two phyla may be due to the low pH and anaerobic conditions during ensiling, which would have been beneficial to the growth of bacteria from these phyla. The percentage of bacteria from the phylum Bacteroidetes decreased gradually and was virtually absent after 30 d of silage. The percentage of bacteria from the phylum Actinobacteria increased at 30 d of silage, but it was not significant. The genus of bacterial communities in alfalfa silage and their relative abundance are shown in Figure 2. Bacteria of the genus Lactobacillus have a high tolerance to low pH and a relatively high capacity of acid production and are, therefore, often screened for use as inoculants in silage. When the pH declined, Lactobacillus became the dominant species and grew vigorously. The relative abundance of Lactobacillus species increased from 0.95 to 28.08% in alfalfa silages after 30 d. Enterococcus species could produce lactic acid, and they could usually survive only in the early stages of silage because of their poor tolerance to acids. The relative abundance of Enterococcus species increased after silage fermentation. Nishino et al. [34] observed a similar result in guinea grass silage, where the results showed that the richness of Enterococcus increased with the extension of time and reached a maximum at 56 d. This result might have been caused by pH being high at a late stage of silage and decreasing slowly. In summary, the total numbers of Lactobacillus and Enterococcus species after 30 d of silage were more than those at 15 d. This indicates that biochar could increase the relative abundance of some LAB and may partially explain the positive effect of biochar in the ensiling of alfalfa. Moreover, more abundant Enterobacter species were discovered after ensiling, but the increase in the number of Enterobacter species after treatment with 2% biochar was less than that for treatment with 1% biochar. Enterobacter species are harmful because they can produce ammonia–N in silage and compete for substrates with LAB [29]. The decrease in the number of Enterobacter species also explained the decrease in ammonia–N content upon the addition of biochar in alfalfa silage.
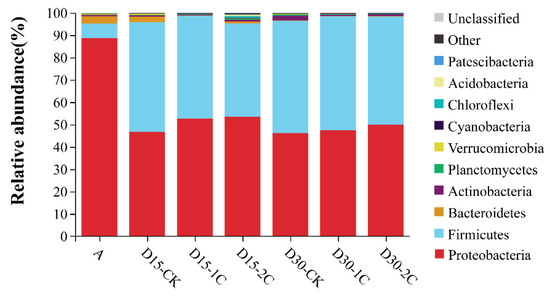
Figure 1.
Microbial community and relative abundance by phylum for alfalfa silage (A, fresh alfalfa; CK, the control; 1C, 1% biochar; 2C, 2% biochar; D15, D30, after 15, 30 days ensiling, respectively).
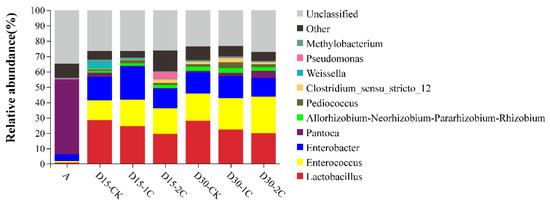
Figure 2.
Microbial community and relative abundance by genus for alfalfa silage (A, fresh alfalfa; CK, the control; 1C, 1% biochar; 2C, 2% biochar; D15, D30, after 15, 30 days ensiling, respectively).
