Directed Synthesis of Humic and Fulvic Derivatives with Enhanced Antioxidant Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Modification Protocols of the Humic Materials Used in This Study
2.3. Synthesis of D-Labeled Derivatives
2.4. Structural Characterization of the Humic Materials Used in This Study
2.5. FT ICR MS Analysis
2.6. Determination of Redox Capacity
2.7. Determination of Antioxidant Capacity of the Humic Materials Using TEAC Method
2.8. Kinetic Measurements on the Rate of ABTS Radical Quenching by Humic Derivatives
3. Results and Discussion
3.1. Synthesis and Structural Characteristics of the Humic Derivatives Obtained in This Study
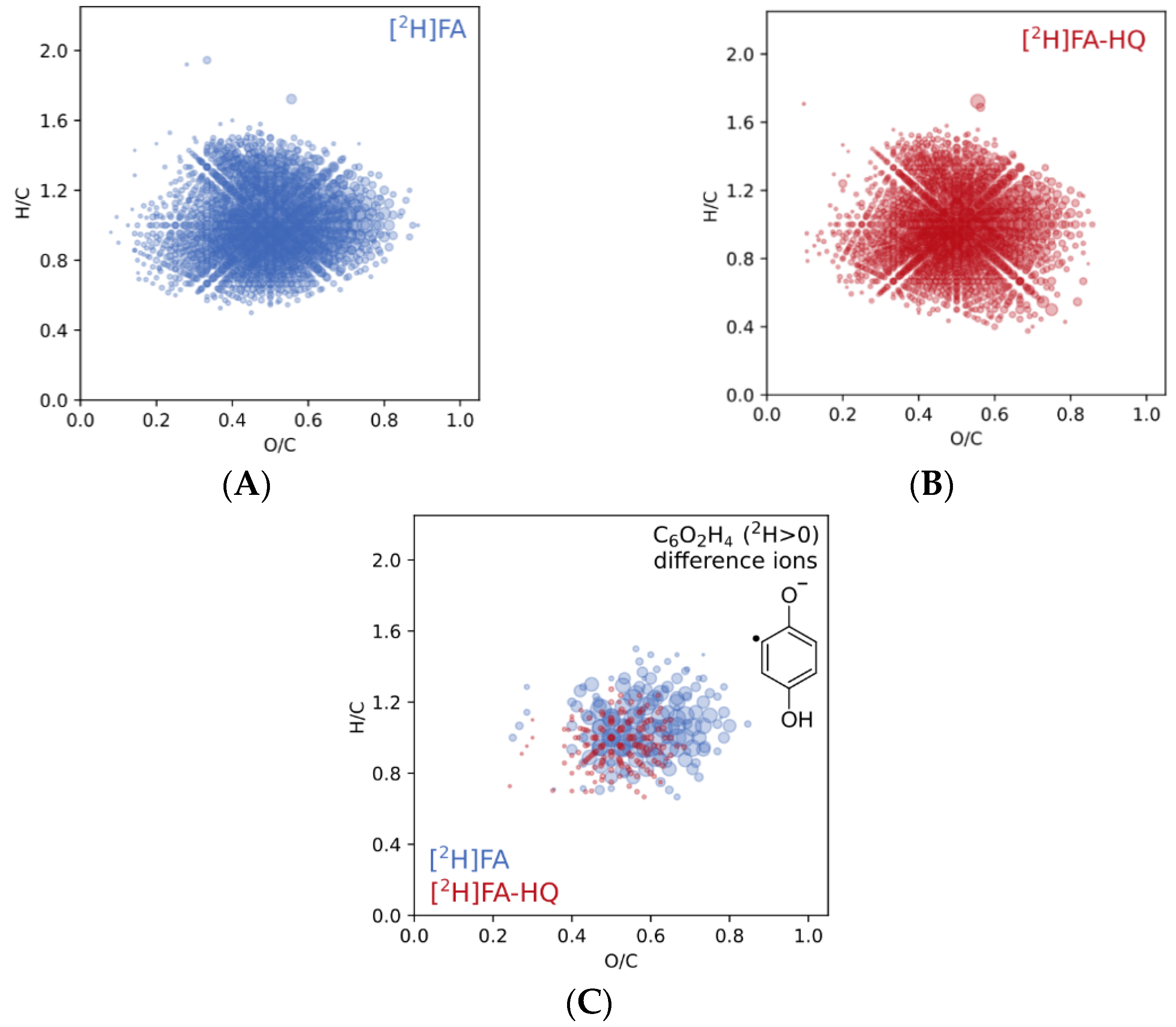
3.2. Characterization of Modification Products Using H/D Exchange Followed by FT ICR MS
3.3. Redox and Antioxidant Capacities of the Synthesized Humic Derivatives
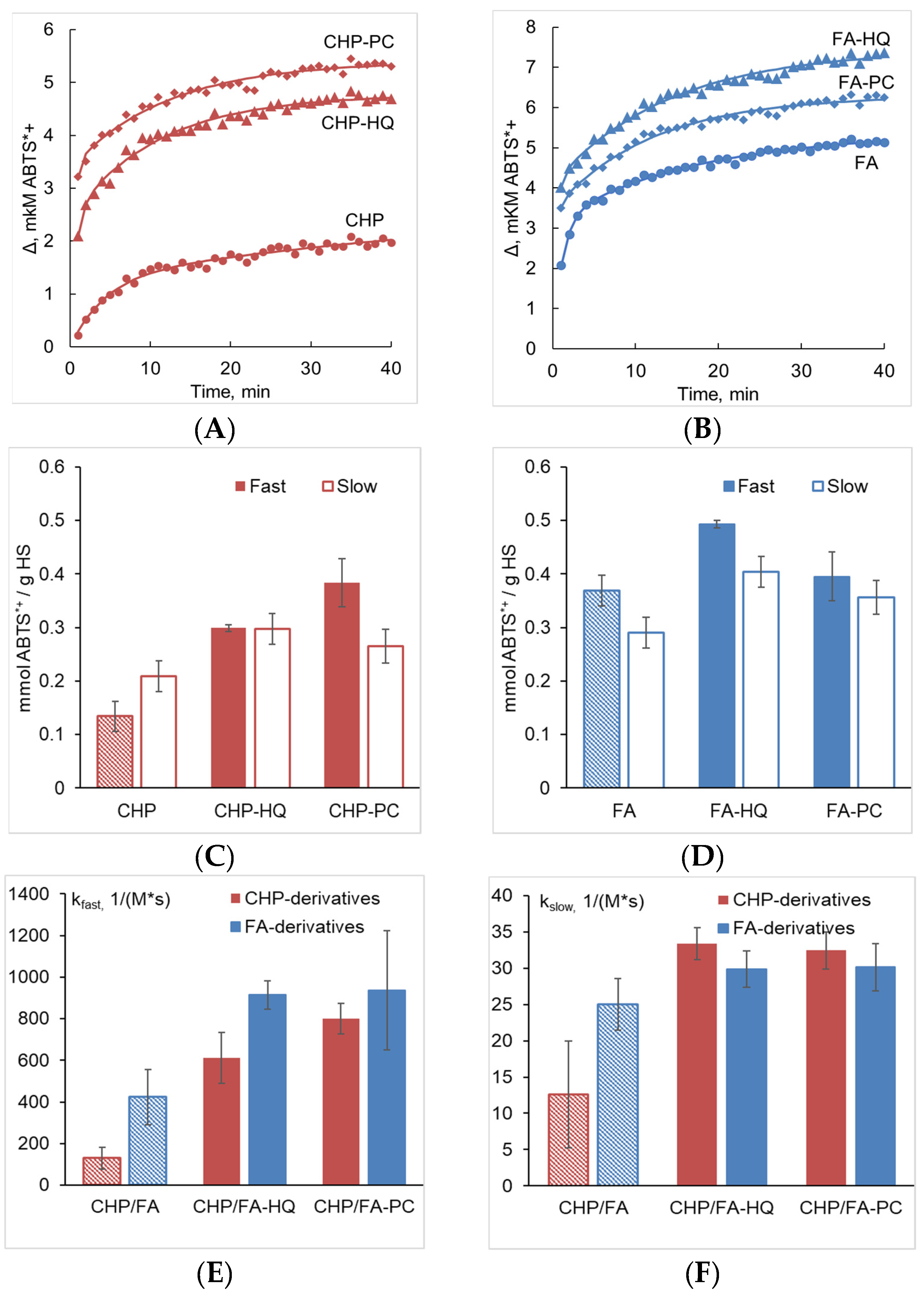
3.4. Quenching Kinetics of ABTS by the Phenolic Derivatives of Humic and Fulvic Acids
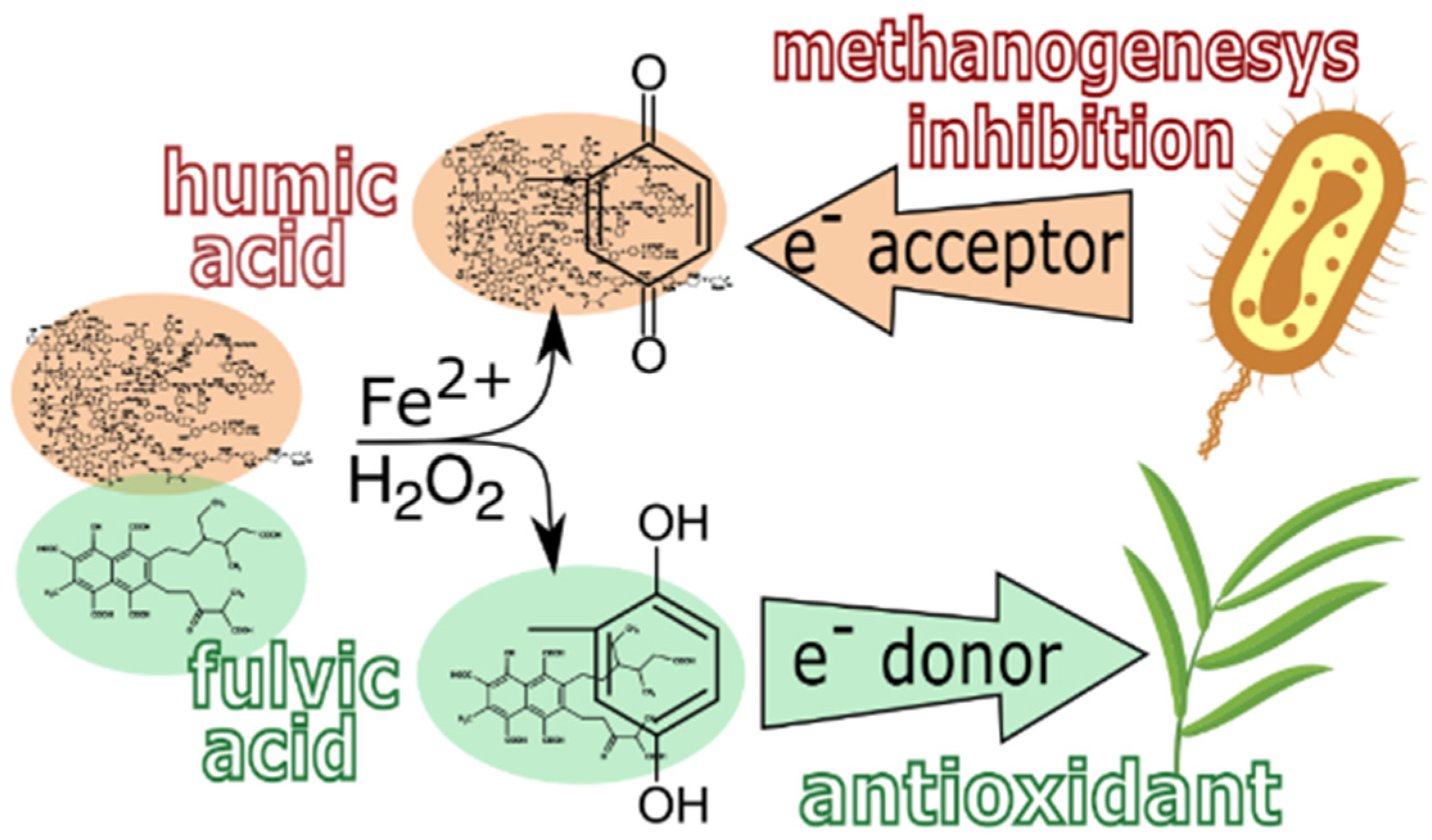
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, K.A.; Achenbach, L.A.; Coates, J.D. Microorganisms pumping iron: Anaerobic microbial iron oxidation and reduction. Nat. Rev. Microbiol. 2006, 4, 752–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez, C.M.; Alvarez, L.H.; Celis, L.B.; Cervantes, F.J. Humus-reducing microorganisms and their valuable contribution in environmental processes. Appl. Microbiol. Biotechnol. 2013, 97, 10293–10308. [Google Scholar] [CrossRef] [PubMed]
- Ratasuk, N.; Nanny, M.A. Characterization and quantification of reversible redox sites in humic substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Sander, M.; Schwarzenbach, R.P. Novel electrochemical approach to assess the redox properties of humic substances. Environ. Sci. Technol. 2010, 44, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, M.; Graf, C.; Schwarzenbach, R.P.; Sander, M. Antioxidant properties of humic substances. Environ. Sci. Technol. 2012, 46, 4916–4925. [Google Scholar] [CrossRef] [PubMed]
