Inoculated Seed Endophytes Modify the Poplar Responses to Trace Elements in Polluted Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains Characterization
2.1.1. In Vitro Plant Growth Promotion Traits and TE Tolerance
2.1.2. Scanning Electron Microscopy (SEM) and EDX Analysis
2.1.3. Genome Sequencing and Assembly
2.2. Inoculation Experiment
2.2.1. Soil and Plant Collection
2.2.2. Plant Inoculation
2.3. Specific Automated Ribosomal Intergenic Spacer Analyses (ARISA)
2.4. Plant Health and Growth
2.5. Trace Element and Nutrient Concentrations in Soil
2.6. Element Concentrations in Plant Tissues
2.7. Bioaccumulation and Translocation Factors
2.8. Statistical Analysis
3. Results
3.1. Characterization of the Bacterial Strains
3.2. Inoculation Experiment
3.2.1. Trace Element Concentrations in Soil
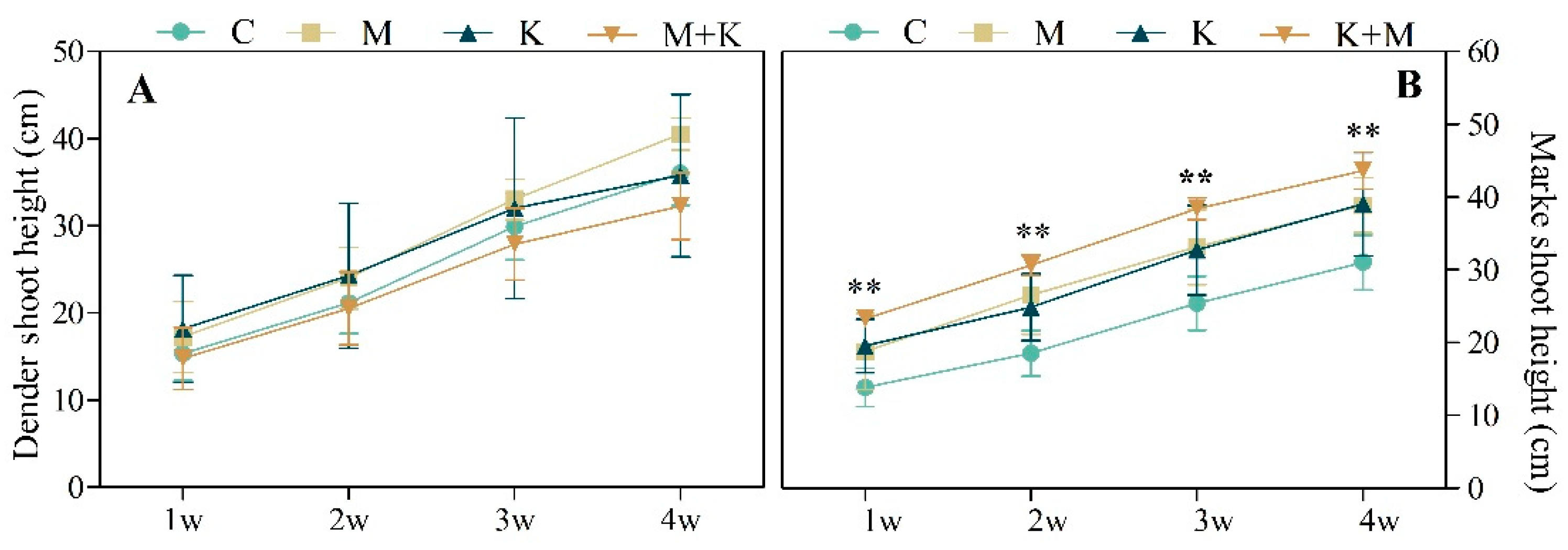
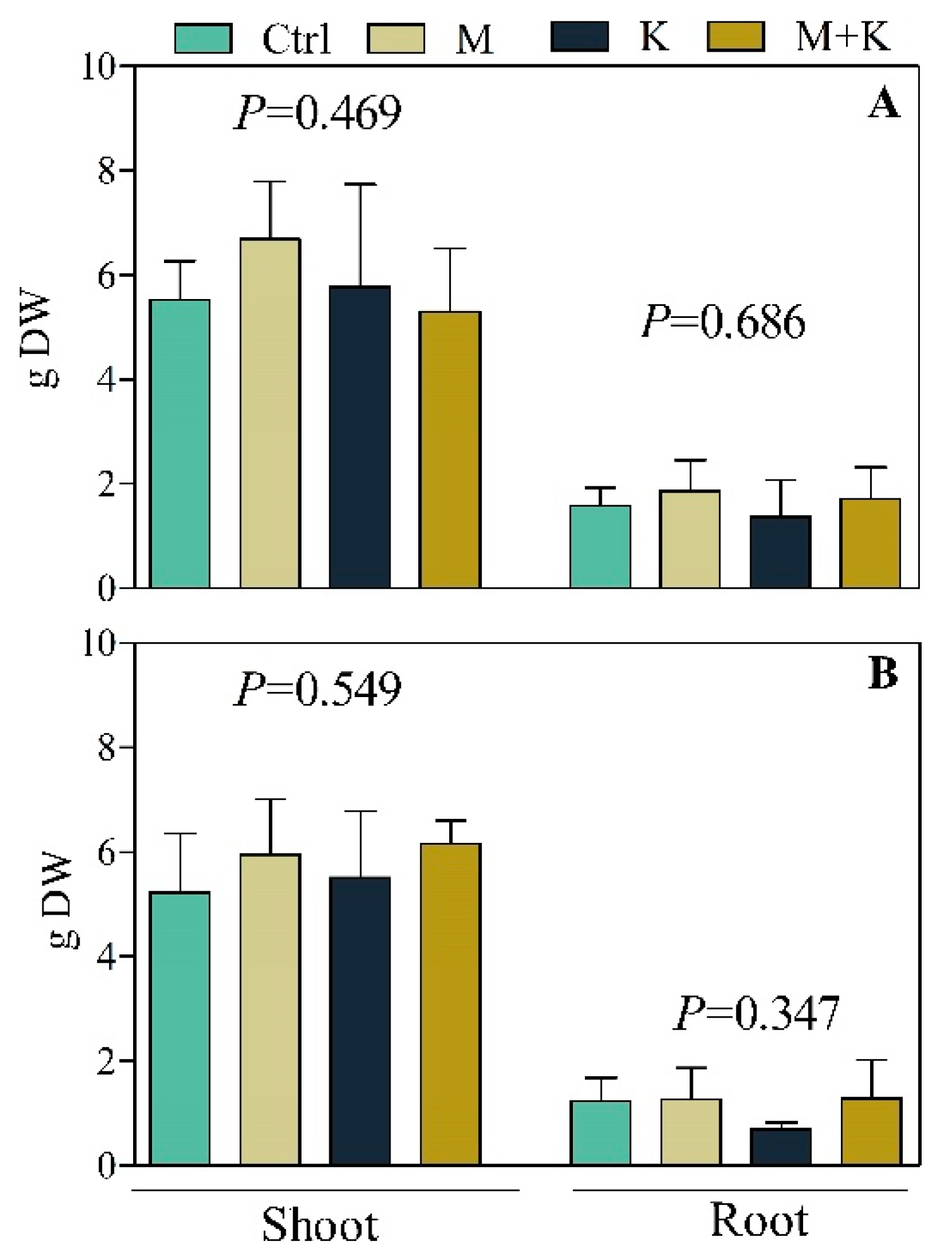
3.2.2. Plant Growth and Biomass Production
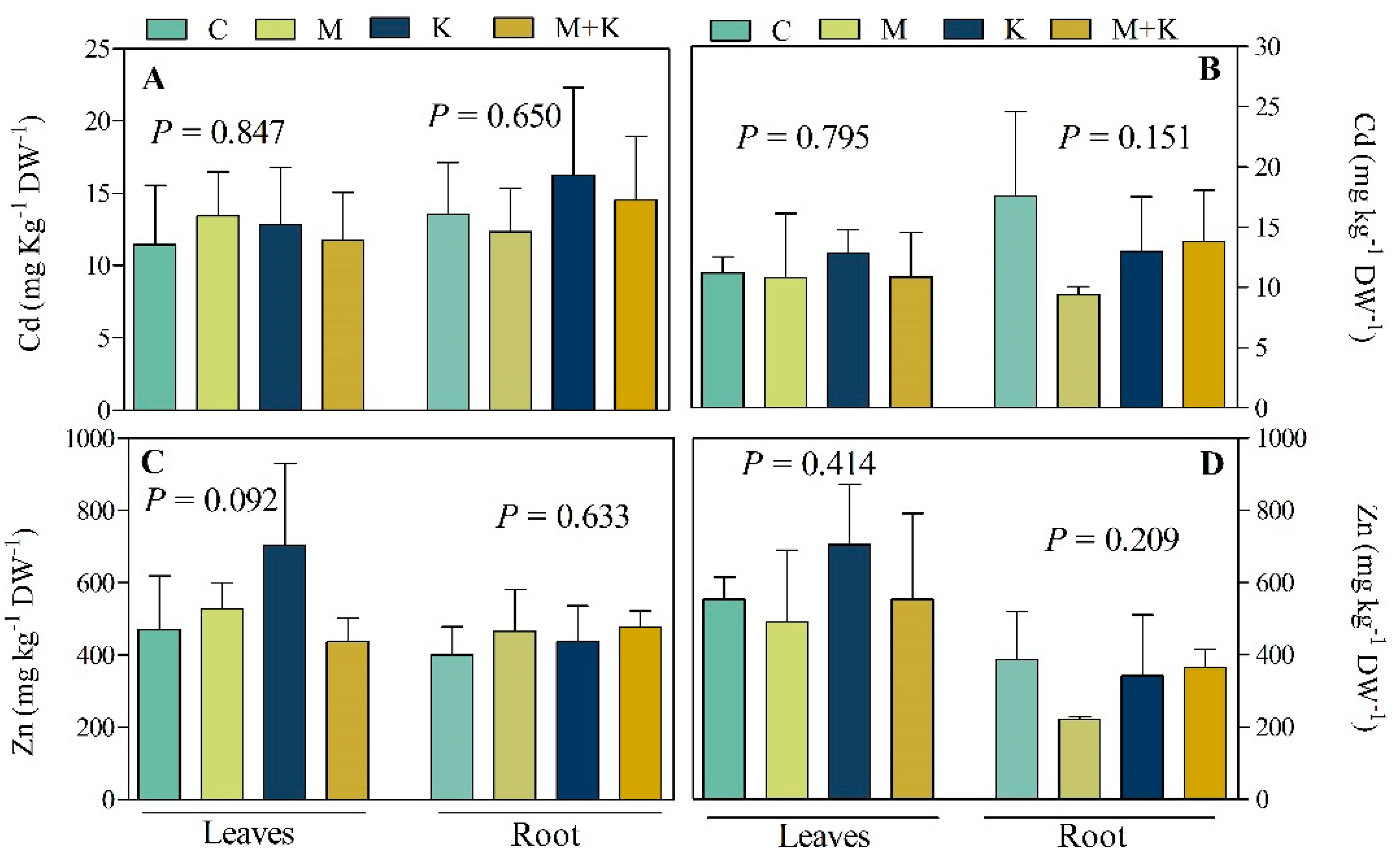
3.2.3. Element Concentrations in Plant Leaves and Roots
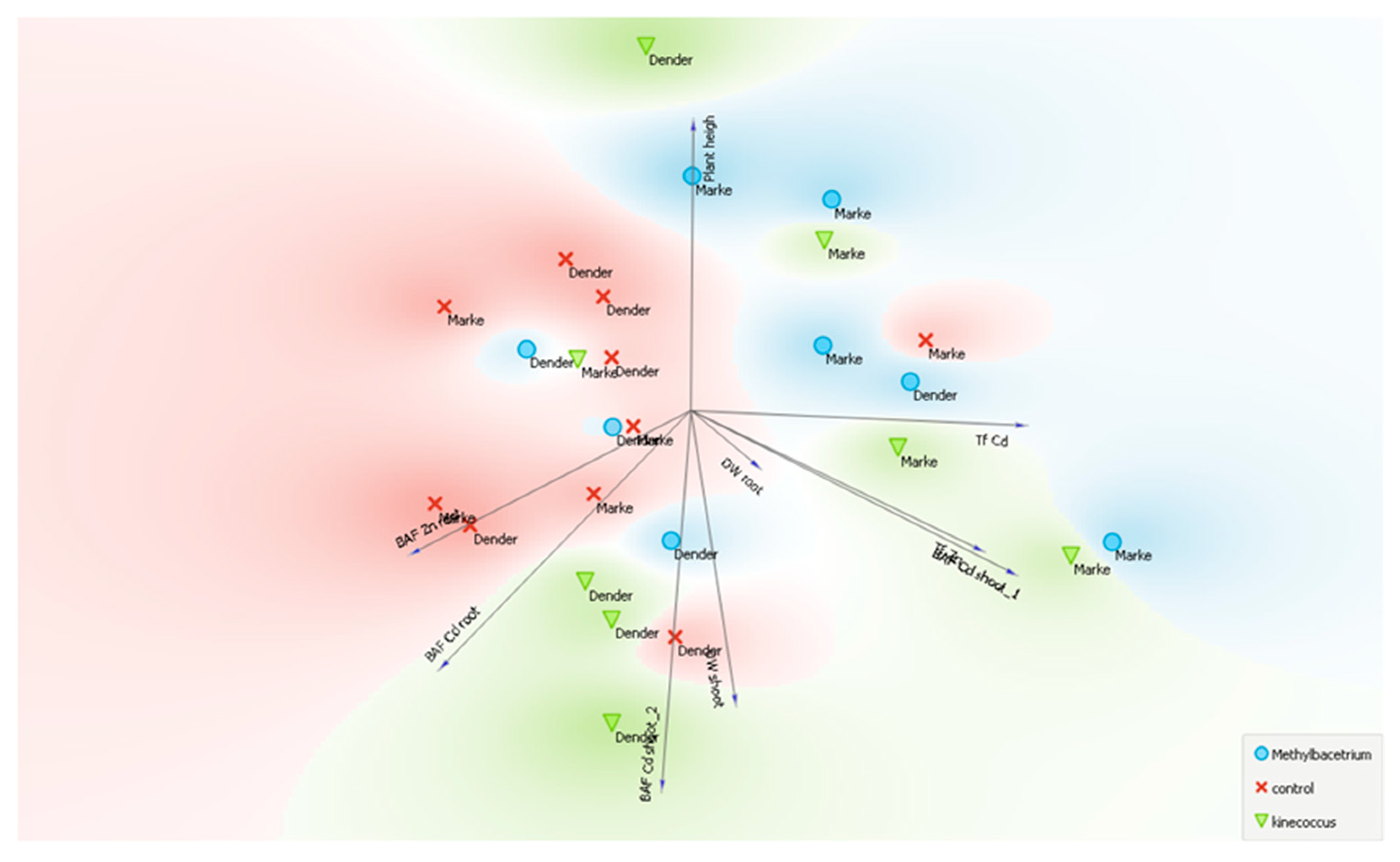
3.2.4. PCA Results
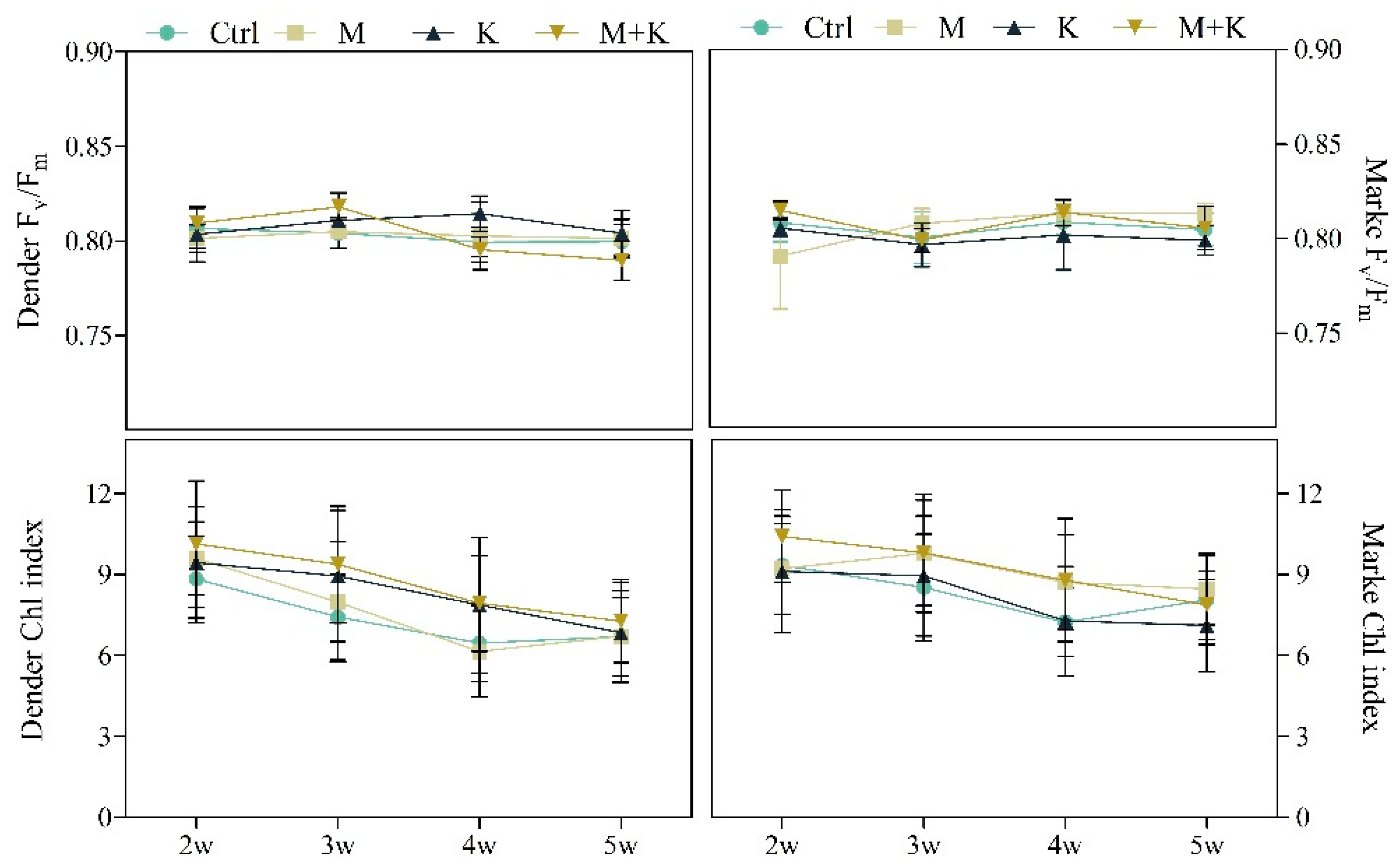
3.2.5. Plant Nutritional Status
3.2.6. Specific Automated Ribosomal Intergenic Spacer Analyses (ARISA)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Shentu, J.; Yang, X.; Baligar, V.C.; Zhang, T.; Stoffella, P.J. Heavy TE contamination of soils: Sources, indicators, and assessment. Environ. Dev. Sustain. 2015, 9, 17–18. [Google Scholar]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Vangronsveld, J.; Van Assche, F.; Clijsters, H. Reclamation of a bare industrial area contaminated by non-ferrous metals–In situ metal immobilization and revegetation. Environ. Pollut. 1995, 87, 51–59. [Google Scholar] [CrossRef]
- Hogervorst, J.; Plusquin, M.; Vangronsveld, J.; Nawrot, T.; Cuypers, A.; Van Hecke, E.; Roels, H.A.; Carleer, R.; Staessen, J.A. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ. Res. 2007, 103, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Vangronsveld, J.; Herzig, R.; Weyens, N.; Boulet, J.; Adriaensen, K.; Ruttens, A.; Thewys, T.; Vassilev, A.; Meers, E.; Nehnevajova, E.; et al. Phytoremediation of contaminated soils and groundwater: Lessons from the field. Environ. Sci. Pollut. Res. 2009, 16, 765–794. [Google Scholar] [CrossRef] [PubMed]
