Breeding for Resilience to Water Deficit and Its Predicted Effect on Forage Mass in Tall Fescue
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Harvests
2.2. Statistical and Genetic Analysis
3. Results
3.1. Average Forage Mass
3.2. Heritability and Genetic Correlation of Forage Mass and Resilience to Deficit Irrigation
4. Discussion
4.1. The Challenge of Multiple Harvests in Forage Breeding for Water Deficit
4.2. Forage Breeding for Reslience Per se to Water Deficit
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahadur, A.V.; Ibrahim, M.; Tanner, T. Characterising resilience: Unpacking the concept for tackling climate change and development. Clim. Dev. 2013, 5, 55–65. [Google Scholar] [CrossRef]
- Moritz, C.; Agudo, R. The Future of Species Under Climate Change: Resilience or Decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef]
- Picasso, V.D.; Casler, M.D.; Undersander, D. Resilience, Stability, and Productivity of Alfalfa Cultivars in Rainfed Regions of North America. Crop Sci. 2019, 59, 800–810. [Google Scholar] [CrossRef]
- Grimm, V.; Wissel, C. Babel, or the ecological stability discussions: An inventory and analysis of terminology and a guide for avoiding confusion. Oecologia 1997, 109, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.; McDonald, J.L.; Hosken, D.J. What do you mean, ‘resilient’? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Tracy, B.F.; Foster, J.L.; Butler, T.J.; Islam, M.A.; Toledo, D.; Vendramini, J.M.B. Resilience in Forage and Grazinglands. Crop Sci. 2018, 58, 31–42. [Google Scholar] [CrossRef]
- Volaire, F.; Barkaoui, K.; Norton, M. Designing resilient and sustainable grasslands for a drier future: Adaptive strategies, functional traits and biotic interactions. Eur. J. Agron. 2014, 52, 81–89. [Google Scholar] [CrossRef]
- De Sá Souza, M.; Da Silva, T.G.F.; De Souza, L.S.B.; Ferraz Jardim, A.M.D.R.; Araújo Júnior, G.D.N.; Nunes Alves, H.K.M. Practices for the improvement of the agricultural resilience of the forage production in semiarid environment: A review. Amazon. J. Plant Res. 2019, 3, 417–430. [Google Scholar] [CrossRef]