- Rakshit, S.; Dibyendu, S. Assessing redox properties of standard humic substances. Int. J. Environ. Sci. Technol. 2017, 14, 1497–1504. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Aeschbacher, M.; Page, S.E.; Wenk, J.; Sander, M.; McNeill, K. Photooxidation-induced changes in optical, electrochemical, and photochemical properties of humic substances. Environ. Sci. Technol. 2014, 48, 2688–2696. [Google Scholar] [CrossRef] [Green Version]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Walpen, N.; Getzinger, G.J.; Schroth, M.H.; Sander, M. Electron-donating phenolic and electron-accepting quinone moieties in peat dissolved organic matter: Quantities and redox transformations in the context of peat biogeochemistry. Environ. Sci. Technol. 2018, 52, 5236–5245. [Google Scholar] [CrossRef]
- Macalady, D.L.; Walton-Day, K. Redox chemistry and natural organic matter (NOM): Geochemists’ dream, analytical chemists’ nightmare. In Aquatic Redox Chemistry; ACS Publications: Washington, DC, USA, 2011; pp. 85–111. [Google Scholar]
- Hernández-Montoya, V.; Alvarez, L.H.; Montes-Morán, M.A.; Cervantes, F.J. Reduction of quinone and non-quinone redox functional groups in different humic acid samples by Geobacter sulfurreducens. Geoderma 2012, 183, 25–31. [Google Scholar] [CrossRef]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voelker, B.M.; Morel, F.M.M.; Sulzberger, B. Iron redox cycling in surface waters: effects of humic substances and light. Environ. Sci. Technol. 1997, 31, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Van der Zee, F.P.; Cervantes, F.J. Impact and application of electron shuttles on the redox (bio)transformation of contaminants: A review. Biotechnol. Adv. 2009, 27, 256–277. [Google Scholar] [CrossRef] [Green Version]
