Allelopathic Activity of Strigolactones on the Germination of Parasitic Plants and Arbuscular Mycorrhizal Fungi Growth
Abstract
:1. Introduction
1.1. Structures of Strigolactones
1.2. Milestones on the Isolation and Study of Strigolactones
2. Material and Methods
3. Review on the Identification and Isolation of Strigolactones
4. Allelopathic Bioactivities of Strigolactones
4.1. Germination Activity on Parasitic Plant Seeds, and Structure-Activity Relationships
4.2. Growth Stimulatory Activity on Arbuscular Mycorrhizal Fungi Hyphae
4.3. Applications of Strigolactones in Agriculture
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macías, F.A.; Durán, A.G.; Molinillo, J.M.G. Allelopathy: The Chemical Language of Plants. In Progress in the Chemistry of Organic Natural Products 112; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–84. [Google Scholar]
- Pons, S.; Fournier, S.; Chervin, C.; Bécard, G.; Rochange, S.; Dit Frey, N.F.; Pagès, V.P. Phytohormone production by the arbuscular mycorrhizal fungus Rhizophagus irregularis. PLoS ONE 2020, 15, e0240886. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Wei, P.; Chen, X.; Fu, Y.; Tam, N.F.; Hu, Z.; Chen, Z.; Li, F.; Zhou, H. Microcosm Study on Allelopathic Effects of Leaf Litter Leachates and Purified Condensed Tannins from Kandelia obovata on Germination and Growth of Aegiceras corniculatum. Forests 2021, 12, 1000. [Google Scholar] [CrossRef]
- Jmii, G.; Molinillo, J.M.G.; Zorrilla, J.G.; Haouala, R. Allelopathic activity of Thapsia garganica L. leaves on lettuce and weeds, and identification of the active principles. S. Afr. J. Bot. 2020, 131, 188–194. [Google Scholar] [CrossRef]
- Sarheed, M.M.; Rajabi, F.; Kunert, M.; Boland, W.; Wetters, S.; Miadowitz, K.; Kaźmierczak, A.; Sahi, V.P.; Nick, P. Cellular Base of Mint Allelopathy: Menthone Affects Plant Microtubules. Front. Plant Sci. 2020, 11, 1320. [Google Scholar] [CrossRef] [PubMed]
- Ćavar, S.; Zwanenburg, B.; Tarkowski, P. Strigolactones: Occurrence, structure, and biological activity in the rhizosphere. Phytochem. Rev. 2015, 14, 691–711. [Google Scholar] [CrossRef]
- Seto, Y.; Sado, A.; Asami, K.; Hanada, A.; Umehara, M.; Akiyama, K.; Yamaguchi, S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. USA 2014, 111, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, K.P.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2018, 69, 2189–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouwmeester, H.J.; Fonne-pfister, R.; Screpanti, C.; Mesmaeker, A. De Strigolactones: Plant Hormones with Promising Features. Angew. Chem. 2019, 12778–12786. [Google Scholar] [CrossRef]
- Chesterfield, R.J.; Vickers, C.E.; Beveridge, C.A. Translation of Strigolactones from Plant Hormone to Agriculture: Achievements, Future Perspectives, and Challenges. Trends Plant Sci. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Brewer, P.B. Strigolactones, how are they synthesized to regulate plant growth and development? Curr. Opin. Plant Biol. 2021, 63, 102072. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science (80-) 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Hauck, C.; Müller, S.; Schildknecht, H. A Germination Stimulant for Parasitic Flowering Plants from Sorghum bicolor, a Genuine Host Plant. J. Plant Physiol. 1992, 139, 474–478. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Prandi, C.; McErlean, C.S.P. The Chemistry of Strigolactones. In Strigolactones—Biology and Applications; Koltai, H., Prandi, C., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 163–198. [Google Scholar]
- Xie, X.; Yoneyama, K.; Kusumoto, D.; Yamada, Y.; Yokota, T.; Takeuchi, Y.; Yoneyama, K. Isolation and identification of alectrol as (+)-orobanchyl acetate, a germination stimulant for root parasitic plants. Phytochemistry 2008, 69, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Regeling, H.; Van Tilburg-Joukema, C.W.; Van Oss, B.; Molenveld, P.; De Gelder, R.; Tinnemans, P. Securing Important Strigolactone Key Structures: Orobanchol and 5-Deoxystrigol. Eur. J. Org. Chem. 2016, 2016, 2163–2169. [Google Scholar] [CrossRef] [Green Version]
- Jamil, M.; Charnikhova, T.; Houshyani, B.; van Ast, A.; Bouwmeester, H.J. Genetic variation in strigolactone production and tillering in rice and its effect on Striga hermonthica infection. Planta 2012, 235, 473–484. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Yoneyama, K.; Kisugi, T.; Uchida, K.; Ito, S.; Akiyama, K.; Hayashi, H.; Yokota, T.; Nomura, T.; Yoneyama, K. Confirming stereochemical structures of strigolactones produced by rice and tobacco. Mol. Plant 2013, 6, 153–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, K.K.; Xie, X.; Yoneyama, K.K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S.P. Which Are the Major Players, Canonical or Non-Canonical Strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef]