- Hofer, D.; Suter, M.; Haughey, E.; Finn, J.A.; Hoekstra, N.J.; Buchmann, N.; Lüscher, A. Yield of temperate forage grassland species is either largely resistant or resilient to experimental summer drought. J. Appl. Ecol. 2016, 53, 1023–1034. [Google Scholar] [CrossRef]
- Easton, H.S.; Stewart, A.V.; Kerr, G.A. Ryegrass in pastures—Breeding for resilience. NZGA Res. Pract. Ser. 2011, 15, 139–147. [Google Scholar] [CrossRef]
- Gilliland, T.J.; Hennessy, D.; Ball, T. Breeding resilient cultivars for European grass based ruminant production systems. In Sustainable Meat and Milk Production from Grasslands, Proceedings of the 27th General Meeting of the European Grassland Federation, Cork, Ireland, 17–18 June 2018; Horan, B., Hennessy, D., O’Donovan, M., Kennedy, E., McCarthy, B., Finn, J.A., O’Brien, B., Eds.; Teagasc, Animal and Grassland Research and Innovation Centre: Cork, Ireland, 2018; pp. 29–42. [Google Scholar]
- Kole, C.; Muthamilarasan, M.; Henry, R.; Edwards, D.; Sharma, R.; Abberton, M.; Batley, J.; Bentley, A.; Blakeney, M.; Bryant, J.; et al. Application of genomics-assisted breeding for generation of climate resilient crops: Progress and prospects. Front. Plant Sci. 2015, 6, 563. [Google Scholar] [CrossRef]
- Urruty, N.; Tailliez-Lefebvre, D.; Huyghe, C. Stability, robustness, vulnerability and resilience of agricultural systems. A review. Agron. Sustain. Dev. 2016, 36, 15. [Google Scholar] [CrossRef]
- Ceccarelli, S.; Grando, S.; Hamblin, J. Relationship between barley grain yield measured in low- and high-yielding environments. Euphytica 1992, 64, 49–58. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantititative Genetics, 3rd ed.; Longman Scientific & Technical/John Wiley & Sons: Essex, UK; New York, NY, USA, 1989. [Google Scholar]
- Conaghan, P.; Casler, M.D.; O’Kiely, P.; Dowley, L.J. Efficiency of indirect selection for dry matter yield based on fresh matter yield in perennial ryegrass sward plots. Crop Sci. 2008, 48, 127–133. [Google Scholar] [CrossRef]