- Witters, N.; Van Slycken, S.; Ruttens, A.; Adriaensen, K.; Meers, E.; Meiresonne, L.; Tack, F.M.; Thewys, T.; Laes, E.; Vangronsveld, J. Short-rotation coppice of willow for phytoremediation of a TE-contaminated agricultural area: A sustainability assessment. Bioenergy Res. 2009, 2, 144–152. [Google Scholar] [CrossRef]
- Janssen, J.; Weyens, N.; Croes, S.; Beckers, B.; Meiresonne, L.; Van Peteghem, P.; Carleer, R.; Vangronsveld, J. Phytoremediation of TE contaminated soil using willow: Exploiting plant-associated bacteria to improve biomass production and TE uptake. Int. J. Phytoremed. 2015, 17, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Thijs, S.; Witters, N.; Janssen, J.; Ruttens, A.; Weyens, N.; Herzig, R.; Mench, M.; Van Slycken, S.; Meers, E.; Meiresonne, L.; et al. Tobacco, sunflower and high biomass SRC clones show potential for trace TE phytoextraction on a moderately contaminated field site in Belgium. Front. Plant Sci. 2018, 9, 1879. [Google Scholar] [CrossRef]
- Sebastiani, L.; Scebba, F.; Tognetti, R. Heavy metal accumulation and growth responses in poplar clones Eridano (Populus deltoides × maximowiczii) and I-214 (P. × euramericana) exposed to industrial waste. Environ. Exper. Bot. 2004, 52, 79–88. [Google Scholar] [CrossRef]
- Giachetti, G.; Sebastiani, L. TE accumulation in poplar plant grown with industrial wastes. Chemosphere 2006, 64, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Pajević, S.; Borišev, M.; Nikolić, N.; Krstić, B.; Pilipović, A.; Orlović, S. Phytoremediation capacity of poplar (Populus spp.) and willow (Salix spp.) clones in relation to photosynthesis. Arch. Biol. Sci. 2009, 61, 239–247. [Google Scholar] [CrossRef]
- Marmiroli, M.; Pietrini, F.; Maestri, E.; Zacchini, M.; Marmiroli, N.; Massacci, A. Growth, physiological and molecular traits in Salicaceae trees investigated for phytoremediation of heavy TEs and organics. Tree Physiol. 2011, 31, 1319–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. Poplars and Other Fast-Growing Trees—Renewable Resources for Future Green Economies. Synthesis of Country Progress Reports. In Proceedings of the 25th Session of the International Poplar Commission, Berlin, Germany, 13–16 September 2016; Working Paper IPC/15; Forestry Policy and Resources Division, FAO: Rome, Italy, 2016; Available online: http://www.fao.org/forestry/ipc2016/en/ (accessed on 25 January 2021).