- Borch, T.; Kretzschmar, R.; Kappler, A.; Cappellen, P.V.; Ginder-Vogel, M.; Voegelin, A.; Campbell, K. Biogeochemical redox processes and their impact on contaminant dynamics. Environ. Sci. Technol. 2010, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.T.; McKnight, D.M.; Blunt-Harris, E.L.; Kolesar, S.E.; Lovley, D.R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 1998, 32, 2984–2989. [Google Scholar] [CrossRef]
- Nurmi, J.T.; Tratnyek, P.G. Electrochemical properties of natural organic matter (NOM), fractions of NOM, and model biogeochemical electron shuttles. Environ. Sci. Technol. 2002, 36, 617–624. [Google Scholar] [CrossRef]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef] [Green Version]
- Pham, A.N.; Rose, A.L.; Waite, T.D. Kinetics of Cu(II) reduction by natural organic matter. J. Phys. Chem. 2012, 116, 6590–6599. [Google Scholar] [CrossRef]
- Aeschbacher, M.; Vergari, D.; Schwarzenbach, R.P.; Sander, M. Electrochemical analysis of proton and electron transfer equilibria of the reducible moieties in humic acids. Environ. Sci. Technol. 2011, 45, 8385–8394. [Google Scholar] [CrossRef]
- Perminova, I.V.; Kovalenko, A.N.; Schmitt-Kopplin, P.; Hatfield, K.; Hertkorn, N.; Belyaeva, E.Y.; Petrosyan, V.S. Design of quinonoid-enriched humic materials with enhanced redox properties. Environ. Sci. Technol. 2005, 39, 8518–8524. [Google Scholar] [CrossRef]
- Dittmar, T.; Koch, B.; Hertkorn, N.; Kattner, G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. Methods 2008, 6, 230–235. [Google Scholar] [CrossRef]
- Zherebker, A.Y.; Perminova, I.V.; Konstantinov, A.I.; Volikov, A.B.; Kostyukevich, Y.I.; Kononikhin, A.S.; Nikolaev, E.N. Extraction of humic substances from fresh waters on solid-phase cartridges and their study by Fourier transform ion cyclotron resonance mass spectrometry. J. Anal. Chem. 2016, 4, 372–378. [Google Scholar] [CrossRef]
- Kim, S.; Kramer, R.W.; Hatcher, P.G. Graphical method for analysis of ultrahigh-resolution broadband mass spectra of natural organic matter, the van Krevelen diagram. J. Anal. Chem. 2003, 75, 5336–5344. [Google Scholar] [CrossRef] [PubMed]