- Ueno, K.; Nakashima, H.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Bioconversion of 5-deoxystrigol stereoisomers to monohydroxylated strigolactones by plants. J. Pestic. Sci. 2018, 43, 198–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kisugi, T.; Xie, X.; Kim, H., II.; Yoneyama, K.; Sado, A.; Akiyama, K.; Hayashi, H.; Uchida, K.; Yokota, T.; Nomura, T.; et al. Strigone, isolation and identification as a natural strigolactone from Houttuynia cordata. Phytochemistry 2013, 87, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kusumoto, D.; Takeuchi, Y.; Yoneyama, K.K.; Yamada, Y.; Yoneyama, K.K. 2′-Epi-orobanchol and solanacol, two unique strigolactones, germination stimulants for root parasitic weeds, produced by tobacco. J. Agric. Food Chem. 2007, 55, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Harada, Y.; Fusegi, N.; Yamada, Y.; Ito, S.; Yokota, T.; Takeuchi, Y.; Yoneyama, K. Fabacyl acetate, a germination stimulant for root parasitic plants from Pisum sativum. Phytochemistry 2009, 70, 211–215. [Google Scholar] [CrossRef]
- Xie, X. Structural diversity of strigolactones and their distribution in the plant kingdom. Jpn. J. Pestic. Sci. 2016, 42, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, T.; Hayashi, H.; Akiyama, K. Medicaol, a strigolactone identified as a putative didehydro-orobanchol isomer, from Medicago truncatula. Phytochemistry 2015, 111, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Alder, A.; Jamil, M.; Marzorati, M.; Bruno, M.; Vermathen, M.; Bigler, P.; Ghisla, S.; Bouwmeester, H.; Beyer, P.; Al-Babili, S. The path from β-carotene to carlactone, a strigolactone-like plant hormone. Science (80-) 2012, 335, 1348–1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abe, S.; Sado, A.; Tanaka, K.; Kisugi, T.; Asami, K.; Ota, S.; Kim, H., II.; Yoneyama, K.; Xie, X.; Ohnishi, T.; et al. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 18084–18089. [Google Scholar] [CrossRef] [Green Version]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.-P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zealactones. Novel natural strigolactones from maize. Phytochemistry 2017, 137, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Kisugi, T.; Yoneyama, K.; Nomura, T.; Akiyama, K.; Uchida, K.; Yokota, T.; McErlean, C.S.P.; Yoneyama, K. Methyl zealactonoate, a novel germination stimulant for root parasitic weeds produced by maize. J. Pestic. Sci. 2017, 42, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Charnikhova, T.V.; Gaus, K.; Lumbroso, A.; Sanders, M.; Vincken, J.P.; De Mesmaeker, A.; Ruyter-Spira, C.P.; Screpanti, C.; Bouwmeester, H.J. Zeapyranolactone—A novel strigolactone from maize. Phytochem. Lett. 2018, 24, 172–178. [Google Scholar] [CrossRef]
- Kim, H., II.; Kisugi, T.; Khetkam, P.; Xie, X.; Yoneyema, K.; Uchida, K.; Yokota, T.; Nomura, T.; McErlean, C.S.P.; Yoneyama, K. Avenaol, a germination stimulant for root parasitic plants from Avena strigosa. Phytochemistry 2014, 103, 85–88. [Google Scholar] [CrossRef]
- Ueno, K.; Furumoto, T.; Umeda, S.; Mizutani, M.; Takikawa, H.; Batchvarova, R.; Sugimoto, Y. Heliolactone, a non-sesquiterpene lactone germination stimulant for root parasitic weeds from sunflower. Phytochemistry 2014, 108, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.; McErlean, C.S.P. Total Synthesis and Stereochemical Confirmation of Heliolactone. Org. Lett. 2019, 21, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Atarashi, T.; Kuse, M.; Sugimoto, Y.; Takikawa, H. Concise synthesis of heliolactone, a non-canonical strigolactone isolated from sunflower. Biosci. Biotechnol. Biochem. 2020, 84, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Baz, L.; Mori, N.; Mi, J.; Jamil, M.; Kountche, B.A.; Guo, X.; Balakrishna, A.; Jia, K.-P.; Vermathen, M.; Akiyama, K.; et al. 3-Hydroxycarlactone, a Novel Product of the Strigolactone Biosynthesis Core Pathway. Mol. Plant 2018, 11, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; Mori, N.; Yoneyama, K.; Nomura, T.; Uchida, K.; Yoneyama, K.; Akiyama, K. Lotuslactone, a non-canonical strigolactone from Lotus japonicus. Phytochemistry 2019, 157, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Sado, A.; Xie, X.; Yoneyama, K.; Asami, K.; Seto, Y.; Nomura, T.; Yamaguchi, S.; Yoneyama, K.; Akiyama, K. Chemical identification of 18-hydroxycarlactonoic acid as an LjMAX1 product and in planta conversion of its methyl ester to canonical and non-canonical strigolactones in Lotus japonicus. Phytochemistry 2020, 174, 112349. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Akiyama, K.; Brewer, P.B.; Mori, N.; Kawano-Kawada, M.; Haruta, S.; Nishiwaki, H.; Yamauchi, S.; Xie, X.; Umehara, M.; et al. Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct 2020, 4, e00219. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Nomura, S.; Muranaka, S.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Ent-2’-epi-Orobanchol and its acetate, as germination stimulants for Striga gesnerioides seeds isolated from cowpea and red clover. J. Agric. Food Chem. 2011, 59, 10485–10490. [Google Scholar] [CrossRef]
- Khetkam, P.; Xie, X.; Kisugi, T.; Kim, H., II.; Yoneyama, K.; Uchida, K.; Yokota, T.; Nomura, T.; Yoneyama, K. 7α- and 7β-Hydroxyorobanchyl acetate as germination stimulants for root parasitic weeds produced by cucumber. J. Pestic. Sci. 2014, 39, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dong, X.; Zheng, H.H.; Zhang, H.; Deng, X.; Chen, Y.P.; Zhu, Y.; Wu, H.H.; Xu, Y.T. Two isonardosinane-type sesquiterpenoids from Nardostachys jatamansi DC. Tetrahedron Lett. 2019, 60, 1992–1995. [Google Scholar] [CrossRef]
- Kohlen, W.; Charnikhova, T.; Bours, R.; López-Ráez, J.A.; Bouwmeester, H. Tomato strigolactones: A more detailed look. Plant Signal Behav. 2012, 8, e22785. [Google Scholar] [CrossRef] [Green Version]