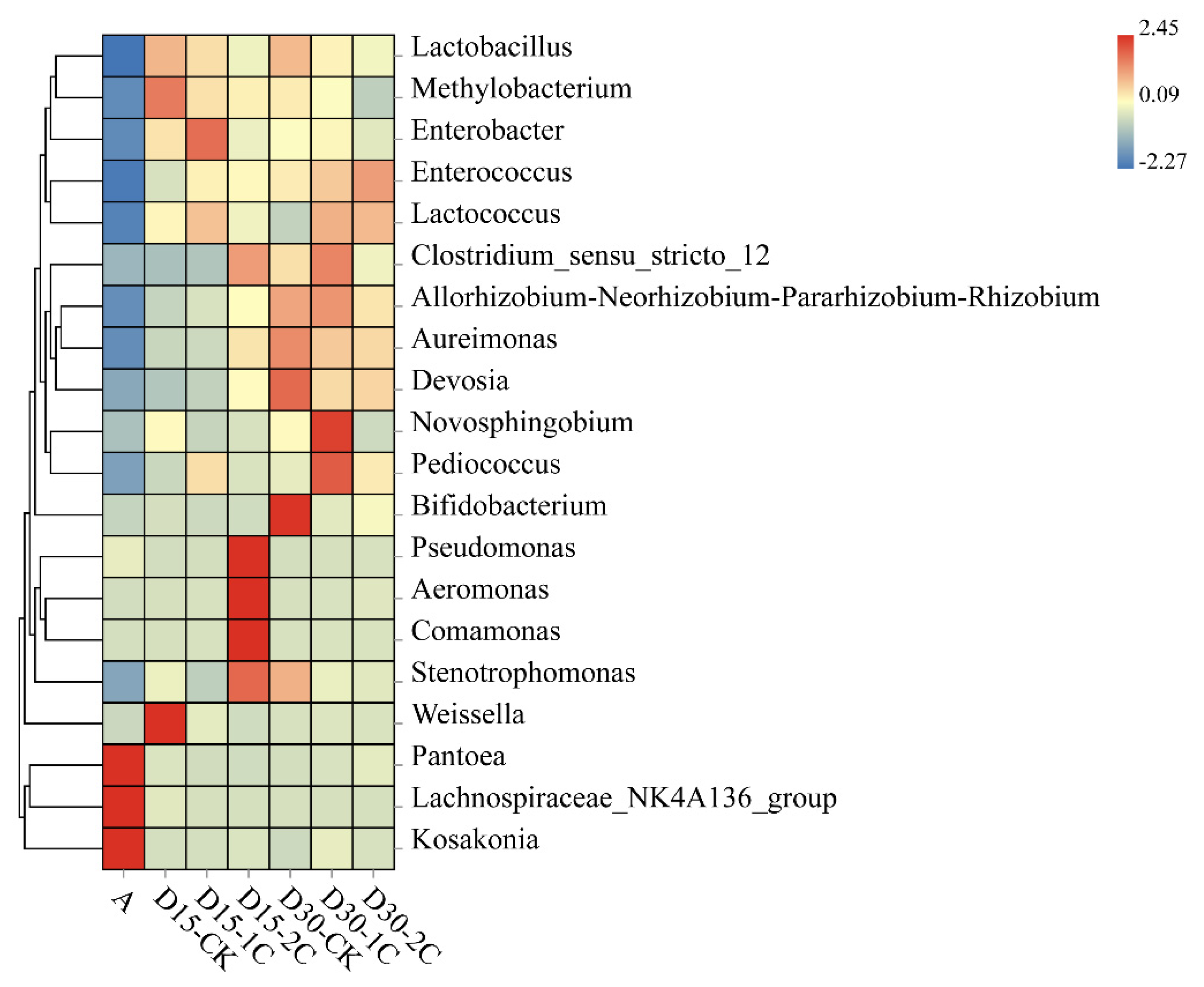
The effect of Pantoea species in silage fermentation is not known. Ogunade and colleagues stated that the number of Pantoea species is inversely associated with ammonia–N content [35]. It has been speculated that Pantoea species could reduce the ammonia–N content in silage. Conversely, Li et al. [36] considered the effect of Pantoea species to be similar to that of Enterobacter species in silage, in that they compete with LAB for nutrients, in turn inferring that Pantoea species in silage would also be undesirable. The relative abundance of Pantoea species was reduced significantly after silage fermentation in our study. However, after 30 d of silage, more abundant Pantoea species were observed after biochar treatment than in the controls. The relative abundance of Pediococcus, Clostridium, and Weissella was detected in silage, and the abundance of Clostridium decreased with the addition of 2% biochar compared with 1% biochar, especially after 30 d ensiling. However, the relative abundance of Lactobacillus decreased after the addition of biochar in 30 d silage (Figure 3). The decline in Lactobacillus may be related to other LABs increasing, such as Enterococcus and Lactococcus. Methylobacterium increased in alfalfa silage, but its relative abundance after adding biochar was less than in the control. The Methylobacterium is strictly aerobic and neutrophilic, and it has also been found in alfalfa silage [35]. At the same time, the relative abundance of Enterobacter species and Clostridium species decreased in 2% of the biochar after 30 d of silage (Figure 3). This finding indicates that the inhibitory effect of biochar upon Enterobacter species and Clostridium species was improved after the addition of 2% biochar. Ogunade et al. [35] suggested that the presence of Pseudomonas species may contribute to protein preservation. Pseudomonas species can survive in anaerobic conditions. After 15 d of silage, the relative abundance of Pseudomonas increased by adding 2% biochar, which partly