- Ruttens, A.; Boulet, J.; Weyens, N.; Smeets, K.; Adriaensen, K.; Meers, E.; Van Slycken, S.; Tack, F.M.G.; Meiresonne, L.; Thewys, T.; et al. Short rotation coppice culture of willows and poplars as energy crops on metal contaminated agricultural soils. Int. J. Phytoremed. 2011, 13, 194–207. [Google Scholar] [CrossRef]
- Mola-Yudego, B.; Díaz-Yáñez, O.; Dimitriou, I. How much yield should we expect from fast-growing plantations for energy? Divergences between experiments and commercial willow plantations. BioEnergy Res. 2015, 8, 1769–1777. [Google Scholar] [CrossRef]
- Mastretta, C.; Barac, T.; Vangronsveld, J.; Newman, L.; Taghavi, S.; Lelie, D.V.D. Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotechnol. Genet. Eng. Rev. 2006, 23, 175–188. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Weyens, N.; van der Lelie, D.; Taghavi, S.; Vangronsveld, J. Phytoremediation: Plant–endophyte partnerships take the challenge. Curr. Opin. Biotechnol. 2009, 20, 248–254. [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Recent trends in microbial biosorption of heavy TEs: A review. Int. J. Biochem. Mol. Biol. 2013, 1, 19–26. [Google Scholar] [CrossRef]
- Herrera, S.D.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Raizada, M.N. Taxonomic and functional diversity of cultured seed associated microbes of the cucurbit family. BMC Microbiol. 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardoim, P.R.; van Overbeek, L.S.; van Elsas, J.D. Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol. 2008, 16, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A. Developmental Peculiarities and Seed-Borne Endophytes in Quinoa: Omnipresent, Robust Bacilli Contribute to Plant Fitness. Front. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truyens, S.; Beckers, B.; Thijs, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Cadmium-induced and trans-generational changes in the cultivable and total seed endophytic community of Arabidopsis thaliana. Plant Biol. 2016, 18, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, A.S.; Thijs, S.; Beckers, B.; González-Chávez, M.C.; Weyens, N.; Carrillo-González, R.; Vangronsveld, J. Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 2018, 422, 51–66. [Google Scholar] [CrossRef]
- Li, H.; Parmar, S.; Sharma, V.K.; White, J.F. Seed Endophytes and Their Potential Applications. In Seed Endophytes; Verma, S., White, J., Jr., Eds.; Springer: Cham, Switzerland, 2019; pp. 35–54. [Google Scholar]