- Atlin, G.N.; Frey, K.J. Predicting the relative effectiveness of direct versus indirect selection for oat yield in three types of stress environments. Euphytica 1989, 44, 137–142. [Google Scholar] [CrossRef]
- Burdon, R.D. Genetic correlation as a concept for studying genotype-environment interaction in forest tree breeding. Silvae Genet. 1977, 26, 5–6. [Google Scholar]
- Cooper, M.; Byth, D.E.; Delacy, I.H.; Woodruff, D.R. Predicting grain-yield in Australian environments using data from Cimmyt International wheat performance trials. 1. Potential for exploiting correlated response to selection. Field Crops Res. 1993, 32, 305–322. [Google Scholar] [CrossRef]
- Stratton, S.D.; Ohm, H.W. Relationship between orchardgrass seed production in Indiana and Oregon. Crop Sci. 1989, 29, 908–913. [Google Scholar] [CrossRef]
- Waldron, B.L.; Ehlke, N.J.; Vellekson, D.J.; White, D.B. Controlled freezing as an indirect selection method for field winterhardiness in turf-type perennial ryegrass. Crop Sci. 1998, 38, 811–816. [Google Scholar] [CrossRef]
- Waldron, B.L.; Robins, J.G.; Peel, M.D.; Jensen, K.B. Predicted efficiency of spaced-plant selection to indirectly improve tall fescue sward yield and quality. Crop Sci. 2008, 48, 443–449. [Google Scholar] [CrossRef]
- Waldron, B.L.; Peel, M.D.; Larson, S.R.; Mott, I.W.; Creech, J.E. Tall fescue forage mass in a grass-legume mixture: Predicted efficiency of indirect selection. Euphytica 2017, 213, 67. [Google Scholar] [CrossRef]
- Hanks, R.J.; Keller, J.; Rasmussen, V.P.; Wilson, G.D. Line source sprinkler for continuous variable irrigation-crop production studies. Soil Sci. Soc. Am. J. 1976, 40, 426–429. [Google Scholar] [CrossRef]
- Hanks, R.J.; Sisson, D.V.; Hurst, R.L.; Hubbard, K.G. Statistical analysis of results from irrigation experiments using the line-source sprinkler system. Soil Sci. Soc. Am. J. 1980, 44, 886–888. [Google Scholar] [CrossRef]