- Rial, C.; Tomé, S.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Phytochemical study of safflower roots (Carthamus tinctorius) on the induction of parasitic plant germination and weed control. J. Chem. Ecol. 2020, 46, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Yoda, A.; Mori, N.; Akiyama, K.; Kikuchi, M.; Xie, X.; Miura, K.; Yoneyama, K.; Sato-Izawa, K.; Yamaguchi, S.; Yoneyama, K.; et al. Strigolactone biosynthesis catalyzed by cytochrome P450 and sulfotransferase in sorghum. New Phytol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ravazzolo, L.; Trevisan, S.; Manoli, A.; Boutet-Mercey, S.; Perreau, F.; Quaggiotti, S. The Control of Zealactone Biosynthesis and Exudation is Involved in the Response to Nitrogen in Maize Root. Plant Cell Physiol. 2019, 60, 2100–2112. [Google Scholar] [CrossRef] [PubMed]
- Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Durán, A.G.; Macías, F.A. Quantification of Strigolactones. In Plant and Food Carotenoids; Rodrıguez-Concepcion, M., Welsch, R., Eds.; Humana: New York, NY, USA, 2020; pp. 199–208. ISBN 9781493999521. [Google Scholar]
- Boutet-Mercey, S.; Perreau, F.; Roux, A.; Clavé, G.; Pillot, J.-P.P.; Schmitz-Afonso, I.; Touboul, D.; Mouille, G.; Rameau, C.; Boyer, F.-D.D. Validated Method for Strigolactone Quantification by Ultra High-Performance Liquid Chromatography—Electrospray Ionisation Tandem Mass Spectrometry Using Novel Deuterium Labelled Standards. Phytochem. Anal. 2018, 29, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Rial, C.; Varela, R.M.; Molinillo, J.M.G.; López-Ráez, J.A.; Macías, F.A. A new UHPLC-MS/MS method for the direct determination of strigolactones in root exudates and extracts. Phytochem. Anal. 2019, 30, 110–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floková, K.; Shimels, M.; Andreo Jimenez, B.; Bardaro, N.; Strnad, M.; Novák, O.; Bouwmeester, H.J. An improved strategy to analyse strigolactones in complex sample matrices using UHPLC–MS/MS. Plant Methods 2020, 16, 125. [Google Scholar] [CrossRef]
- Halouzka, R.; Zeljković, S.Ć.; Klejdus, B.; Tarkowski, P. Analytical methods in strigolactone research. Plant Methods 2020, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Cochetel, N.; Météier, E.; Merlin, I.; Hévin, C.; Pouvreau, J.B.; Coutos-Thévenot, P.; Hernould, M.; Vivin, P.; Cookson, S.J.; Ollat, N.; et al. Potential contribution of strigolactones in regulating scion growth and branching in grafted grapevine in response to nitrogen availability. J. Exp. Bot. 2018, 69, 4099–4112. [Google Scholar] [CrossRef] [PubMed]
- Aquino, B.; Bradley, J.M.; Lumba, S. On the outside looking in: Roles of endogenous and exogenous strigolactones. Plant J. 2021, 105, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Borghi, L.; Kang, J.; de Brito Francisco, R. Filling the gap: Functional clustering of ABC proteins for the investigation of hormonal transport in planta. Front. Plant Sci. 2019, 10, 422. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Cala, A.; Rial, C.; Mejías, F.J.R.; Molinillo, J.M.G.; Varela, R.M.; Macías, F.A. Synthesis of Active Strigolactone Analogues Based on Eudesmane- and Guaiane-Type Sesquiterpene Lactones. J. Agric. Food Chem. 2020, 68, 9636–9645. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Razvi, S.S.I.; Kuerban, A.; Balamash, K.S.; Al-Bishri, W.M.; Abulnaja, K.O.; Choudhry, H.; Khan, J.A.; Moselhy, S.S.; Ma, Z.; et al. Strigolactones-A novel class of phytohormones as anti-cancer agents. J. Pestic. Sci. 2018, 43, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.Q.; Jiang, K.; Takahashi, I.; Li, G.D. Strigolactone-induced senescence of a bamboo leaf in the dark is alleviated by exogenous sugar. J. Pestic. Sci. 2018, 43, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Ding, F.; Li, M.; Zhang, X.; Zhang, S.; Huang, B. Strigolactone and ethylene inhibitor suppressing dark-induced leaf senescence in perennial ryegrass involving transcriptional downregulation of chlorophyll degradation. J. Am. Soc. Hortic. Sci. 2021, 146, 79–86. [Google Scholar] [CrossRef]
- Takahashi, I.; Jiang, K.; Asami, T. Counteractive effects of sugar and strigolactone on leaf senescence of rice in darkness. Agronomy 2021, 11, 1044. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M.; Wegmann, K.; Joel, D.M. Revisiting strategies for reducing the seedbank of Orobanche and Phelipanche spp. Weed Res. 2009, 49, 23–33. [Google Scholar] [CrossRef]
- Macías, F.A.; Mejías, F.J.; Molinillo, J.M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 2019, 75, 2413–2436. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Macías, F.A. Facile synthesis of anhydrojudaicin and 11,13-dehydroanhydrojudaicin, two eudesmanolide-skeleton lactones with potential allelopathic activity. Phytochem. Lett. 2019, 31, 229–236. [Google Scholar] [CrossRef]
- Lumbroso, A.; Villedieu-Percheron, E.; Zurwerra, D.; Screpanti, C.; Lachia, M.; Dakas, P.Y.; Castelli, L.; Paul, V.; Wolf, H.C.; Sayer, D.; et al. Simplified strigolactams as potent analogues of strigolactones for the seed germination induction of Orobanche cumana Wallr. Pest Manag. Sci. 2016, 72, 2054–2068. [Google Scholar] [CrossRef]
- Pouvreau, J.-B.; Poulin, L.; Huet, S.; Delavault, P. Strigolactone-Like Bioactivity via Parasitic Plant Germination Bioassay. In Strigolactones; Cardinale, F., Prandi, C., Eds.; Humana: New York, NY, USA; pp. 59–74. ISBN 9781071614280.