- Sánchez-López, A.S.; González-Chávez, M.D.C.A.; Carrillo-González, R.; Vangronsveld, J.; Díaz-Garduño, M. Wild flora of mine tailings: Perspectives for use in phytoremediation of potentially toxic elements in a semi-arid region in Mexico. Int. J. Phytoremed. 2015, 17, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, A.; Pintelon, I.; Stevens, V.; Imperato, V.; Timmermans, J.P.; González-Chávez, C.; Carrillo-González, R.; Van Hamme., J.; Vangronsveld, J.; Thijs, S. Seed endophyte microbiome of Crotalaria pumila unpeeled: Identification of plant-beneficial Methylobacteria. Int. J. Mol. Sci. 2018, 19, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mergeay, M.; Nies, D.; Schlegel, H.; Gerits, J.; Charles, P.; Van Gijsegem, F. Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy TEs. J. Bacteriol. Res. 1985, 162, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Patten, C.L.; Glick, B.R. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl. Environ. Microbiol. 2002, 68, 3795–3801. [Google Scholar] [CrossRef] [Green Version]
- Jorquera, M.A.; Hernández, M.T.; Rengel, Z.; Marschner, P.; de la Luz Mora, M. Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Bio. Fertil. Soils 2008, 44, 1025–1034. [Google Scholar] [CrossRef]
- Schwyn, B.; Neilands, J. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Cosson, J.P.; Baker, A.J.M. Nichel-hyperaccumulating plants provide a nichel for nichel-resistant bacteria. Bot. Acta 1961, 194, 18–25. [Google Scholar]
- Cunningham, J.E.; Kuiack, C. Production of citric and oxalic acids and solubilization of calcium phosphate by Penicillium bilaii. Appl. Environ. Microbiol. 1992, 58, 1451–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, J.D.; Smith, P.R.; Evans-Gowing, R.; Richardson, D.J.; Russell, D.A.; Sodeau, J.R. Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch. Microbiol. 1995, 163, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, T.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Vallenet, D.; Labarre, L.; Rouy, Z.; Barbe, V.; Bocs, S.; Cruveiller, S.; Lajus, A.; Pascal, G.; Scarpelli, C.; Médigue, C. MaGe: A microbial genome annotation system supported by synteny results. Nucleic Acids Res. 2006, 34, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [Green Version]
