Wheat Density Alters but Does Not Repress the Expression of a Fluroxypyr-Resistant Kochia (Bassia scoparia) Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Treatment Structure
2.2. Plant Material
2.3. Experimental Logistics
2.4. Data Collection
2.5. Statistical Analyses
3. Results and Discussion
3.1. Population Differences Absent of Crop Interference
3.2. Impact of Crop Interference
3.3. Management Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friesen, L.F.; Beckie, H.J.; Warwick, S.I.; Van Acker, R.C. The biology of Canadian weeds. 138. Kochia scoparia (L.) Schrad. Can. J. Plant Sci. 2009, 89, 141–167. [Google Scholar] [CrossRef]
- Manthey, G.A.; Hareland, G.A.; Zollinger, R.K.; Huseby, D.J. Kochia (Kochia scoparia) interference with oat (Avena sativa). Weed Technol. 1996, 10, 522–525. [Google Scholar] [CrossRef]
- Yadav, T.; Kumar, V.; Jha, P. Herbicide programs to manage glyphosate/dicamba-resistant kochia (Bassia scoparia) in glyphosate/dicamba-resistant soybean. Weed Technol. 2020, 34, 568–574. [Google Scholar] [CrossRef]
- Leeson, J.Y.; Hall, L.M.; Neeser, C.; Tidemann, B.; Harker, K.N. Alberta weed survey of annual crops in 2017. In Weed Survey Series Publication 19-1; Agriculture and Agri-Food Canada: Saskatoon, SK, Canada, 2019; p. 275. [Google Scholar]
- Schwinghamer, T.D.; Van Acker, R.C. Emergence timing and persistence of kochia (Kochia scoparia). Weed Sci. 2008, 56, 37–41. [Google Scholar] [CrossRef]
- Martin, S.L.; Benedict, L.; Wei, W.; Sauder, C.A.; Beckie, H.J.; Hall, L.M. High gene flow maintains genetic diversity following selection for high EPSPS copy number in the weed kochia (Amaranthaceae). Sci. Rep. 2020, 10, 18864. [Google Scholar] [CrossRef] [PubMed]
- Beckie, H.J.; Blackshaw, R.E.; Hall, L.M.; Johnson, E.N. Pollen- and seed-mediated gene flow in kochia (Kochia scoparia). Weed Sci. 2016, 64, 624–633. [Google Scholar] [CrossRef]
- Beckie, H.J.; Blackshaw, R.E.; Leeson, J.Y.; Stahlman, P.W.; Gaines, T.A.; Johnson, E.N. Seedbank persistence, germination and early growth of glyphosate-resistant Kochia scoparia. Weed Res. 2018, 58, 177–187. [Google Scholar] [CrossRef]
- Beckie, H.J.; Hall, L.M.; Shirriff, S.W.; Martin, E.; Leeson, J.Y. Triple-resistant kochia [Kochia scoparia L. Schrad.] in Alberta. Can. J. Plant Sci. 2019, 99, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Geddes, C.M.; Ostendorf, T.E.; Owen, M.L.; Leeson, J.Y.; Sharpe, S.M.; Shirriff, S.W.; Beckie, H.J. Fluroxypyr-resistant kochia [Bassia scoparia (L.) A.J. Scott] confirmed in Alberta. Can. J. Plant Sci. 2021, in press. [Google Scholar] [CrossRef]
- Geddes, C.M.; Owen, M.L.; Ostendorf, T.E.; Leeson, J.Y.; Sharpe, S.M.; Shirriff, S.W.; Beckie, H.J. Herbicide diagnostics reveal multiple patterns of synthetic auxin resistance in kochia (Bassia scoparia). Weed Technol. 2021, in press. [Google Scholar] [CrossRef]
- Geddes, C.M.; Pittman, M.M.; Gulden, R.H.; Jones, T.; Leeson, J.Y.; Sharpe, S.M.; Shirriff, S.W.; Beckie, H.J. Rapid increase in glyphosate resistance and confirmation of dicamba-resistant kochia (Bassia scoparia) in Manitoba. Can. J. Plant Sci. 2021, in press. [Google Scholar] [CrossRef]
- Heap, I.M. The International Herbicide Resistant Weed Database. Available online: http://www.weedscience.org (accessed on 14 August 2021).