- Evidente, A.; Fernández-Aparicio, M.; Cimmino, A.; Rubiales, D.; Andolfi, A.; Motta, A. Peagol and peagoldione, two new strigolactone-like metabolites isolated from pea root exudates. Tetrahedron Lett. 2009, 50, 6955–6958. [Google Scholar] [CrossRef]
- Yoneyama, K. Recent progress in the chemistry and biochemistry of strigolactones. J. Pestic. Sci. 2020, 45, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, S.; Nakashima, H.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Structural requirements of strigolactones for germination induction and inhibition of Striga gesnerioides seeds. Plant Cell Rep. 2013, 32, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.; Whichard, L.; Wall, M.; Egley, G. Germination stimulants. II. Structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea). J. Am. Chem. Soc. 1972, 94, 6198–6199. [Google Scholar] [CrossRef]
- Kim, H., II.; Xie, X.; Kim, H.S.; Chun, J.C.; Yoneyama, K.; Nomura, T.; Takeuchi, Y.; Yoneyama, K. Structure-activity relationship of naturally occurring strigolactones in Orobanche minor seed germination stimulation. J. Pestic. Sci. 2010, 35, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, Y.; Ueyama, T. Production of (+)-5-deoxystrigol by Lotus japonicus root culture. Phytochemistry 2008, 69, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ueno, K.; Fujiwara, M.; Nomura, S.; Mizutani, M.; Sasaki, M.; Takikawa, H.; Sugimoto, Y. Structural requirements of strigolactones for germination induction of Striga gesnerioides seeds. J. Agric. Food Chem. 2011, 59, 9226–9231. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.; Ohashi, K.; Sasako, H.; Tagawa, N.; Takano, Y.; Ioka, Y.; Nabeta, K.; Yoshihara, T. Germination stimulant from root exudates of Vigna unguiculata. Plant Growth Regul. 2008, 54, 31–36. [Google Scholar] [CrossRef]
- Xie, X.; Yoneyama, K.; Kurita, J.; Harada, Y.; Yamada, Y.; Takeuchi, Y.; Yoneyama, K. 7-Oxoorobanchyl acetate and 7-Oxoorobanchol as germination stimulants for root parasitic plants from flax (Linum usitatissimum). Biosci. Biotechnol. Biochem. 2009, 73, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, M.; Fonné-Pfister, R.; Screpanti, C.; Hermann, K.; Rendine, S.; Dieckmann, M.; Quinodoz, P.; De Mesmaeker, A. Total Synthesis and Biological Evaluation of Heliolactone. Helv. Chim. Acta 2019, 102. [Google Scholar] [CrossRef]
- Lachia, M.; Wolf, H.C.; De Mesmaeker, A. Synthesis of strigolactones analogues by intramolecular [2+2] cycloaddition of ketene-iminium salts to olefins and their activity on Orobanche cumana seeds. Bioorg. Med. Chem. Lett. 2014, 24, 2123–2128. [Google Scholar] [CrossRef] [PubMed]
- Ashida, K.; Hoshimoto, Y.; Tohnai, N.; Scott, D.E.; Ohashi, M.; Imaizumi, H.; Tsuchiya, Y.; Ogoshi, S. Enantioselective Synthesis of Polycyclic γ-Lactams with Multiple Chiral Carbon Centers via Ni(0)-Catalyzed Asymmetric Carbonylative Cycloadditions without Stirring. J. Am. Chem. Soc. 2020, 142, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Ronellenfitsch, H.; Liesche, J.; Jensen, K.H.; Michele Holbrook, N.; Schulz, A.; Katifori, E. Scaling of phloem structure and optimality of photoassimilate transport in conifer needles. Proc. R. Soc. B Biol. Sci. 2015, 282. [Google Scholar] [CrossRef]