- Zherebker, A.Y.; Airapetyan, D.; Konstantinov, A.I.; Kostyukevich, Y.I.; Kononikhin, A.S.; Popov, I.A.; Zaitsev, K.V.; Nikolaev, E.N.; Perminova, I.V. Synthesis of model humic substances: A mechanistic study using controllable H/D exchange and Fourier transform ion cyclotron resonance mass spectrometry. Analyst 2015, 140, 4708–4719. [Google Scholar] [CrossRef] [PubMed]
- Zherebker, A.Y.; Perminova, I.V.; Kostyukevich, Y.I.; Kononikhin, A.S.; Kharyabin, O.S.; Nikolaev, E.N. Structural investigation of coal humic substances by selective isotopic exchange and high-resolution mass spectrometry. Faraday Discuss. 2019, 218, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Matthiessen, A. Determining the redox capacity of humic substances as a function of pH. Vom Wasser. 1995, 84, 229–235. [Google Scholar]
- Peretyazhko, T.; Garrison, S. Reducing capacity of terrestrial humic acids. Geoderma 2006, 137, 140–146. [Google Scholar] [CrossRef]
- Bravo, C.; De Nobili, M.; Gambi, A.; Martin-Neto, L.; Nascimento, O.R.; Toniolo, R. Kinetics of electron transfer reactions by humic substances: Implications for their biogeochemical roles and determination of their electron donating capacity. Chemosphere 2022, 286 Pt 2, 131755. [Google Scholar] [CrossRef]
- Re, R.; Pelligrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Van den Berg, R.; Haenen, G.R.M.M.; van den Berg, H.; Bast, A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Antioxidant activity of humic substances via bioluminescent monitoring in vitro. Environ. Monit. Assess. 2015, 89, 187–201. [Google Scholar] [CrossRef]
- Klein, O.I.; Kulikova, N.A.; Filimonov, I.S.; Koroleva, O.V.; Konstantinov, A.I. Long-term kinetics study and quantitative characterization of the antioxidant capacities of humic and humic-like substances. J. Soils Sediments 2018, 18, 1355–1364. [Google Scholar] [CrossRef]