- Moore, K.J.; Moser, L.E.; Vogel, K.P.; Waller, S.S.; Johnson, B.E.; Pedersen, J.F. Describing and quantifying growth-stages of perennial forage grasses. Agron. J. 1991, 83, 1073–1077. [Google Scholar] [CrossRef]
- Jahufer, M.Z.Z.; Luo, D. DeltaGen: A Comprehensive Decision Support Tool for Plant Breeders. Crop Sci. 2018, 58, 1118–1131. [Google Scholar] [CrossRef]
- Pour-Aboughadareh, A.; Yousefian, M.; Moradkhani, H.; Poczai, P.; Siddique, K.H.M. STABILITYSOFT: A new online program to calculate parametric and non-parametric stability statistics for crop traits. Appl. Plant Sci. 2019, 7, e01211. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; 4.0.3; R Foundation for Statistical Computing: Vienna, Australia, 2020. [Google Scholar]
- Nguyen, H.T.; Sleper, D.A. Theory and application of half-sib matings in forage grass breeding. Theor. Appl. Genet. 1983, 64, 187–196. [Google Scholar] [CrossRef]
- Asay, K.H.; Jensen, K.B.; Waldron, B.L. Responses of tall fescue cultivars to an irrigation gradient. Crop Sci. 2001, 41, 350–357. [Google Scholar] [CrossRef]
- Finlay, K.W.; Wilkinson, G.N. The analysis of adaptation in a plant-breeding programme. Aust. J. Agric. Res. 1963, 14, 742. [Google Scholar] [CrossRef]
- Waldron, B.L.; Asay, K.H.; Jensen, K.B. Stability and yield of cool-season pasture grass species grown at five irrigation levels. Crop Sci. 2002, 42, 890–896. [Google Scholar] [CrossRef]
- Jensen, K.B.; Asay, K.H.; Waldron, B.L. Dry matter production of orchardgrass and perennial ryegrass at five irrigation levels. Crop Sci. 2001, 41, 479–487. [Google Scholar] [CrossRef]
- Casler, M.D.; Fales, S.L.; McElroy, A.R.; Hall, M.H.; Hoffman, L.D.; Undersander, D.J.; Leath, K.T. Half-sib family selection for forage yield in orchardgrass. Plant Breed. 2002, 121, 43–48. [Google Scholar] [CrossRef]
- Casler, M.D. Among-and-within-family selection in eight forage grass populations. Crop Sci. 2008, 48, 434–442. [Google Scholar] [CrossRef][Green Version]