- Caspi, R.; Billington, R.; Fulcher, C.A.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Midford, P.E.; Ong, Q.; Ong, W.K.; et al. The MetaCyc database of metabolic pathways and enzymes. Nucleic Acids Res. 2017, 46, D633–D639. [Google Scholar] [CrossRef] [Green Version]
- Meers, E.; Samson, R.; Tack, F.; Ruttens, A.; Vandegehuchte, M.; Vangronsveld, J.; Verloo, M. Phytoavailability assessment of heavy metals in soils by single extractions and accumulation by Phaseolus vulgaris. Environ. Exp. Bot. 2007, 60, 385–396. [Google Scholar] [CrossRef]
- Cardinale, M.; Brusetti, L.; Quatrini, P.; Borin, S.; Puglia, A.M.; Rizzi, A.; Zanardini, E.; Sorlini, C.; Corselli, C.; Daffonchio, D. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Appl. Environ. Microbiol. 2004, 70, 6147–6156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelland, R.J.; Dejean, S.; Combes, S.; Fortun-Lamothe, L.; Cauquil, L. StatFingerprints: A friendly graphical interface program for processing and analysis of microbial fingerprint profiles. Mol. Ecol. Res. 2009, 9, 1359–1363. [Google Scholar] [CrossRef]
- U.S. EPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils; Revision 2; U.S. EPA: Washington, DC, USA, 1996. [Google Scholar]
- U.S. EPA. Method 3051A Acid Digestion of Sediments, Sludge, and Soils; U.S. EPA: Washington, DC, USA, 1994. [Google Scholar]
- Pandey, S.; Rai, R.; Rai, L.C. Biochemical and Molecular Basis of Arsenic Toxicity and Tolerance in Microbes and Plants. In Handbook of Arsenic Toxicology; Flora, S.J.S., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 627–674. [Google Scholar]
- De Temmerman, L.; Vanongeval, L.; Boon, W.; Hoenig, M.; Geypens, M. Heavy metal content of arable soils in Northern Belgium. Water Air Soil Pollut. 2003, 148, 61–76. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Ann. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.F.; Huang, H.I.; Shen, F.T.; Young, C.C. The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresour. Technol. 2006, 97, 957–960. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Carrasco, L.; Roldan, A. Interactions between a plant growth-promoting rhizobacterium, an AM fungus and a phosphate-solubilising fungus in the rhizosphere of Lactuca sativa. Appl. Soil Ecol. 2007, 35, 480–487. [Google Scholar] [CrossRef]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Viruel, E.; Lucca, M.E.; Siñeriz, F. Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina. Arch. Microbiol. 2011, 193, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Bagwell, C.E.; Hixson, K.K.; Milliken, C.E.; Lopez-Ferrer, D.; Weitz, K.K. Proteomic and physiological responses of Kineococcus radiotolerans to copper. PLoS ONE 2010, 5, e12427. [Google Scholar] [CrossRef] [Green Version]