- Walpen, N.; Lau, M.P.; Fiskal, A.; Getzinger, G.J.; Meyer, S.A.; Nelson, T.F.; Lever, M.A.; Schroth, M.H.; Sander, M. Oxidation of reduced peat particulate organic matter by dissolved oxygen: Quantification of apparent rate constants in the field. Environ. Sci. Technol. 2018, 52, 11151–11160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Kappler, A. Kinetics of microbial and chemical reduction of humic substances: Implications for electron shuttling. Environ. Sci. Technol. 2008, 42, 3563–3569. [Google Scholar] [CrossRef]
- Ozdoba, D.M.; Blyth, J.C.; Engler, R.F.; Dinel, H.; Schnitzer, M. Leonardite and humified organic matter. In Humic Substances: Structures, Models and Functions; Ghabbour, E.A., Davies, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2001; p. 388. [Google Scholar]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: the new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef] [PubMed]
- Lawson, G.J.; Stewart, D. Coal humic acids. In Humic Substances II; In Search of Structure; Hayes, M.H.B., MacCarthy, P., Malcolm, R.L., Swift, R.S., Eds.; John Wiley & Sons: Chichester, UK, 1989; p. 642. [Google Scholar]
- Kappler, A.; Haderlein, S.B. Natural organic matter as reductant for chlorinated aquatic pollutants. Environ. Sci. Technol. 2003, 37, 2714–2719. [Google Scholar] [CrossRef]
- Benz, M.; Schink, B.; Brune, A. Humic acid reduction by Propionibacterium freudenreichii and other fermenting bacteria. Appl. Environ. Microbiol. 1998, 64, 4507–4512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struyk, Z.; Sposito, G. Redox properties of standard humic acids. Geoderma 2001, 102, 329–346. [Google Scholar] [CrossRef]
- Klein, O.I.; Kulikova, N.A.; Konstantinov, A.I.; Zykova, M.V.; Perminova, I.V. A systematic study of the antioxidant capacity of humic substances against peroxyl radicals: Relation to structure. Polymers 2021, 13, 3262. [Google Scholar] [CrossRef]