explained the decrease in ammonia–N content in this treatment. The relative abundance of Aeromonas, Comamonas, and Weissella significantly increased in 15 d silage but declined in 30 d silage. The relative abundance of Pantoea, Lachnospiraceae, and Kosakonia was significantly decreased in silage compared with raw material. However, some of these genera had low relative abundance, and their role in silage has not been extensively studied. It was also considered possible that the predominance of LAB inhibits their growth over longer silage times. These phenomena indicate the positive effects of biochar on the control of undesirable microbes, which may explain the improvement in fermentation quality during ensiling.
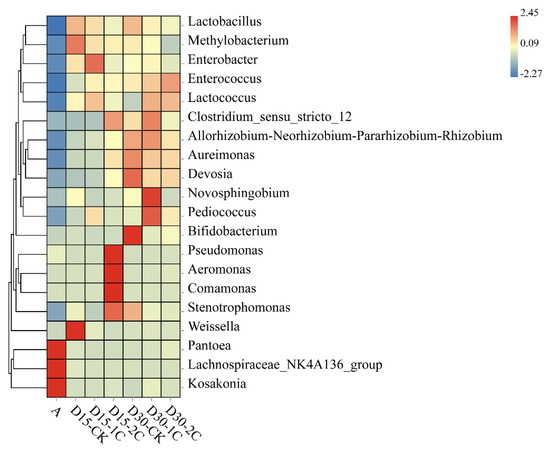
Figure 3.
Heatmap of prominent microbial genera (20 most abundant genera) for alfalfa silage (A, fresh alfalfa; CK, the control; 1C, 1% biochar; 2C, 2% biochar; D15, D30, after 15, 30 days ensiling, respectively).
4. Conclusions
This study revealed that mixing biochar to alfalfa silage positively asserted its fermentation quality and bacterial diversity. pH, the number of coliform bacteria, nonprotein-N content, and ammonia–N content of alfalfa silage decreased significantly following the addition of biochar. The relative abundance of Pantoea species, as well as bacteria of the phyla Proteobacteria and Bacteroidetes, decreased, while the relative abundance of Lactobacillus and Enterococcus increased, and the abundance of Pantoea decreased after biochar treatment. Our data suggest that mixing biochar from the pyrolysis of waste furniture could improve the fermentation quality and bacterial diversity of high-moisture alfalfa silage. Therefore, this method avoids the growth and reproduction of undesirable microorganisms and the loss of nutrients; it is a highly suitable method for improving the fermentation quality of alfalfa silage. However, further experiments should be performed to better understand microbial change mechanisms after adding biochar to alfalfa silage.