- Ojuederie, O.; Babalola, O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Fang, L.; Cai, P.; Huang, Q.; Chen, H.; Liang, W.; Rong, X. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ. Pollut. 2011, 159, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.; Wu, H.F.; Gopal, J.; Sivanesan, I.; Chun, S. Exploiting microbial polysaccharides for biosorption of trace elements in aqueous environments—Scope for expansion via nanomaterial intervention. Polymers 2017, 9, 721. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, J.H.; Kim, C.J.; Oh, D.K. TE adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol. Lett. 1996, 18, 1161–1164. [Google Scholar] [CrossRef]
- Kumari, D.; Qian, X.Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially–induced carbonate precipitation for immobilization of toxic TEs. Adv. Appl. Microbiol. 2016, 94, 79–108. [Google Scholar] [PubMed] [Green Version]
- Igiri, B.E.; Okoduwa, S.I.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy TEs contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Diels, L.; Dong, Q.; van der Lelie, D.; Baeyens, W.; Mergeay, M. The czc operon of Alcaligenes eutrophus CH34: From resistance mechanism to the removal of heavy metals. J. Ind. Microbiol. Biotechnol. 1995, 14, 142–153. [Google Scholar]
- Nies, D. Heavy metal-resistant bacteria as extremophiles: Molecular physiology and biotechnological use of Ralstonia sp. CH34. Extremophiles 2000, 4, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Intorne, A.C.; de Oliveira, M.V.V.; Pereira, L.D.M.; de Souza Filho, G.A. Essential role of the czc determinant for cadmium, cobalt and zinc resistance in Gluconacetobacter diazotrophicus PAl 5. Int. Microbiol. 2012, 15, 69–78. [Google Scholar] [PubMed]
- Chandra, B.R.; Yogavel, M.; Sharma, A. Structural analysis of ABC-family periplasmic zinc binding protein provides new insights into mechanism of ligand uptake and release. J. Mol. Biol. 2007, 367, 970–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhaiyan, M.; Poonguzhali, S.; Sa, T. TE tolerating methylotrophic bacteria reduces nickel and cadmium toxicity and promotes plant growth of tomato (Lycopersicon esculentum L.). Chemosphere 2007, 69, 220–228. [Google Scholar] [CrossRef]