- Jensen, K.B.; Waldron, B.L.; Robins, J.G.; Monaco, T.A.; Peel, M.D. Breeding meadow bromegrass for forage characteristics under a line-source irrigation design. Can. J. Plant Sci. 2008, 88, 695–703. [Google Scholar] [CrossRef]
- Holland, J.B.; Nyquist, W.E.; Cervantes-Martínez, C.T. Estimating and interpreting heritability for plant breeding: An update. Plant Breed. Rev. 2010, 22, 9–112. [Google Scholar] [CrossRef]
- Robins, J.G.; Waldron, B.L.; Jensen, K.B. Productivity, stability, and resilience of cool-season perennial grasses used for rangeland revegetation. Agrosyst. Geosci. Environ. 2020, 3, e20002. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russell, W.A. Stability parameters for comparing varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Abtahi, M.; Majidi, M.M.; Mirlohi, A. Marker-based parental selection to improve performance of orchadgrass polycross populations under water deficit environments. Euphytica 2018, 214, 232. [Google Scholar] [CrossRef]
- Waldron, B.L. Data used in Agronomy MDPI manuscript, Breeding for resilience to water deficit and its predicted effect on forage mass in tall fescue. Figshare, Dataset. 2021. Available online: https://doi.org/10.6084/m9.figshare.16528446 (accessed on 28 September 2021).
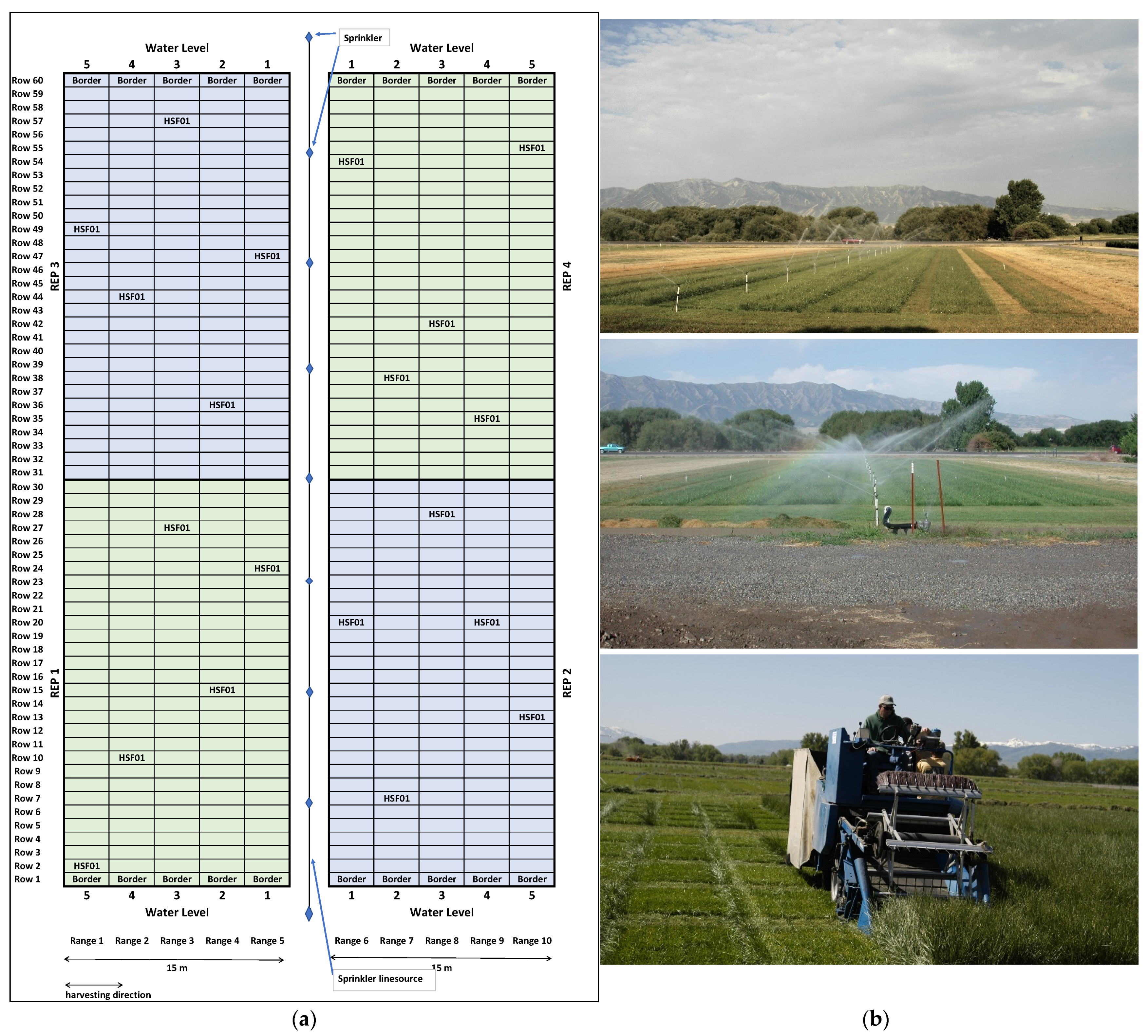
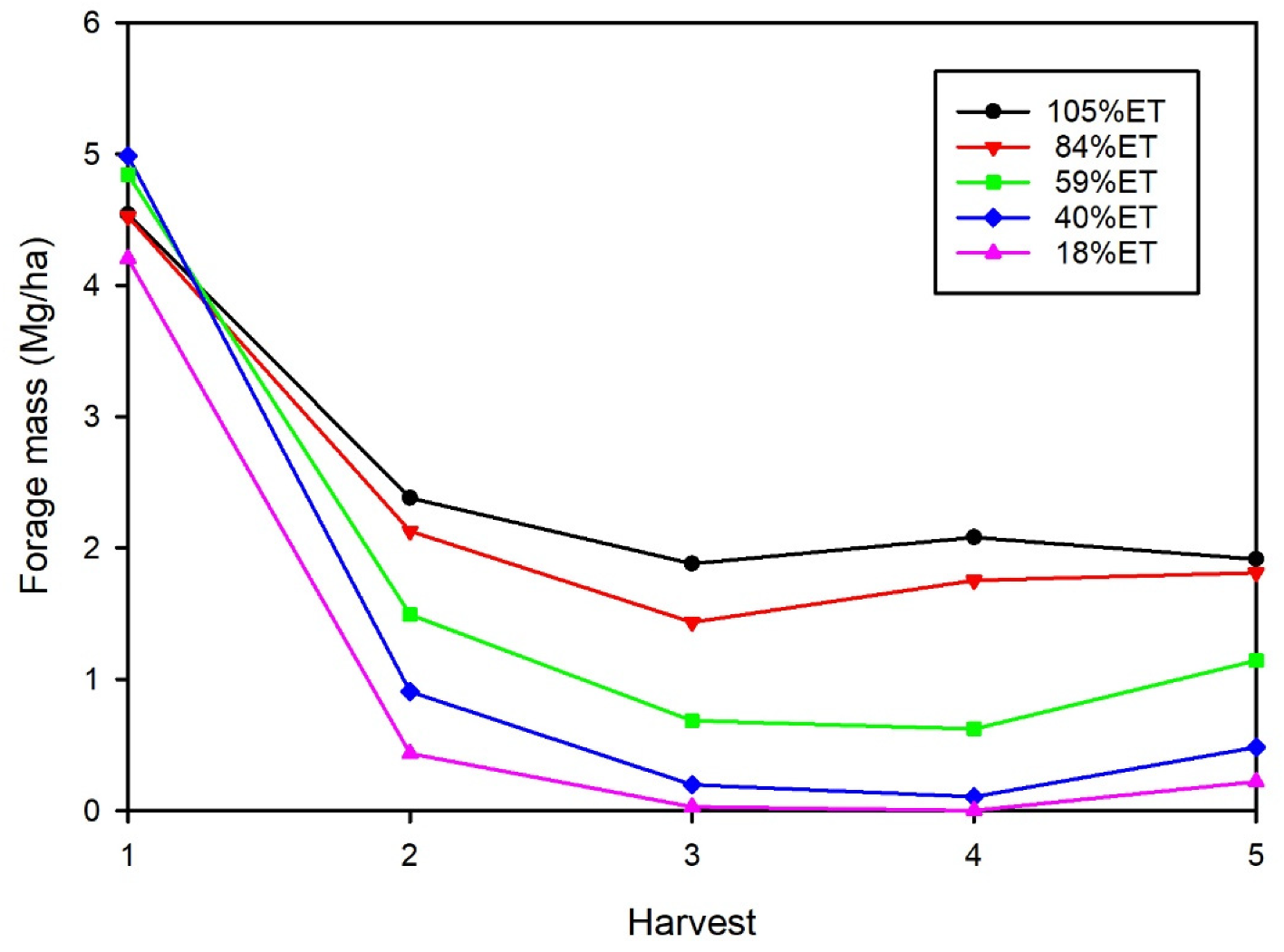
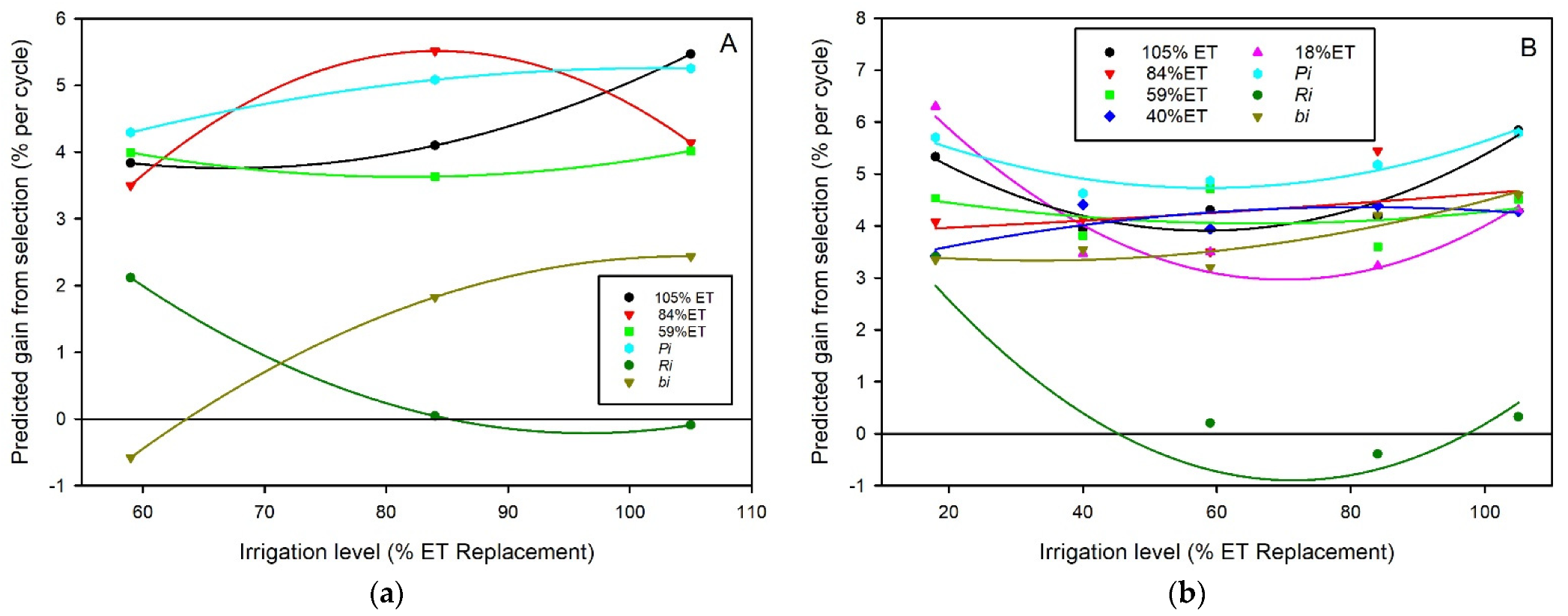
| 2001 | 2002 | 2003 | Mean | ||||
|---|---|---|---|---|---|---|---|
| Water Level | Ave. mm/wk | % ETo Replaced | Ave. mm/wk | % ETo Replaced | Ave. mm/wk | % ETo Replaced | % ETo Replaced |
| 1 | 40 | 102 | 41 | 106 | 41 | 107 | 105 |
| 2 | 30 | 78 | 34 | 89 | 33 | 84 | 84 |
| 3 | 20 | 50 | 24 | 63 | 24 | 62 | 59 |
| 4 | 13 | 32 | 17 | 44 | 17 | 44 | 40 |
| 5 | 4 | 12 | 10 | 25 | 7 | 19 | 18 |
| Statistic 1 | Water Level 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Yi | Ri | bi | 105%ET | 84%ET | 59%ET | 40%ET | 18%ET | |
| Mg/ha | unitless | unitless | Mg/ha | |||||
| Across Harvests | ||||||||
| HSF | ||||||||
| Mean | 2.22 | 0.70 | 1.00 | 2.57 | 2.34 | 1.76 | 1.34 | 0.98 |