- Piccolo, A. The supramolecular structure of humic substances. Soil Sci. 2001, 166, 810–832. [Google Scholar] [CrossRef] [Green Version]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavanoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Munoz, J.L.; Garcia-Molina, F.; Varon, R.; Tudela, J.; García-Cánovas, F.; Rodriguez-Lopez, J.N. Quantification of the antioxidant capacity of different molecules and their kinetic antioxidant efficiencies. J. Agric. Food Chem. 2010, 58, 2062–2070. [Google Scholar] [CrossRef] [PubMed]
- Efremenko, E.; Senko, O.; Stepanov, N.; Mareev, N.; Volikov, A.; Perminova, I. Humic compounds as redox regulators of complicated biocatalytic systems in methane generation. Antioxidants 2020, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Larmar, R.T. Possible Role for Electron Shuttling Capacity in Elicitation of PB Activity of Humic Substances on Plant Growth Enhancement. In The Chemical Biology of Plant Biostimulants; Geelen, D., Xu, L., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; Chapter 4; pp. 97–112. [Google Scholar] [CrossRef]





| Name | Synthesis |
|---|---|
| CHP-HQ | coal HA (CHP) and 1,4-hydroquinone |
| CHP-MHQ | coal HA (CHP) and 2-methy-1,4-hydroquinone |
| CHP-PC | coal HA (CHP) and 1,2-hydroquinone |
| CHP-1,4-NQ | coal HA (CHP) and 1,4-naphthoquinone |
| CHP-2-OH-1,4-NQ | coal HA (CHP) and 2-hydroxy-1,4-naphthoquinone |
| FA-HQ | peat FA (FA) and 1,4-hydroquinone |
| FA-MHQ | peat FA (FA) and 2-methyl-1,4-hydroquinone |
| FA-PC | peat FA (FA) and 1,2-hydroquinone |
| FA-1,4-NQ | peat FA (FA) and 1,4-naphthoquinone |
| FA-2-OH-1,4-NQ | peat FA (FA) and 2-hydroxy-1,4-naphthoquinone |
| Sample | SUVA254, L/mgC × cm | E2/E3 | E4/E6 | Λ, nm | Asm350 | I350, L/mgC × cm | λ350, nm |
|---|---|---|---|---|---|---|---|
| CHP | 0.066 | 2.65 | 3.51 | 95 | 2.23 | 1.83 × 107 | 445 |
| CHP-HQ | 0.063 | 2.54 | 3.26 | 97 | 3.10 | 1.02 × 107 | 432 |
| CHP-MHQ | 0.052 | 2.67 | 3.30 | 101 | 3.20 | 1.44 × 107 | 440 |
| CHP-PC | 0.050 | 2.74 | 2.54 | 156 | 2.69 | 9.91 × 106 | 443 |