- Mesa, J.; Mateos-Naranjo, E.; Caviedes, M.A.; Redondo-Gómez, S.; Pajuelo, E.; Rodríguez-Llorente, I.D. Endophytic cultivable bacteria of the TE bioaccumulator Spartina maritima improve plant growth but not TE uptake in polluted marshes soils. Front. Microbiol. 2015, 6, 1450. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy TE challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Rengel, Z.; Bose, J.; Chen, Q.; Tripathi, B.N. Magnesium alleviates plant toxicity of aluminium and heavy metals. Crop. Pasture Sci. 2016, 66, 1298–1307. [Google Scholar] [CrossRef]


| Metal Tolerance | PGP Traits | |||||||
|---|---|---|---|---|---|---|---|---|
| Bacteria Strains | Cd (0.4 mM) | Cd (0.8 mM) | Zn (0.6 mM) | Zn (1 mM) | IAA | Sid | Org acid | P-mi |
| K. endophyticus CP19 | − | − | + | + | + | − | − | − |
| Methylobacterium sp. CP3 | + | + | + | + | + | − | − | + |
| Trace Element | Gene | Encoded Protein/Enzyme | Bacteria |
|---|---|---|---|
| Cd, Zn | czcD | CzcD, cation efflux system protein | Methylobacterium K. endophyticus |
| Cd, Zn | czcA | CzcA, proton antiport (CzcCBA chemiosmotic transporter) | Methylobacterium |
| Cd, Zn | czcB | CzcB, proton antiport (CzcCBA chemiosmotic transporter) | Methylobacterium K. endophyticus |
| Cd, Zn | cadA3 | Cd2+ -P-type exporting ATPase | Methylobacterium K. endophyticus |
| Zn, Cd | zntA | Zn2+ -P-type exporting ATPase | Methylobacterium K. endophyticus |
| Zn | Zur | Zur, Zinc uptake regulation protein | Methylobacterium K. endophyticus |
| Zn | znuA | ZnuA, Zn2+ ABC transporter, periplasmic-binding protein | Methylobacterium K. endophyticus |
| Zn | znuB | ZnuB, Zn2+ ABC transporter, inner membrane permease subunit | Methylobacterium K. endophyticus |
| Zn | znuC | ZnuC, Zn2+ ABC transporter, ATP-binding subunit | Methylobacterium K. endophyticus |
| Organ | Ctrl | M | K | M + K | ANOVA | |
|---|---|---|---|---|---|---|
| Cu | L | 6 ± 1.35 | 8 ± 1.5 | 9 ± 2.8 | 6.3 ± 0.8 | 0.3019 |
| R | 33 ± 9.9 | 40 ± 8.5 | 34 ± 7.5 | 41 ± 1.0 | 0.051 | |
| Mn | L | 14.1 ± 3.5 | 16 ± 2.6 | 26 ± 16.2 | 16.5 ± 1.4 | 0.311 |
| R | 49 ± 22.3 | 81 ± 11.9 | 71 ± 4.9 | 73 ± 4. 8 | 0.214 | |
| Ca | L | 8771 ± 1574.1 | 10167 ± 1521.7 | 11443 ± 2510.3 | 10712 ± 728.6 | 0.160 |