- Cardoso, C.; Ruyter-Spira, C.; Bouwmeester, H.J. Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche spp. Plant Sci. 2011, 180, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Duran, H.G.S.; van Haarst, J.C.; Schijlen, E.G.W.M.; Ruyter-Spira, C.; Medema, M.H.; Dong, L.; Bouwmeester, H.J. The role of strigolactones in P deficiency induced transcriptional changes in tomato roots. BMC Plant Biol. 2021, 21, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Banasiak, J.; Borghi, L.; Stec, N.; Martinoia, E.; Jasiński, M. The Full-Size ABCG Transporter of Medicago truncatula Is Involved in Strigolactone Secretion, Affecting Arbuscular Mycorrhiza. Front. Plant Sci. 2020, 11, 18. [Google Scholar] [CrossRef] [Green Version]
- Abdelhalim, T.; Jannoura, R.; Joergensen, R.G. Mycorrhiza response and phosphorus acquisition efficiency of sorghum cultivars differing in strigolactone composition. Plant Soil 2019, 437, 55–63. [Google Scholar] [CrossRef]
- Zhou, Y.; Ge, S.; Jin, L.; Yao, K.; Wang, Y.; Wu, X.; Zhou, J.; Xia, X.; Shi, K.; Foyer, C.H.; et al. A novel CO2-responsive systemic signaling pathway controlling plant mycorrhizal symbiosis. New Phytol. 2019, 224, 106–116. [Google Scholar] [CrossRef] [PubMed]
- López-Ráez, J.A. How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta 2016, 243, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.C.; Ren, C.G.; Li, R.Z.; Xie, Z.H.; Wang, J.P. Hydrogen Peroxide and Strigolactones Signaling Are Involved in Alleviation of Salt Stress Induced by Arbuscular Mycorrhizal Fungus in Sesbania cannabina Seedlings. J. Plant Growth Regul. 2017, 36, 734–742. [Google Scholar] [CrossRef]
- Ruth, B.; Khalvati, M.; Schmidhalter, U. Quantification of Mycorrhizal water uptake via high-resolution on-line water content sensors. Plant Soil 2011, 342, 459–468. [Google Scholar] [CrossRef]
- Nasir, F.; Shi, S.; Tian, L.; Chang, C.; Ma, L.; Li, X.; Gao, Y.; Tian, C. Strigolactones shape the rhizomicrobiome in rice (Oryza sativa). Plant Sci. 2019, 286, 118–133. [Google Scholar] [CrossRef]
- Mitra, D.; Guerra Sierra, B.E.; Khoshru, B.; De Los Santos Villalobos, S.; Belz, C.; Chaudhary, P.; Shahri, F.N.; Djebaili, R.; Adeyemi, N.O.; El-Ballat, E.M.; et al. Impacts of Arbuscular Mycorrhizal Fungi on Rice Growth, Development, and Stress Management With a Particular Emphasis on Strigolactone Effects on Root Development. Commun. Soil Sci. Plant Anal. 2021, 52, 1591–1621. [Google Scholar] [CrossRef]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef]
- Mori, N.; Nishiuma, K.; Sugiyama, T.; Hayashi, H.; Akiyama, K. Carlactone-type Strigolactones and their Synthetic Analogues as Inducers of Hyphal Branching in Arbuscular Mycorrhizal Fungi. Phytochemistry 2016, 130, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- López-Raez, J.A.; Shirasu, K.; Foo, E. Strigolactones in Plant Interactions with Beneficial and Detrimental Organisms: The Yin and Yang. Trends Plant Sci. 2017, 22, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Chai, M.; Zhu, X.; Cui, H.; Jiang, C.; Zhang, J.; Shi, L. Lily Cultivars Have Allelopathic Potential in Controlling Orobanche aegyptiaca Persoon. PLoS ONE 2015, 10, e0142811. [Google Scholar] [CrossRef] [Green Version]
- Vurro, M.; Boari, A.; Thiombiano, B.; Bouwmeester, H. Strigolactones and Parasitic Plants. In Strigolactones—Biology and Applications; Koltai, H., Prandi, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 89–120. [Google Scholar]