| CHP-NQ | 0.065 | 3.44 | 4.25 | 81 | 2.26 | 7.33 × 106 | 441 |
| CHP-2OHNQ | 0.059 | 3.77 | - | 83 | 3.47 | 1.96 × 107 | 422 |
| FA | 0.033 | 6.01 | 2.65 | 168 | 5.69 | 1.72 × 107 | 447 |
| FA-HQ | 0.038 | 3.45 | 2.66 | 113 | 6.13 | 1.69 × 107 | 431 |
| FA-MHQ | 0.035 | 4.61 | 3.90 | 74 | 5.86 | 8.81 × 108 | 442 |
| FA-PC | 0.038 | 4.66 | 3.50 | 94 | 5.66 | 1.09 × 109 | 442 |
| FA-NQ | 0.039 | 5.31 | 4.08 | 93 | 6.26 | 9.53 × 108 | 447 |
| FA-2OHNQ | 0.031 | 8.46 | 36 | 6.23 | 8.15 × 108 | 442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volikov, A.B.; Mareev, N.V.; Konstantinov, A.I.; Molodykh, A.A.; Melnikova, S.V.; Bazhanova, A.E.; Gasanov, M.E.; Nikolaev, E.N.; Zherebker, A.Y.; Volkov, D.S.; et al. Directed Synthesis of Humic and Fulvic Derivatives with Enhanced Antioxidant Properties. Agronomy 2021, 11, 2047. https://doi.org/10.3390/agronomy11102047
Volikov AB, Mareev NV, Konstantinov AI, Molodykh AA, Melnikova SV, Bazhanova AE, Gasanov ME, Nikolaev EN, Zherebker AY, Volkov DS, et al. Directed Synthesis of Humic and Fulvic Derivatives with Enhanced Antioxidant Properties. Agronomy. 2021; 11(10):2047. https://doi.org/10.3390/agronomy11102047
Chicago/Turabian StyleVolikov, Alexander B., Nikita V. Mareev, Andrey I. Konstantinov, Alexandra A. Molodykh, Sofia V. Melnikova, Alina E. Bazhanova, Mikhail E. Gasanov, Evgeny N. Nikolaev, Alexander Ya. Zherebker, Dmitry S. Volkov, and et al. 2021. "Directed Synthesis of Humic and Fulvic Derivatives with Enhanced Antioxidant Properties" Agronomy 11, no. 10: 2047. https://doi.org/10.3390/agronomy11102047
APA StyleVolikov, A. B., Mareev, N. V., Konstantinov, A. I., Molodykh, A. A., Melnikova, S. V., Bazhanova, A. E., Gasanov, M. E., Nikolaev, E. N., Zherebker, A. Y., Volkov, D. S., Zykova, M. V., & Perminova, I. V. (2021). Directed Synthesis of Humic and Fulvic Derivatives with Enhanced Antioxidant Properties. Agronomy, 11(10), 2047. https://doi.org/10.3390/agronomy11102047