| Greatest | 2.37 | 0.73 | 1.07 | 2.73 | 2.51 | 1.85 | 1.36 | 1.02 |
| Least | 2.12 | 0.68 | 0.91 | 2.44 | 2.18 | 1.68 | 1.31 | 0.95 |
| Range | 0.25 | 0.05 | 0.16 | 0.29 | 0.32 | 0.17 | 0.06 | 0.07 |
| std. error | 0.052 | 0.012 | 0.059 | 0.070 | 0.063 | 0.047 | 0.030 | 0.029 |
| Checks 3 | ||||||||
| Fawn | 2.15 | 0.67 | 1.05 | 2.52 | 2.29 | 1.67 | 1.32 | 0.95 |
| KY31E− | 2.06 | 0.70 | 0.91 | 2.34 | 2.18 | 1.69 | 1.32 | 0.97 |
| KY31E+ | 2.29 | 0.71 | 1.01 | 2.65 | 2.39 | 1.81 | 1.35 | 0.99 |
| Seasonal Total | ||||||||
| HSF | ||||||||
| Mean | 8.96 | 0.54 | 1.00 | 12.80 | 11.65 | 8.79 | 6.68 | 4.89 |
| Greatest | 9.52 | 0.57 | 1.09 | 13.68 | 12.52 | 9.32 | 6.98 | 5.31 |
| Least | 8.37 | 0.51 | 0.91 | 11.63 | 10.90 | 8.26 | 6.35 | 4.53 |
| Range | 1.15 | 0.06 | 0.18 | 2.05 | 1.62 | 1.06 | 0.63 | 0.78 |
| std. error | 0.190 | 0.014 | 0.036 | 0.345 | 0.313 | 0.237 | 0.174 | 0.170 |
| Checks | ||||||||
| Fawn | 8.62 | 0.53 | 1.01 | 12.56 | 11.44 | 8.26 | 6.53 | 4.60 |
| KY31E− | 8.37 | 0.56 | 0.91 | 11.63 | 10.90 | 8.39 | 6.55 | 4.78 |
| KY31E+ | 9.28 | 0.54 | 1.03 | 13.26 | 11.96 | 9.07 | 6.83 | 5.02 |
| Across Harvests | Seasonal Total | |||||
|---|---|---|---|---|---|---|
| WL or Statistic 2 | σ2F ± s.e. | p-Value 1 | h2 ± s.e. | σ2F ± s.e. | p-Value 1 | h2 ± s.e. |
| Yi | 0.0076 ± 0.0030 | 0.0002 | 0.66 ± 0.11 | 0.1264 ± 0.0443 | 0.0001 | 0.73 ± 0.07 |
| Ri | 0.0003 ± 0.0002 | 0.0372 | 0.43 ± 0.16 | 0.0003 ± 0.0002 | 0.0918 | 0.42 ± 0.17 |
| bi | 0.0054 ± 0.0044 | 0.1611 | 0.35 ± 0.21 | 0.0025 ± 0.0014 | 0.0273 | 0.49 ± 0.16 |
| 105%ET | 0.0128 ± 0.0054 | 0.0006 | 0.63 ± 0.11 | 0.3447 ± 0.1320 | 0.0001 | 0.67 ± 0.09 |
| 84%ET | 0.0106 ± 0.0044 | 0.0008 | 0.65 ± 0.12 | 0.2605 ± 0.1047 | 0.0001 | 0.64 ± 0.10 |
| 59%ET | 0.0042 ± 0.0024 | 0.0263 | 0.49 ± 0.16 | 0.1252 ± 0.0570 | 0.0021 | 0.57 ± 0.12 |
| 40%ET | 0.0012 ± 0.0014 | 0.3441 | 0.25 ± 0.23 | 0.0653 ± 0.0317 | 0.0092 | 0.55 ± 0.14 |
| 18%ET | 0.0011 ± 0.0012 | 0.2734 | 0.27 ± 0.21 | 0.0671 ± 0.0306 | 0.0040 | 0.59 ± 0.13 |