Author Contributions
Conceptualization, X.G., M.W. and Q.Z.; methodology, X.G., M.W. and Q.Z.; formal analysis and investigation, M.Z., S.W. and X.Z.; writing—original draft preparation, X.G., M.Z. and S.W.; writing—review and editing, X.C. and Q.Z.; funding acquisition, M.W. and Q.Z.; resources, M.W. and Q.Z.; supervision, M.W. and Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Guangzhou Forestry Science and Technology Innovation Commission (Grant No. 2018KJCX001), Scientific Research Foundation for the Introduction of Talent in Hebei Agricultural University (YJ201826).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Q.; Yu, Z.; Wang, X.; Risu, N. Effects of chlorpyrifos and chlorantraniliprole on fermentation quality of alfalfa (Medicago sativa L.) silage inoculated with or without Lactobacillus plantarum LP. Anim. Sci. J. 2017, 88, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Marshall, S.A.; Campbell, C.P.; Buchanan-Smith, J.G. Proteolysis and rumen degradability of alfalfa silages preserved with a microbial inoculant, spent sulfite liquor, formic acid or formaldehyde. Can. J. Anim. Sci. 1993, 73, 559–570. [Google Scholar] [CrossRef]
- Ni, K.K.; Lu, Y.; Wang, X.K.; Guo, L.N.; Li, X.M.; Yang, F.Y. Exploring the silage quality of alfalfa ensiled with the residues of astragalus and hawthorn. Bioresour. Technol. 2020, 297, 122249. [Google Scholar] [CrossRef]
- Calvelo, P.R.; Muetzel, S.; Camps, A.M.; Bishop, P.; Hina, K.; Hedley, M. Assessment of the influence of biochar on rumen and silage fermentation: A laboratory-scale experiment. Anim. Feed Sci. Technol. 2014, 196, 22–31. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhao, Z.J.; Chen, L.; Wang, L.Q.; Ji, L.Z.; Xiao, Y. Influences of arbuscular mycorrhizae, phosphorus fertiliser and biochar on alfalfa growth, nutrient status and cadmium uptake. Ecotoxicol. Environ. Saf. 2020, 196, 110537. [Google Scholar] [CrossRef]
- Agnieszka, W.M.; Tomasz, P.; Alicja, N.; Adam, K.; Dariusz, K.; Aleksandra, G.; Agnieszka, A.P. The Effect of Biochar-Based Organic Amendments on the Structure of Soil Bacterial Community and Yield of Maize (Zea mays L.). Agronomy 2021, 11, 7. [Google Scholar]
- Ajeng, A.A.; Abdullah, R.; Ling, T.C.; Ismail, S.; Lau, B.F.; Ong, H.C.; Chew, K.W.; Show, P.L.; Chang, J. Bioformulation of biochar as a potential inoculant carrier for sustainable agriculture. Environ. Technol. Innov. 2020, 20, 101168. [Google Scholar] [CrossRef]
- Andrey, V.G.; Tatiana, M.M.; Saglara, S.M.; Leonid, V.P.; Gerhard, S.; Inna, V.Z.; Vishnu, D.R.; Svetlana, N.S.; Dinesh, M.; Jun, Y. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health Off. J. Soc. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Leng, R.; Preston, T.; Inthapanya, S. Biochar reduces enteric methane and improves growth and feed conversion in local “Yellow” cattle fed cassava root chips and fresh cassava foliage. Livest. Res. Rural Dev. 2012, 11, 24. [Google Scholar]
- Toth, J.D.; Dou, Z. Use and impact of biochar and charcoal in animal production systems. Agric. Environ. Appl. Biochar Adv. Barriers 2016, 63, 199–224. [Google Scholar]
- Joseph, S.; Pow, D.; Dawson, K.; Mitchell, D.R.G.; Rawal, A.; Hook, J.; Solaiman, Z.M. Feeding biochar to cows: An innovative solution for improving soil fertility and farm productivity. Pedosphere 2015, 25, 666–679. [Google Scholar] [CrossRef]