- Torbiak, A.T.; Blackshaw, R.E.; Brandt, R.W.; Hall, L.M.; Hamman, B.; Geddes, C.M. Herbicide mixtures control glyphosate-resistant kochia (Bassia scoparia) in chemical fallow, but their longevity warrants careful stewardship. Can. J. Plant Sci. 2021, 101, 188–198. [Google Scholar] [CrossRef]
- Torbiak, A.T.; Blackshaw, R.E.; Brandt, R.W.; Hamman, B.; Geddes, C.M. Herbicide strategies for managing glyphosate-resistant and -susceptible kochia (Bassia scoparia) in spring wheat. Can. J. Plant Sci. 2021, 101, 607–621. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Beckie, H.J.; Molnar, L.J.; Entz, T.; Moyer, J.R. Combining agronomic practices and herbicides improves weed management in wheat-canola rotations within zero-tillage production systems. Weed Sci. 2005, 53, 528–535. [Google Scholar] [CrossRef]
- Lemerle, D.; Lockley, P.; Koetz, E.; Diffey, S. Herbicide efficacy for control of annual ryegrass (Lolium rigidum Gaud.) is influenced more by wheat seeding rate than row spacing. Crop Pasture Sci. 2013, 64, 708–715. [Google Scholar] [CrossRef]
- O’Donovan, J.T.; Blackshaw, R.E.; Harker, K.N.; Clayton, G.W. Wheat seeding rate influences herbicide performance in wild oat (Avena fatua L.). Agron. J. 2006, 98, 815–822. [Google Scholar] [CrossRef]
- Redlick, C.; Duddu, H.S.N.; Syrovy, L.D.; Willenborg, C.J.; Johnson, E.N.; Shirtliffe, S.J. Effect of seeding rate on dose-response of wild mustard (Sinapis arvensis) to fluthiacet-methyl. Weed Sci. 2017, 65, 525–533. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Harker, K.N.; O’Donovan, J.T.; Beckie, H.J.; Smith, E.G. Ongoing development of integrated weed management systems on the Canadian prairies. Weed Sci. 2008, 56, 146–150. [Google Scholar] [CrossRef]
- Mohler, C.L. Enhancing the competitive ability of crops. In Ecological Management of Agricultural Weeds; Liebman, M., Mohler, C.L., Staver, C.P., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 269–322. [Google Scholar]
- Blackshaw, R.E.; Semach, G.; O’Donovan, J.T. Utilization of wheat seeding rate to manage redstem filaree (Erodium cicutarium) in a zero-till cropping system. Weed Technol. 2000, 14, 389–396. [Google Scholar] [CrossRef]
- Champion, G.T.; Froud-Williams, R.J.; Holland, J.M. Interactions between wheat (Triticum aestivum L.) cultivar, row spacing and density and the effect on weed suppression and crop yield. Ann. Appl. Biol. 1998, 133, 443–453. [Google Scholar] [CrossRef]
- Kristensen, L.; Olsen, J.; Weiner, J. Crop density, sowing pattern, and nitrogen fertilization effects on weed suppression and yield in spring wheat. Weed Sci. 2008, 56, 97–102. [Google Scholar] [CrossRef]