- Nelson, D.C. The mechanism of host-induced germination in root parasitic plants. Plant Physiol. 2021, 185, 1353–1373. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Pospíšil, T.; Ćavar Zeljković, S. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bürger, M.; Chory, J. The Many Models of Strigolactone Signaling. Trends Plant Sci. 2020, 25, 395–405. [Google Scholar] [CrossRef]
- Hailu, G.; Niassy, S.; Zeyaur, K.R.; Ochatum, N.; Subramanian, S. Maize–legume intercropping and push–pull for management of fall armyworm, stemborers, and Striga in Uganda. Agron. J. 2018, 110, 2513–2522. [Google Scholar] [CrossRef] [Green Version]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Nomura, T.; Takahashi, I.; Asami, T.; Mori, N.; Akiyama, K.; Kusajima, M.; Nakashita, H. Regulation of biosynthesis, perception, and functions of strigolactones for promoting arbuscular mycorrhizal symbiosis and managing root parasitic weeds. Pest Manag. Sci. 2019, 75, 2353–2359. [Google Scholar] [CrossRef] [PubMed]
- Kawada, K.; Takahashi, I.; Arai, M.; Sasaki, Y.; Asami, T.; Yajima, S.; Ito, S. Synthesis and Biological Evaluation of Novel Triazole Derivatives as Strigolactone Biosynthesis Inhibitors. J. Agric. Food Chem. 2019, 67, 6143–6149. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.Z.; Yao, Z.Q.; Cao, X.L.; Peng, J.F.; Xu, Y.; Chen, M.X.; Zhao, S.F. Transcriptome analysis of Phelipanche aegyptiaca seed germination mechanisms stimulated by fluridone, TIS108, and GR24. PLoS ONE 2017, 12, e0187539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holbrook-Smith, D.; Toh, S.; Tsuchiya, Y.; McCourt, P. Small-molecule antagonists of germination of the parasitic plant Striga hermonthica. Nat. Chem. Biol. 2016, 12, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Khosla, A.; Nelson, D.C. Strigolactones, super hormones in the fight against Striga. Curr. Opin. Plant Biol. 2016, 33, 57–63. [Google Scholar] [CrossRef] [Green Version]

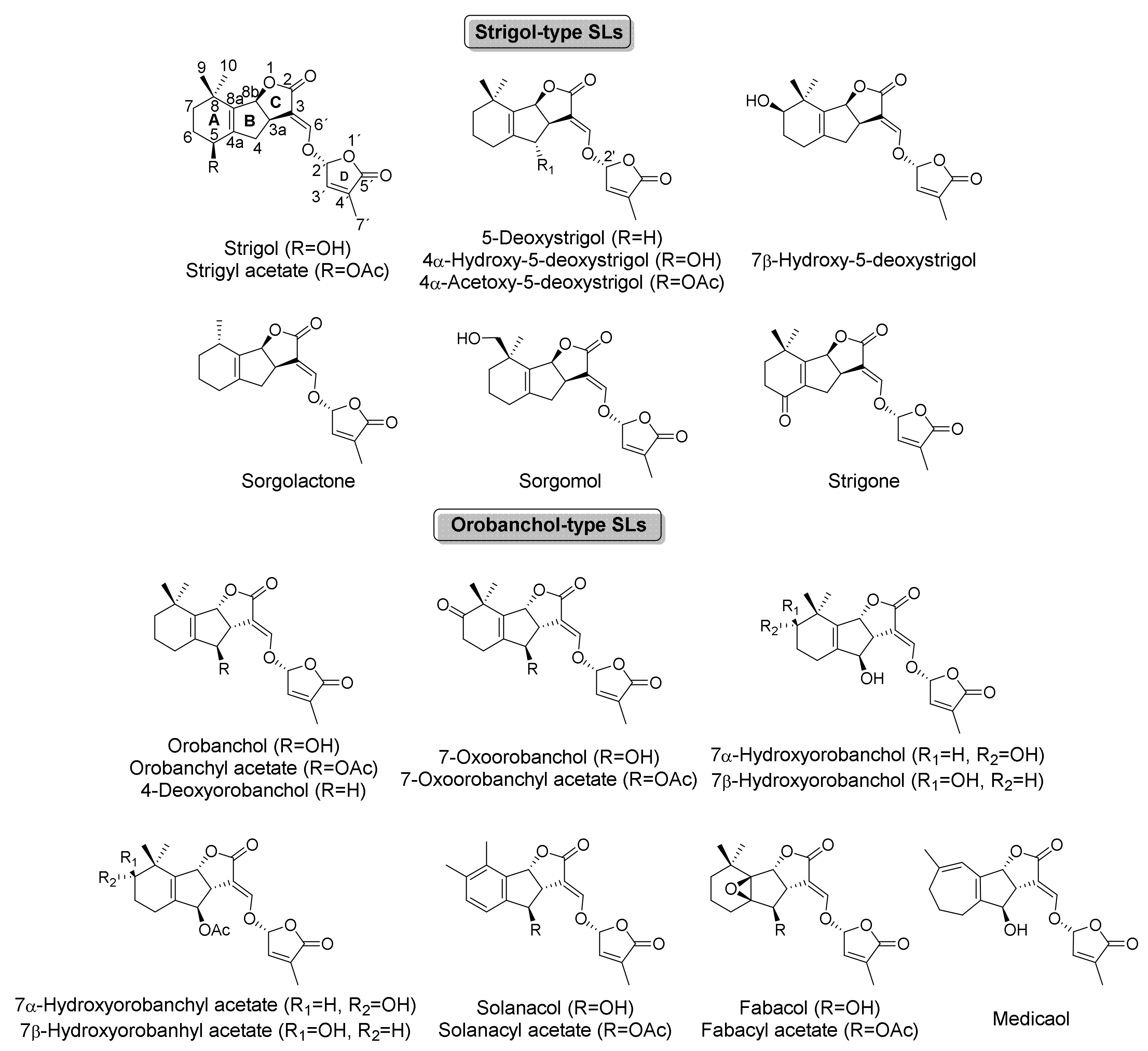


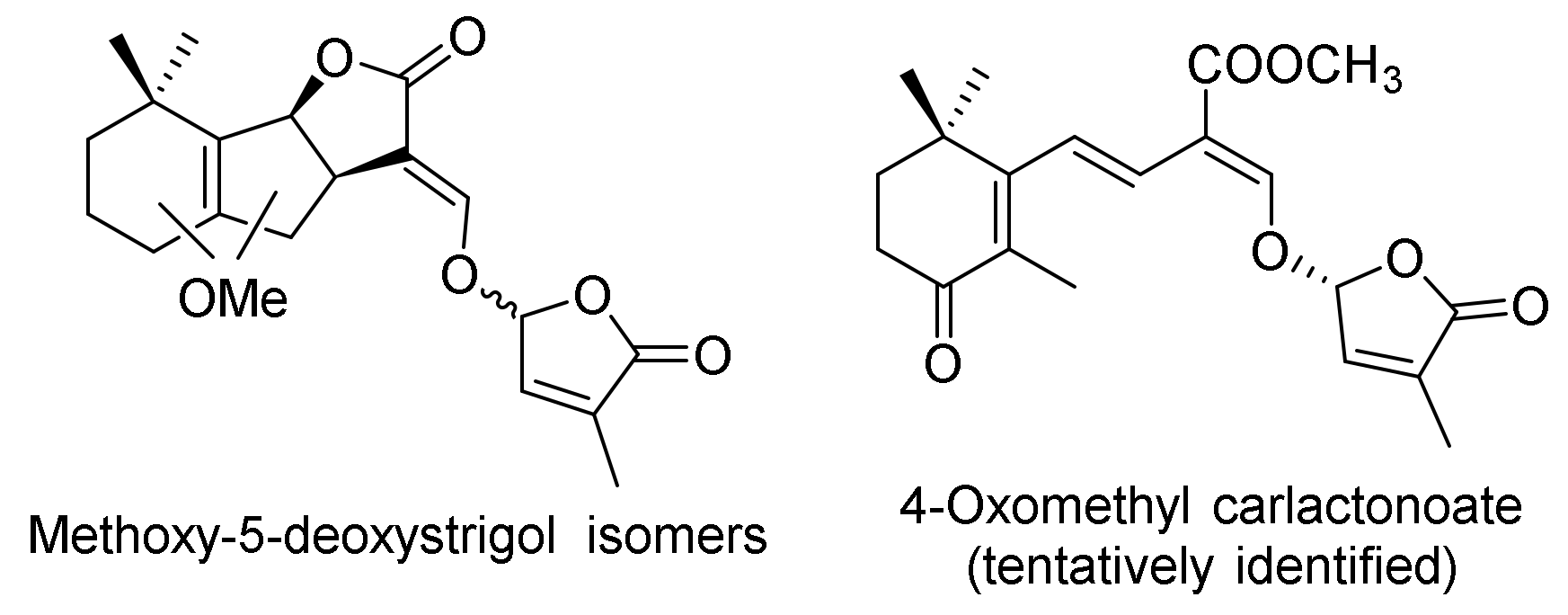



| Strigolactones | Identified in | Isolated | References |
|---|---|---|---|
| Canonical strigolactones | |||
| Strigol | Fragaria x ananassa | [27] | |
| Strigyl acetate | Fragaria x ananassa Sorghum bicolor | [27] [27] | |
| 5-Deoxystrigol | Fragaria x ananassa Nicotiana tabacum | [27] [21,27] | |
| 4α-Hydroxy-5-deoxystrigol | Nicotiana tabacum Trifolium pretense Vigna unguiculata | X X | [21] [42] [42] |
| 4α-Acetoxy-5-deoxystrigol | Nicotiana tabacum | [21] | |
| 7β-Hydroxy-5 deoxystrigol | Houttuynia cordata | [22] | |
| Orobanchol | Cryptomeria japonica Cucumis sativus Ginkgo biloba Pinus thunbergii | [22] [43] [27] [27] | |
| Orobanchyl acetate | Cryptomeria japonica Cucumis sativus Ginkgo biloba Nardostachys jatamansi Nicotiana tabacum Oryza sativa Pinus thunbergii | X | [22] [43] [27] [44] [21] [21] [27] |
| 4-Deoxyorobanchol | Cryptomeria japónica Cucumis sativus Gynkgo biloba Nicotiana tabacum Oryza sativa Pisum sativum Populus spp. Selaginella moellendorfii Solanum lycopersicum | X X | [22] [43] [27] [21] [21] [27] [27] [27] [27] |
| 7-Oxoorobanchol | Solanum lycopersicum | X | [45] |
| 7-Oxoorobanchyl acetate | Oryza sativa | [27] | |
| 7 α-Hydroxyorobanchol | Solanum lycopersicum | [45] | |
| 7 β-Hydroxyorobanchol | Solanum lycopersicum | [45] | |