- Bu, J.; Wei, H.L.; Wang, Y.T.; Cheng, J.R.; Zhu, M.J. Biochar boosts dark fermentative H2 production from sugarcane bagasse by selective enrichment/colonization of functional bacteria and enhancing extracellular electron transfer. Water Res. 2021, 202, 117440. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.; Wiseman, J.; Udén, P.; Mateos, G. Some experimental design and statistical criteria for analysis of studies in manuscripts submitted for consideration for publication. Anim. Feed Sci. Technol. 2006, 129, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Liu, B.; Liu, Q.; Zheng, H.; You, X.; Sun, K.; Luo, X.; Li, F. Comparative study of individual and Co-Application of biochar and wood vinegar on blueberry fruit yield and nutritional quality. Chemosphere 2020, 246, 125699. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Effects of wilting and Lactobacillus plantarum addition on the fermentation quality and microbial community of Moringa oleifera leaf silage. Front. Microbiol. 2018, 9, 1817. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; He, L.W.; Xing, Y.Q.; Zhou, W.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Fermentation quality and microbial community of alfalfa and stylo silage mixed with Moringa oleifera leaves. Bioresour. Technol. 2019, 284, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determinations of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertsom, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 4, 347–358. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Xing, Y.; Zhou, W.; Yang, F.; Chen, X.; Zhang, Q. Effects of mixing Neolamarckia cadamba leaves on fermentation quality, microbial community of high moisture alfalfa and stylo silage. Microb. Biotechnol. 2019, 5, 869–878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.H. Theoretical Carbohydrates Requirement for Alfalfa Silage Production. Agron. J. 1962, 4, 291–293. [Google Scholar] [CrossRef]
- Cai, Y.M.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Nakase, T. Influence of Lactobacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991. [Google Scholar]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Kung, L.; Shaver, R.D.; Grant, R.J.; Schmidt, R.J. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, P.K.; de Araujo, L.C.; Sanches, L.A.; Dos Santos Araujo, S.N.; de Lima, T.O.; Lino, A.D.A.; Ferreira, E.M. Effect of sward height on the fermentability coefficient and chemical composition of guinea grass silage. Grass Forage Sci. 2018, 3, 588–598. [Google Scholar] [CrossRef]
- He, L.W.; Chen, N.; Lv, H.J.; Wang, C.; Zhou, W.; Chen, X.Y.; Zhang, Q. Gallic acid influencing fermentation quality, nitrogen distribution and bacterial community of high-moisture mulberry leaves and stylo silage. Bioresour. Technol. 2020, 295, 122255. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.; Wang, Z.; Xing, B. Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 1, 951–960. [Google Scholar] [CrossRef]
- He, L.W.; Wang, C.; Xing, Y.Q.; Zhou, W.; Pian, R.Q.; Yang, F.Y.; Chen, X.Y.; Zhang, Q. Dynamics of proteolysis, protease activity and bacterial community of Neolamarckia cadamba leaves silage and the effects of formic acid and Lactobacillus farciminis. Bioresour. Technol. 2019, 294, 122127. [Google Scholar] [CrossRef]
- Liu, B.; Huan, H.; Gu, H.; Xu, N.; Shen, Q.; Ding, C. Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 2019, 273, 212–219. [Google Scholar] [CrossRef]
- Nishino, N.; Li, Y.; Wang, C.; Parvin, S. Effects of wilting and molasses addition on fermentation and bacterial community in guinea grass silage. Lett. Appl. Microbiol. 2010, 54, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Ogunade, I.M.; Jiang, Y.; Cervantes, A.A.P.; Kim, D.H.; Oliveira, A.S.; Vyas, D.; Weinberg, Z.G.; Jeong, K.C.; Adesogan, A.T. Bacterial diversity and composition of alfalfa silage as analyzed by Illumina MiSeq sequencing: Effects of Escherichia coli O157:H7 and silage additives. J. Dairy Sci. 2018, 101, 2048–2059. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Yuan, Z.H.; Sun, Y.M.; Kong, X.Y.; Dong, P.Y.; Zhang, J. A reused method for molasses-processed wastewater: Effect on silage quality and anaerobic digestion performance of Pennisetum. Bioresour. Technol. 2017, 241, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).