- Lemerle, D.; Cousens, R.D.; Gill, G.S.; Peltzer, S.J.; Moerkerk, M.; Murphy, C.W.; Collins, D.; Cullis, B.R. Reliability of higher seeding rates of wheat for increased competitiveness with weeds in low rainfall environments. J. Agric. Sci. 2004, 142, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Olsen, J.; Kristensen, L.; Weiner, J.; Griepentrog, H.W. Increased density and spatial uniformity increase weed suppression by spring wheat. Weed Res. 2005, 45, 315–321. [Google Scholar] [CrossRef]
- Olsen, J.; Kristensen, L.; Weiner, J. Influence of sowing density and spatial pattern of spring wheat (Triticum aestivum) on the suppression of different weed species. Weed Biol. Manag. 2006, 6, 165–173. [Google Scholar] [CrossRef]
- Olsen, J.; Griepentrog, H.W.; Nielsen, J.; Weiner, J. How important are crop spatial pattern and density for weed suppression by spring wheat? Weed Sci. 2012, 60, 501–509. [Google Scholar] [CrossRef]
- Weiner, J.; Griepentrog, H.W.; Kristensen, L. Suppression of weeds by spring wheat (Triticum aestivum) increases with crop density and spatial uniformity. J. Appl. Ecol. 2001, 38, 784–790. [Google Scholar] [CrossRef]
- Walker, S.R.; Medd, R.W.; Robinson, G.R.; Cullis, B.R. Improved management of Avena ludoviciana and Phalaris paradoxa with more densely sown wheat and less herbicide. Weed Res. 2002, 42, 257–270. [Google Scholar] [CrossRef]
- Geddes, C.M.; Davis, A.S. The critical period for weed seed control: A proposed framework to limit weed seed return. Weed Res. 2021, 61, 282–287. [Google Scholar] [CrossRef]
- Ramseier, D.; Weiner, J. Competitive effect is a linear function of neighbor biomass in experimental populations of Kochia scoparia. J. Ecol. 2006, 94, 305–309. [Google Scholar] [CrossRef]
- Menalled, F.D.; Smith, R.G. Competitiveness of herbicide-resistant and herbicide-susceptible kochia (Kochia scoparia [L.] Schrad.) under contrasting management practices. Weed Biol. Manag. 2007, 7, 115–119. [Google Scholar] [CrossRef]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Evidence for an ecological cost of enhanced herbicide metabolism in Lolium rigidum. J. Ecol. 2009, 97, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Sheldrake, R., Jr.; Boodley, J.W. Plant growing in lightweight artificial mixes. Acta Hortic. 1966, 4, 155–157. [Google Scholar] [CrossRef]
- Canadian Weed Science Society—Société Canadienne de Malherbologie. Description of 0–100 Rating Scale for Herbicide Efficacy and Crop Phytotoxicity. Available online: http://www.weedscience.ca/cwss-visual-ratings-scale/ (accessed on 14 August 2021).