| R | 5007 ± 667.8 | 5015 ± 823.0 | 5130 ± 597.7 | 5062 ± 684.1 | 0.993 | |
| Mg | L | 1906 ± 105.3b | 2118 ± 189.4b | 2091 ± 190.0b | 2286 ± 66.6a | 0.015 |
| R | 1532 ± 133.6 | 1400 ± 129.9 | 1451 ± 181.1 | 1402 ± 49.8 | 0.135 | |
| K | L | 3374 ± 306.2 | 3792 ± 562.3 | 3765 ± 828.9 | 4229 ± 456.8 | 0.058 |
| R | 2960 ± 524.4 | 2636 ± 462.9 | 3699 ± 701.5 | 2553 ± 431.1 | 0.581 |
| Ctrl | M | K | M + K | ANOVA | ||
|---|---|---|---|---|---|---|
| Cu | L | 6 ± 1.1 | 6 ± 0.8 | 6 ± 0.9 | 6 ± 1.2 | 0.971 |
| R | 28 ± 11.3 | 23 ± 1.5 | 22 ± 6.1 | 34 ± 5.2 | 0.1212 | |
| Mn | L | 14.2 ± 3.1 | 16 ± 4.1 | 18 ± 4.8 | 16 ± 3.6 | 0.652 |
| R | 35.3 ± 23.5ab | 21 ± 2.5ab | 22.5 ± 7.6b | 41.4 ± 14.6a | 0.029 | |
| Ca | L | 10767 ± 948.1 | 11099 ± 1756.6 | 12904 ± 1732.7 | 11661 ± 956.3 | 0.233 |
| R | 4284 ± 765.5ab | 3880 ± 231.4ab | 3630 ± 410.9b | 4842 ± 283.4a | 0.022 | |
| Mg | L | 2030 ± 129.6a | 2203 ± 221.3ab | 2196 ± 67.6ab | 2267 ± 57.9b | 0.029 |
| R | 1442 ± 2018.4 | 1269 ± 71.9 | 1282 ± 119.4 | 1502 ± 176.7 | 0.135 | |
| K | L | 3799 ± 429.0 | 3818 ± 661.8 | 4134 ± 366.3 | 4442 ± 497.3 | 0.241 |
| R | 2941 ± 514.0 | 2435 ± 461.5 | 2354 ± 56.2 | 2339 ± 320.2 | 0.107 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vannucchi, F.; Imperato, V.; Saran, A.; Staykov, S.; D’Haen, J.; Sebastiani, L.; Vangronsveld, J.; Thijs, S. Inoculated Seed Endophytes Modify the Poplar Responses to Trace Elements in Polluted Soil. Agronomy 2021, 11, 1987. https://doi.org/10.3390/agronomy11101987
Vannucchi F, Imperato V, Saran A, Staykov S, D’Haen J, Sebastiani L, Vangronsveld J, Thijs S. Inoculated Seed Endophytes Modify the Poplar Responses to Trace Elements in Polluted Soil. Agronomy. 2021; 11(10):1987. https://doi.org/10.3390/agronomy11101987
Chicago/Turabian StyleVannucchi, Francesca, Valeria Imperato, Anabel Saran, Svetoslav Staykov, Jan D’Haen, Luca Sebastiani, Jaco Vangronsveld, and Sofie Thijs. 2021. "Inoculated Seed Endophytes Modify the Poplar Responses to Trace Elements in Polluted Soil" Agronomy 11, no. 10: 1987. https://doi.org/10.3390/agronomy11101987
APA StyleVannucchi, F., Imperato, V., Saran, A., Staykov, S., D’Haen, J., Sebastiani, L., Vangronsveld, J., & Thijs, S. (2021). Inoculated Seed Endophytes Modify the Poplar Responses to Trace Elements in Polluted Soil. Agronomy, 11(10), 1987. https://doi.org/10.3390/agronomy11101987