| 7 α-Hydroxyorobanchyl acetate | Cucumis sativus Solanum lycopersicum | X | [43] [45] |
| 7 β-Hydroxyorobanchyl acetate | Cucumis sativus Solanum lycopersicum | X | [43] [45] |
| Solanacol | Carthamus tinctorius | [46] | |
| Medicaol | Medicago truncatula | X | [28] |
| Fabacol | Pisum sativum | [26,27] | |
| Non-canonical strigolactones | |||
| Carlactone | Arabidopsis thaliana Oryza sativa | [7,41] [7] | |
| Carlactonoic acid | Arabidopsis thaliana Helianthus annuus Pinus thunbergii Populus spp. Selaginella moellendorffii Zea Mays | [30] [27] [27] [27] [27] [27] | |
| Methyl carlactonoate | Arabidopsis thaliana Populus spp. | [30] [27] | |
| 3-Hydroxycarlactone 3-Hydroxycarlactonoic acid 4-Hydroxycarlactone 4-Hydroxycarlactonoic acid Methyl 4-hydroxycarlactonoate 16-Hydroxycarlactone 16-Hydroxycarlactonoic acid Methyl 16-hydroxycarlactonoate | Arabidopsis thaliana | [41] | |
| 18-Hydroxycarlactonoic acid | Lotus japonicus Sorghum bicolor | [40] [47] | |
| Hydroxymethyl carlactonoate | Arabidopsis thaliana | [41] | |
| Heliolactone | Helianthus annuus Zea mays | X X | [35] [31] |
| Methyl zealactonoate (two diastereomers) | Zea mays | X | [31,32,48] |
| Zeapyranolactone | Zea mays | X | [33] |
| Avenaol | Avena strigose | X | [34] |
| Lotuslactone | Lotus japonicus | X | [39] |
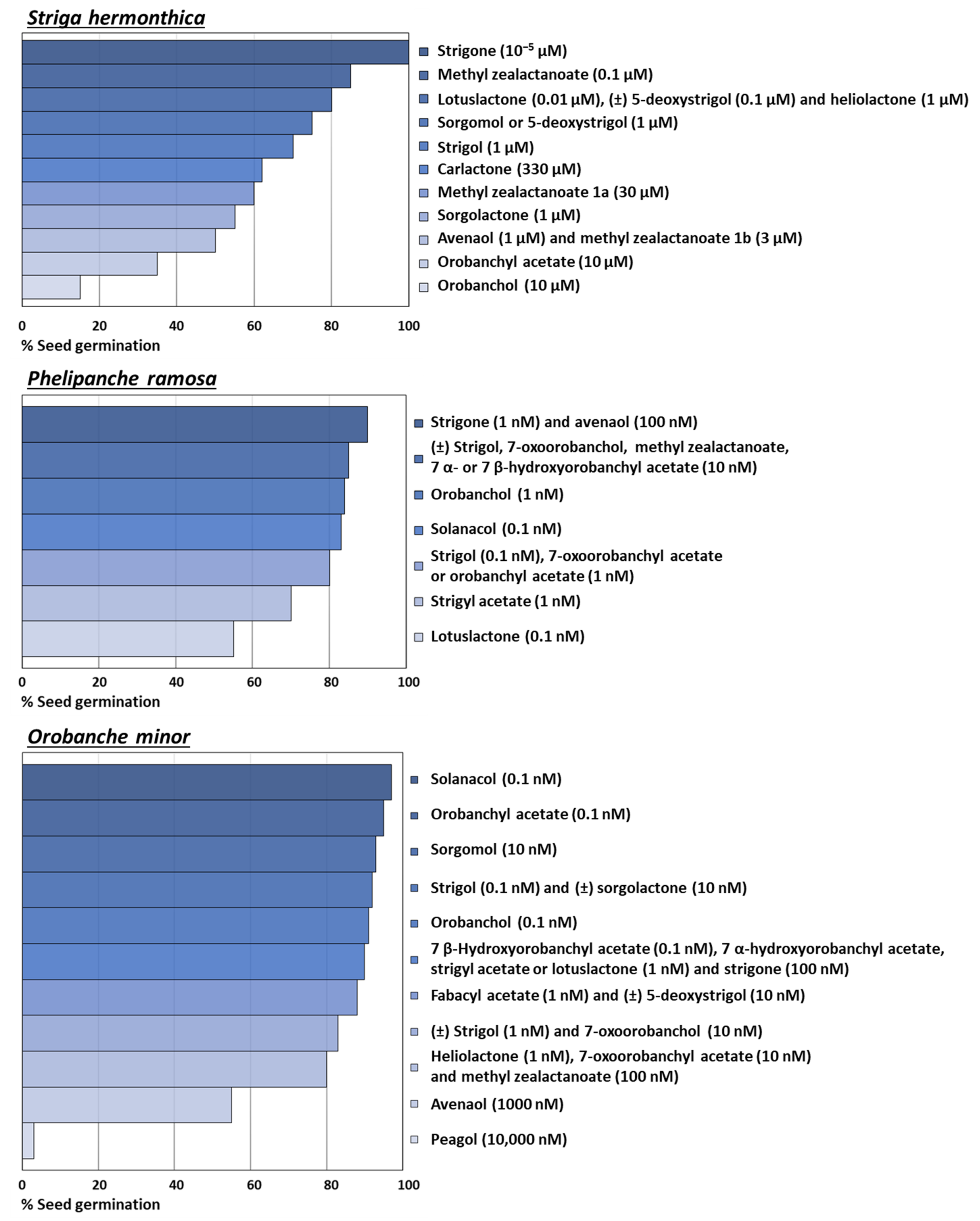
| Strigolactones | Parasitic Plant Species (Activity and Concentration) | References |
|---|---|---|
| Canonical strigolactones | ||
| Strigol | Alectra vogelii (50% 100 nM) Orobanche cumana (19% 100 nM) Orobanche minor (92% 0.1 nM; 83% 1 nM for (±) strigol) Phelipanche aegyptiaca (50% 100 pM) Phelipanche ramosa (80% 0.1 nM; 85% 10 nM for (±) strigol) Striga asiatica (50% 100 pM) Striga gesnerioides (0% 10 nM or 1 µM) Striga hermonthica (70% 1 µM) Striga lutea (50% 10 pM) | [13] [68] [18,25] [13] [18,25] [13] [69] [69] [70] |
| Strigyl acetate | Phelipanche ramosa (70% 1 nM) | [18] |
| 5-Deoxystrigol | Orobanche minor (87% 1 nM for (±) 5-deoxystrigol) Orobanche crenata (45% 10 µM for (±) 5-deoxystrigol) Striga gesnerioides (0% 10 nM or 1 µM) Striga hermonthica (75% 1 µM; 80% 0.1 µM for (±) 5-deoxystrigol) | [71] [72] [69] [69] [72] |
| Sorgolactone | Alectra vogelii (50% 1 µM) Orobanche minor (92% 10 nM for (±) sorgolactone) Phelipanche aegyptiaca (50% 100 nM) Striga asiatica (50% 10 pM) Striga gesnerioides (0% 10 nM or 1 µM) Striga hermonthica (55% 1 µM) | [13] [71] [13] [13] [69] [69] |
| Sorgomol | Orobanche minor (93% 10 nM) Striga gesnerioides (0% 10 nM or 1 µM) Striga hermonthica (75% 1 µM) | [71] [69] [69] |
| Orobanchol | Orobanche cumana (64% 100 nM) Striga gesnerioides (50% 1 nM) Striga hermonthica (15% 10 µM) | [68] [42] [73] |
| Orobanchyl acetate | Orobanche minor (95% 100 pM) Striga gesnerioides (40% 0.1 nM; 69% 350 nM) Striga hermonthica (35% 10 µM) | [71] [42,74] [73] |
| 7-Oxoorobanchol | Orobanche minor (83% 10 nM) Phelipanche ramosa (85% 10 nM) | [71] [75] |
| 7-Oxoorobanchyl acetate | Orobanche cumana (94% 100 nM) Orobanche minor (95% 1 nM) | [68] [75] |
| 7 α-Hydroxyorobanchyl acetate | Orobanche minor (90% 1 nM) Phelipanche ramosa (85% 10 nM) | [43] |
| 7 β-Hydroxyorobanchyl acetate | Orobanche minor (90% 0.1 nM) Phelipanche ramosa (85% 10 nM) | [43] |
| Solanacol | Orobanche minor (97% 100 pM) Phelipanche ramosa (83% 0.1 nM) | [71] [25] |
| Fabacyl acetate | Orobanche minor (88% 1 nM) | [71] |