- Littell, R.C.; Milken, G.A.; Stroup, W.W.; Wolfinger, R.R.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006; p. 834. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R software package for dose-response studies: The concept and data analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
- Ritz, C. Toward a unified approach to dose-response modeling in ecotoxicology. Environ. Toxicol. Chem. 2010, 29, 220–229. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, V.; Lim, C.A. Variable response of kochia [Kochia scoparia (L.) Schrad.] to auxinic herbicides dicamba and fluroxypyr in Montana. Can. J. Plant Sci. 2015, 95, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Kumar, C.; Jha, P.; Jugulam, M.; Yadav, R.; Stahlman, P.W. Herbicide-resistant kochia (Bassia scoparia) in North America: A review. Weed Sci. 2019, 67, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Jha, P. Differences in germination, growth, and fecundity characteristics of dicamba-fluroxypyr-resistant and susceptible Kochia scoparia. PLoS ONE 2016, 11, e0161533. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.J.; Messersmith, C.G.; Nalewaja, J.D.; Duysen, M.E. Interference between spring cereals and Kochia scoparia related to environment and photosynthetic pathways. Agron. J. 2000, 92, 173–181. [Google Scholar] [CrossRef]
- Christoffoleti, P.J.; Westra, P. Competition effects with mixed stands of wheat and kochia (Kochia scoparia) biotypes resistant and susceptible to acetolactase synthase inhibitor herbicides. Sci. Agric. Piracicaba 1994, 51, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Geddes, C.M. Burial environment drives seed mortality of kochia (Bassia scoparia), wild oat (Avena fatua), and volunteer canola (Brassica napus) irrespective of crop species. Plants 2021, 10, 1961. [Google Scholar] [CrossRef] [PubMed]
- Tidemann, B.D.; Hall, L.M.; Harker, K.N.; Beckie, H.J. Factors affecting weed seed devitalization with the Harrington Seed Destructor. Weed Sci. 2017, 65, 650–658. [Google Scholar] [CrossRef]
- Kumar, V.; Jha, P. Influence of herbicides applied postharvest in wheat stubble on control, fecundity, and progeny fitness of Kochia scoparia in the US Great Plains. Crop Prot. 2015, 71, 144–149. [Google Scholar] [CrossRef] [Green Version]
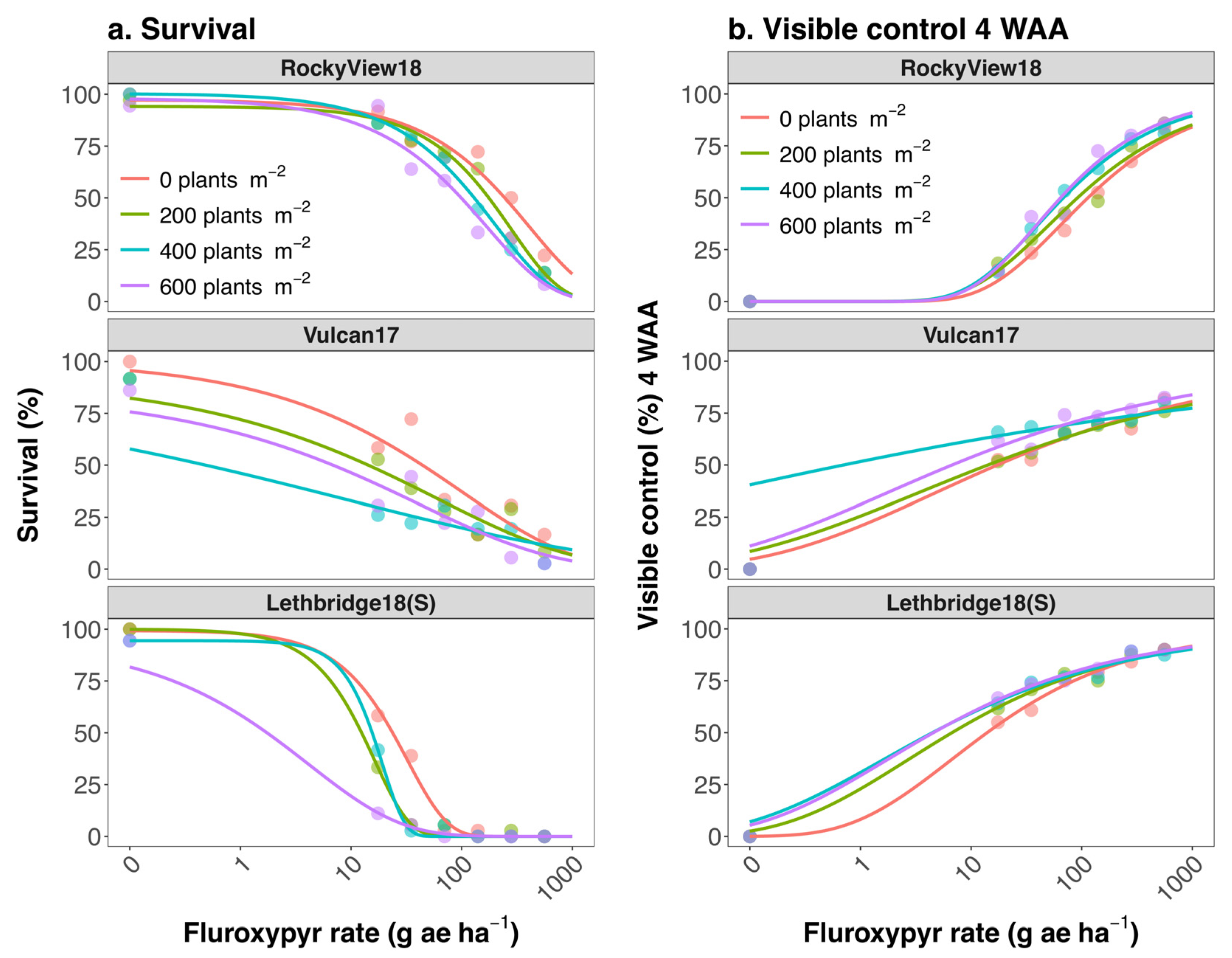


| Population | Wheat Density (Plants m−2) | b (± SE) a | (± SE) | e (± SE) | LD50/GR50 b (± SE) (g ae ha−1) | Population R/S c | Wheat Density R/S d | Population by Wheat Density R/S e |