| WL 1 or Statistic 2 | Yi | Ri | bi | 105%ET | 84%ET | 59%ET | 40%ET | 18%ET |
|---|---|---|---|---|---|---|---|---|
| Yi | 0.06 | 0.91 | 0.95 | 0.89 | 0.91 | 0.91 | 0.81 | |
| Ri | 0.14 | −0.22 | 0.07 | −0.09 | 0.05 | −0.28 | 0.64 | |
| bi | 0.36 | −0.78 | 0.92 | 0.88 | 0.73 | 0.85 | 0.58 | |
| 105%ET | 0.94 | −0.02 | 0.60 | 0.75 | 0.84 | 0.81 | 0.79 | |
| 84%ET | 0.91 | 0.01 | 0.45 | 0.75 | 0.70 | 0.87 | 0.62 | |
| 59%ET | 0.92 | 0.56 | −0.17 | 0.84 | 0.76 | 0.85 | 0.73 | |
| 40%ET | 0.56 | |||||||
| 18%ET |
| WL 1 or Statistic 2 | Yi | Ri | bi | 105%ET | 84%ET | 59%ET | 40%ET | 18%ET |
|---|---|---|---|---|---|---|---|---|
| Yi | −0.01 | 0.70 | 0.85 | 0.85 | 0.86 | 0.83 | 0.67 | |
| Ri | 0.05 | −0.47 | −0.09 | −0.04 | −0.10 | −0.03 | 0.65 | |
| bi | −0.69 | 0.45 | 0.82 | 0.69 | 0.50 | 0.43 | 0.12 | |
| 105%ET | −0.16 | 0.88 | 0.69 | 0.61 | 0.64 | 0.61 | 0.54 | |
| 84%ET | −0.05 | 0.85 | 0.39 | 0.61 | 0.65 | 0.67 | 0.50 | |
| 59%ET | 0.43 | 0.85 | −0.01 | 0.65 | 0.65 | 0.72 | 0.50 | |
| 40%ET | 0.52 | |||||||
| 18%ET |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waldron, B.L.; Jensen, K.B.; Peel, M.D.; Picasso, V.D. Breeding for Resilience to Water Deficit and Its Predicted Effect on Forage Mass in Tall Fescue. Agronomy 2021, 11, 2094. https://doi.org/10.3390/agronomy11112094
Waldron BL, Jensen KB, Peel MD, Picasso VD. Breeding for Resilience to Water Deficit and Its Predicted Effect on Forage Mass in Tall Fescue. Agronomy. 2021; 11(11):2094. https://doi.org/10.3390/agronomy11112094
Chicago/Turabian StyleWaldron, Blair L., Kevin B. Jensen, Michael D. Peel, and Valentin D. Picasso. 2021. "Breeding for Resilience to Water Deficit and Its Predicted Effect on Forage Mass in Tall Fescue" Agronomy 11, no. 11: 2094. https://doi.org/10.3390/agronomy11112094
APA StyleWaldron, B. L., Jensen, K. B., Peel, M. D., & Picasso, V. D. (2021). Breeding for Resilience to Water Deficit and Its Predicted Effect on Forage Mass in Tall Fescue. Agronomy, 11(11), 2094. https://doi.org/10.3390/agronomy11112094