| Equimolar mixture of orobanchol and strigol | Orobanche cumana (24% 100 nM) Orobanche minor (91% 100 nM) | [68] |
| Equimolar mixture of orobanchol and 7-oxoorobanchyl acetate | Orobanche cumana (0% 100 nM) Orobanche minor (80% 100 nM) | [68] |
Peagol * 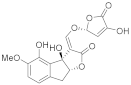 | Orobanche crenata (7% 10 µM) Orobanche foetida (27% 10 µM) Orobanche minor (3% 10 µM) Phelipanche aegyptiaca (42% 10 µM) | [67] |
| Non-canonical strigolactones | ||
| Carlactone | Striga hermonthica (62% 330 µM) | [29] |
| Methyl carlactanoate | Orobanche cumana (80% 30 nM of racemate) | [76] |
| Heliolactone | Orobanche crenata (80% 1000 nM) Orobanche cumana (88% 30 nM; 91% for racemate) Orobanche minor (80% 1 nM) Phelipanche aegyptiaca (30% 1000 nM) Striga hermonthica (80% 1000 nM) | [35] [76] [35] [35] [35] |
| Methyl zealactonoate | Orobanche minor (80% 100 nM) Phelipanche ramosa (85% 10 nM) Striga hermonthica (85% 100 nM) | [32] |
| Methyl zealactonoate 1aMethyl zealactonoate 1b | Striga hermonthica (60% 30 µM) Striga hermonthica (50% 3 µM) | [31] |
| Avenaol | Orobanche minor (55% 1000 nM) Phelipanche ramosa (90 % 100 nM) Striga hermonthica (50 % 1000 nM) | [34] |
| Lotuslactone | Orobanche minor (90% 1 nM) Phelipanche ramosa (55% 0.1 nM) Striga hermonthica (80% 10 nM) | [39] |
| Strigolactone | AMF Species and MEC Values | References |
|---|---|---|
| Medicaol | Gigaspora margarita (10 pg/disc) | [28] |
| 4-Deoxyorobanchol | Gigaspora margarita (3 pg/disc) | [28] |
| (±) Strigone | Gigaspora margarita (10 pg/disc) | [90] |
| Carlactone (racemate) | Gigaspora margarita (100,000 pg/disc) | [91] |
| Methyl carlactonoate (racemate) | Gigaspora margarita (1000 pg/disc) | [91] |
| Carlactonoic acid (racemate) | Gigaspora margarita (100 pg/disc) | [91] |
| Methyl zealactonoate | Gigaspora margarita (10,000 pg/disc) | [39] |
| Avenaol | Gigaspora margarita (1,000,000 pg/disc) | [39] |
| Heliolactone | Gigaspora margarita (10,000 pg/disc) | [39] |
| Lotuslactone | Gigaspora margarita (1000 pg/disc) | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Cruz, F.J.; Zorrilla, J.G.; Rial, C.; Varela, R.M.; Molinillo, J.M.G.; Igartuburu, J.M.; Macías, F.A. Allelopathic Activity of Strigolactones on the Germination of Parasitic Plants and Arbuscular Mycorrhizal Fungi Growth. Agronomy 2021, 11, 2174. https://doi.org/10.3390/agronomy11112174
Soto-Cruz FJ, Zorrilla JG, Rial C, Varela RM, Molinillo JMG, Igartuburu JM, Macías FA. Allelopathic Activity of Strigolactones on the Germination of Parasitic Plants and Arbuscular Mycorrhizal Fungi Growth. Agronomy. 2021; 11(11):2174. https://doi.org/10.3390/agronomy11112174
Chicago/Turabian StyleSoto-Cruz, Francisco J., Jesús G. Zorrilla, Carlos Rial, Rosa M. Varela, José M. G. Molinillo, José M. Igartuburu, and Francisco A. Macías. 2021. "Allelopathic Activity of Strigolactones on the Germination of Parasitic Plants and Arbuscular Mycorrhizal Fungi Growth" Agronomy 11, no. 11: 2174. https://doi.org/10.3390/agronomy11112174
APA StyleSoto-Cruz, F. J., Zorrilla, J. G., Rial, C., Varela, R. M., Molinillo, J. M. G., Igartuburu, J. M., & Macías, F. A. (2021). Allelopathic Activity of Strigolactones on the Germination of Parasitic Plants and Arbuscular Mycorrhizal Fungi Growth. Agronomy, 11(11), 2174. https://doi.org/10.3390/agronomy11112174