|---|---|---|---|---|---|---|---|---|
| Plant Survival (%) | ||||||||
| Lethbridge18(S) | 0 | 1.2 (0.32) | 99 (7.6) | 33 (4.7) | 24 (4.3) | ― | ― | ― |
| 200 | 1.4 (0.81) | 100 (7.5) | 16 (3.0) | 12 (3.8) | ― | 0.5 | 0.5 | |
| 400 | 2.1 (1.16) | 94 (7.5) | 19 (2.1) | 16 (2.2) | ― | 0.7 | 0.7 | |
| 600 | 0.5 (0.63) | 94 (7.5) | 4 (8.7) | 2 (6.0) | ― | 0.1 | 0.1 | |
| Vulcan17 | 0 | 0.4 (0.10) | 101 (7.4) | 102 (29.5) | 43 (15.8) | 1.8 | ― | 1.8 |
| 200 | 0.3 (0.09) | 92 (7.5) | 61 (23.7) | 21 (11.7) | 1.8 | 0.5 | 0.9 | |
| 400 | 0.2 (0.09) | 92 (7.2) | 9 (11.6) | 1 (2.4) | 0.1 *** | <0.1 | <0.1 *** | |
| 600 | 0.4 (0.11) | 86 (7.6) | 40 (18.5) | 14 (9.7) | 7.0 | 0.3 | 0.6 | |
| RockyView18 | 0 | 0.8 (0.24) | 97 (7.5) | 413 (83.3) | 258 (62.6) | 10.8 ** | ― | 10.8 ** |
| 200 | 1.0 (0.26) | 94 (6.9) | 283 (47.6) | 193 (40.4) | 16.1 * | 0.7 | 8.0 ** | |
| 400 | 0.8 (0.17) | 100 (6.9) | 202 (36.7) | 129 (27.4) | 8.1 *** | 0.5 | 5.4 ** | |
| 600 | 0.7 (0.14) | 98 (6.9) | 168 (33.9) | 103 (23.4) | 51.5 | 0.4 | 4.3 ** | |
| Biomass FW (g pot−1) | ||||||||
| Lethbridge18(S) | 0 | 0.3 (0.07) | 30 (6.7) | 6 (6.9) | 2 (2.3) | ― | ― | ― |
| 200 | 0.4 (0.11) | 12 (3.4) | 23 (20.5) | 8 (9.6) | ― | 4.0 * | 4.0 * | |
| 400 | 0.5 (0.14) | 10 (3.1) | 25 (19.5) | 12 (11.5) | ― | 6.0 *** | 6.0 *** | |
| 600 | 0.6 (0.18) | 5 (1.8) | 46 (31.6) | 25 (21.1) | ― | 12.5 *** | 12.5 *** | |
| Vulcan17 | 0 | 0.4 (0.11) | 30 (6.5) | 56 (34.1) | 25 (19.5) | 12.5 | ― | 12.5 |
| 200 | 0.3 (0.11) | 16 (4.2) | 39 (46.5) | 10 (16.7) | 1.3 | 0.4 | 5.0 | |
| 400 | 0.2 (0.13) | 10 (2.9) | 29 (46.4) | 6 (14.4) | 0.5 | 0.2 | 3.0 | |
| 600 | 0.3 (0.14) | 8 (2.5) | 37 (42.5) | 12 (19.1) | 0.5 | 0.5 | 6.0 | |
| RockyView18 | 0 | 0.5 (0.11) | 43 (8.6) | 64 (32.5) | 30 (20.0) | 15.0 | ― | 15.0 |
| 200 | 0.5 (0.13) | 16 (4.0) | 59 (39.1) | 26 (22.6) | 3.3 | 0.9 | 13.0 | |
| 400 | 0.4 (0.14) | 11 (2.9) | 61 (39.6) | 29 (24.1) | 2.4 | 1.0 | 14.5 | |
| 600 | 0.6 (0.13) | 11 (2.9) | 38 (20.6) | 20 (13.5) | 0.8 | 0.7 | 10.0 |
| Population | Wheat Density (Plants m−2) | b (± SE) a | e (± SE) | ED50 b (± SE) (g ae ha−1) | Population R/S c | Wheat Density R/S d | Population by Wheat Density R/S e |
|---|---|---|---|---|---|---|---|
| Visible Control (%) 2 WAA | |||||||
| Lethbridge18(S) | 0 | −0.4 (0.06) | 4 (2.0) | 11 (3.8) | ― | ― | ― |
| 200 | −0.3 (0.06) | 4 (2.0) | 11 (4.2) | ― | 1.0 | 1.0 | |
| 400 | −0.3 (0.06) | 2 (1.5) | 7 (3.6) | ― | 0.6 | 0.6 | |
| 600 | −0.2 (0.06) | 1 (0.3) | 1 (1.7) | ― | 0.1 | 0.1 | |
| Vulcan17 | 0 | −0.3 (0.05) | 8 (2.9) | 23 (5.3) | 2.1 | ― | 2.1 |
| 200 | −0.3 (0.05) | 5 (2.7) | 20 (6.4) | 1.8 | 0.9 | 1.8 | |
| 400 | −0.1 (0.05) | 1 (0.4) | 2 (3.3) | 0.3 | 0.1 | 0.2 ** | |
| 600 | −0.2 (0.05) | 1 (1.4) | 8 (4.7) | 8.0 | 0.3 | 0.7 | |
| RockyView18 | 0 | −0.6 (0.06) | 44 (4.0) | 77 (6.7) | 7.0 * | ― | 7.0 * |
| 200 | −0.5 (0.05) | 59 (6.1) | 116 (11.7) | 10.5 * | 1.5 *** | 10.5 ** | |
| 400 | −0.5 (0.05) | 42 (4.8) | 87 (9.1) | 12.4 | 1.1 | 7.9 * | |
| 600 | −0.5 (0.05) | 42 (4.6) | 82 (8.1) | 82.0 | 1.1 | 7.5 * | |
| Visible Control (%) 4 WAA | |||||||
| Lethbridge18(S) | 0 | −0.5 (0.07) | 7 (2.2) | 14 (3.2) | ― | ― | ― |
| 200 | −0.4 (0.08) | 3 (1.6) | 7 (2.9) | ― | 0.5 | 0.5 | |
| 400 | −0.4 (0.08) | 2 (1.3) | 4 (2.6) | ― | 0.3 | 0.3 | |
| 600 | −0.4 (0.08) | 2 (1.3) | 5 (2.5) | ― | 0.4 | 0.4 | |
| Vulcan17 | 0 | −0.3 (0.05) | 5 (2.5) | 17 (5.4) | 1.2 | ― | 1.2 |
| 200 | −0.3 (0.05) | 3 (2.2) | 14 (5.5) | 2.0 | 0.8 | 1.0 | |
| 400 | −0.1 (0.05) | 0 (0.1) | 1 (1.3) | 0.3 ** | 0.1 | 0.1 *** | |
| 600 | −0.3 (0.06) | 2 (1.4) | 7 (3.6) | 1.4 | 0.4 | 0.5 | |
| RockyView18 | 0 | −0.6 (0.06) | 64 (6.0) | 114 (10.2) | 8.1 *** | ― | 8.1 *** |
| 200 | −0.6 (0.05) | 50 (4.9) | 92 (8.4) | 13.1 * | 0.8 | 6.6 *** | |
| 400 | −0.7 (0.06) | 40 (3.6) | 68 (5.8) | 17.0 | 0.6 *** | 4.9 ** | |
| 600 | −0.7 (0.06) | 39 (3.4) | 65 (5.2) | 13.0 | 0.6 *** | 4.6 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 Her Majesty the Queen in Right of Canada, as represented by the Minister of Agriculture and Agri-Food. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geddes, C.M.; Kimmins, M.T. Wheat Density Alters but Does Not Repress the Expression of a Fluroxypyr-Resistant Kochia (Bassia scoparia) Phenotype. Agronomy 2021, 11, 2160. https://doi.org/10.3390/agronomy11112160
Geddes CM, Kimmins MT. Wheat Density Alters but Does Not Repress the Expression of a Fluroxypyr-Resistant Kochia (Bassia scoparia) Phenotype. Agronomy. 2021; 11(11):2160. https://doi.org/10.3390/agronomy11112160
Chicago/Turabian StyleGeddes, Charles M., and Michael T. Kimmins. 2021. "Wheat Density Alters but Does Not Repress the Expression of a Fluroxypyr-Resistant Kochia (Bassia scoparia) Phenotype" Agronomy 11, no. 11: 2160. https://doi.org/10.3390/agronomy11112160
APA StyleGeddes, C. M., & Kimmins, M. T. (2021). Wheat Density Alters but Does Not Repress the Expression of a Fluroxypyr-Resistant Kochia (Bassia scoparia) Phenotype. Agronomy, 11(11), 2160. https://doi.org/10.3390/agronomy11112160






