Amending Potassic Fertilizer with Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Potassium Availability in a Tropical Acid Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Preparation
2.2. Initial Characterization of Soil, Charcoal, and Sago Bark Ash
2.3. Incubation Set-Up
2.4. Soil Chemical Analysis before and after Incubation
2.5. Statistical Analysis
3. Results and Discussion
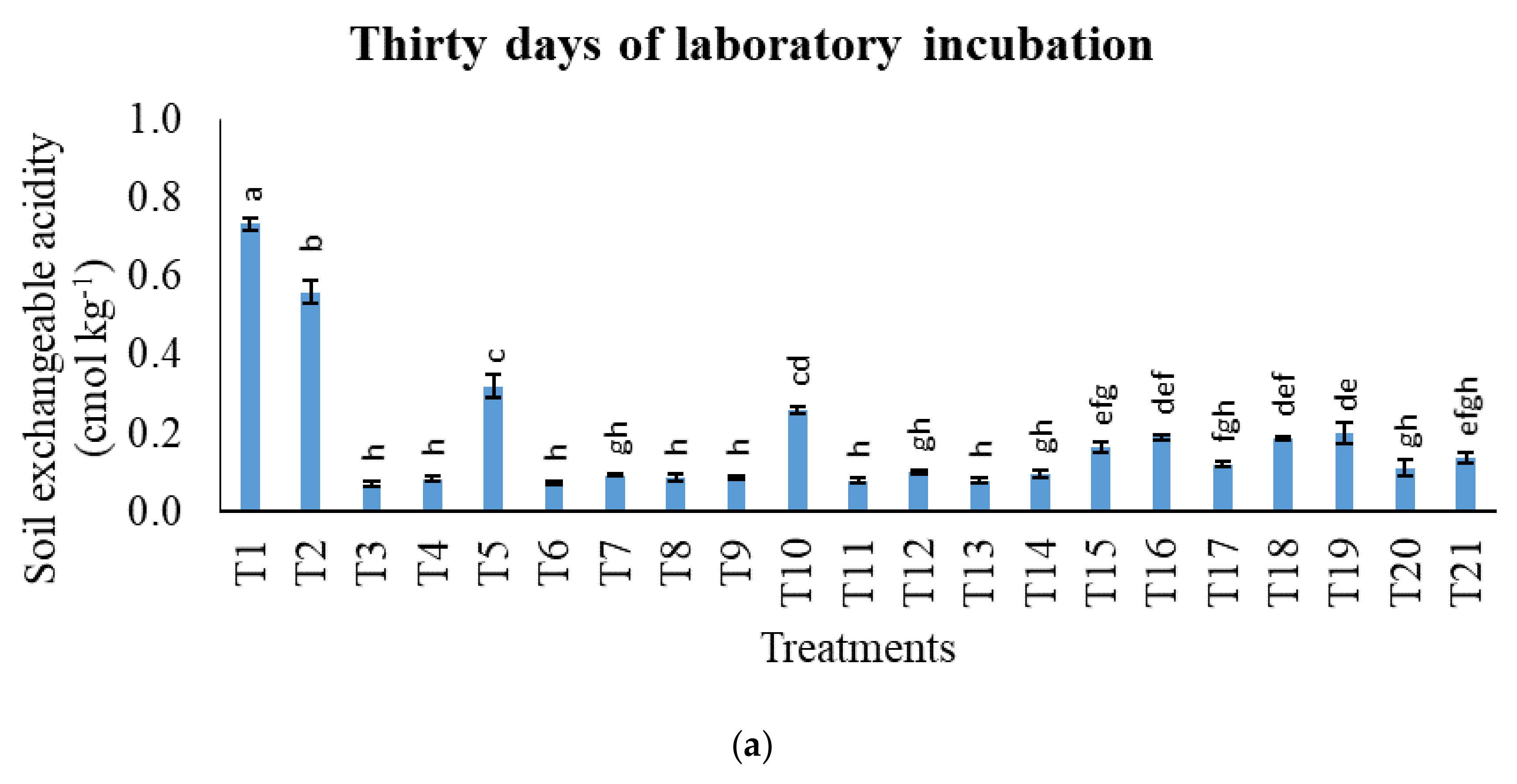
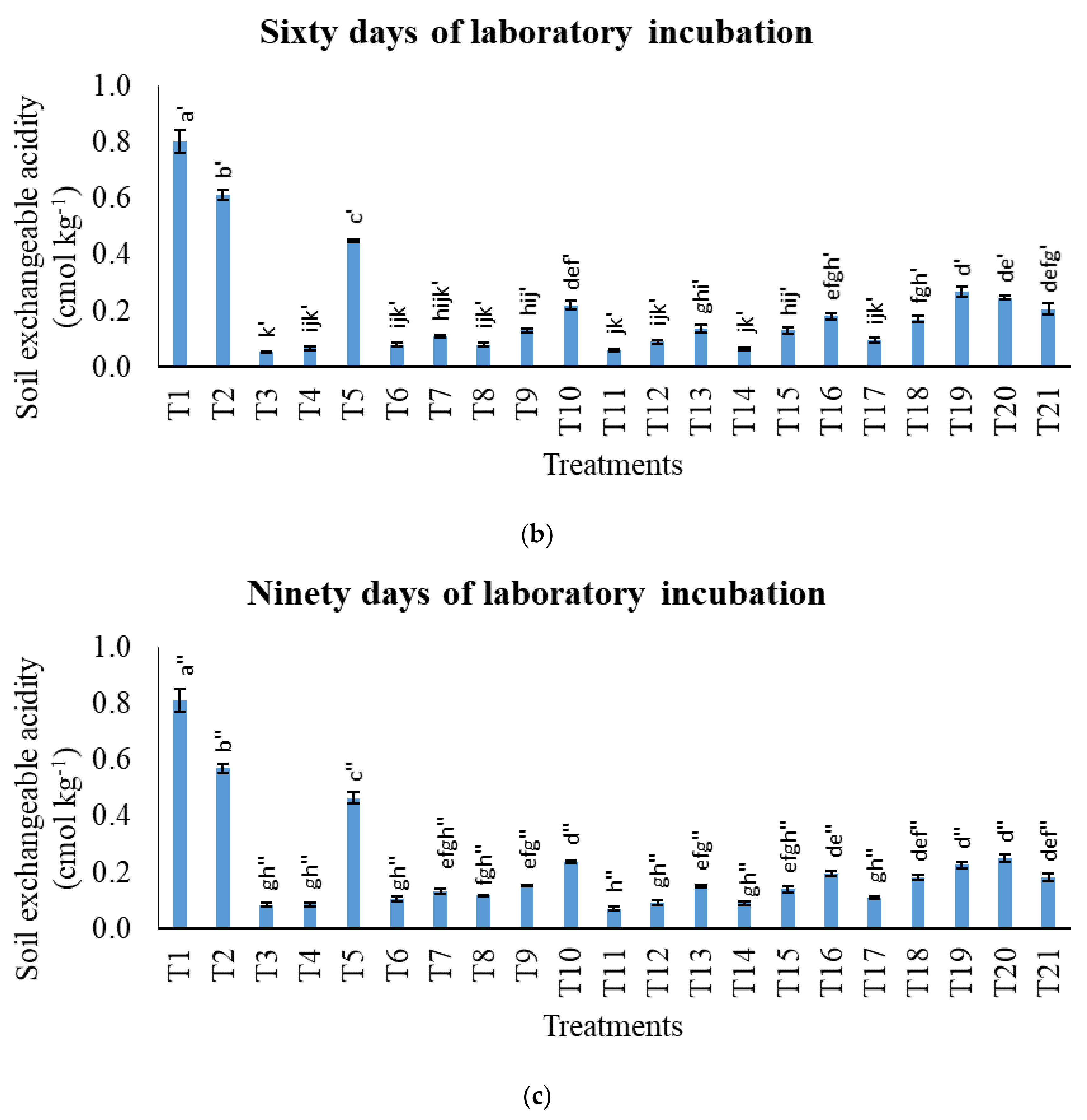
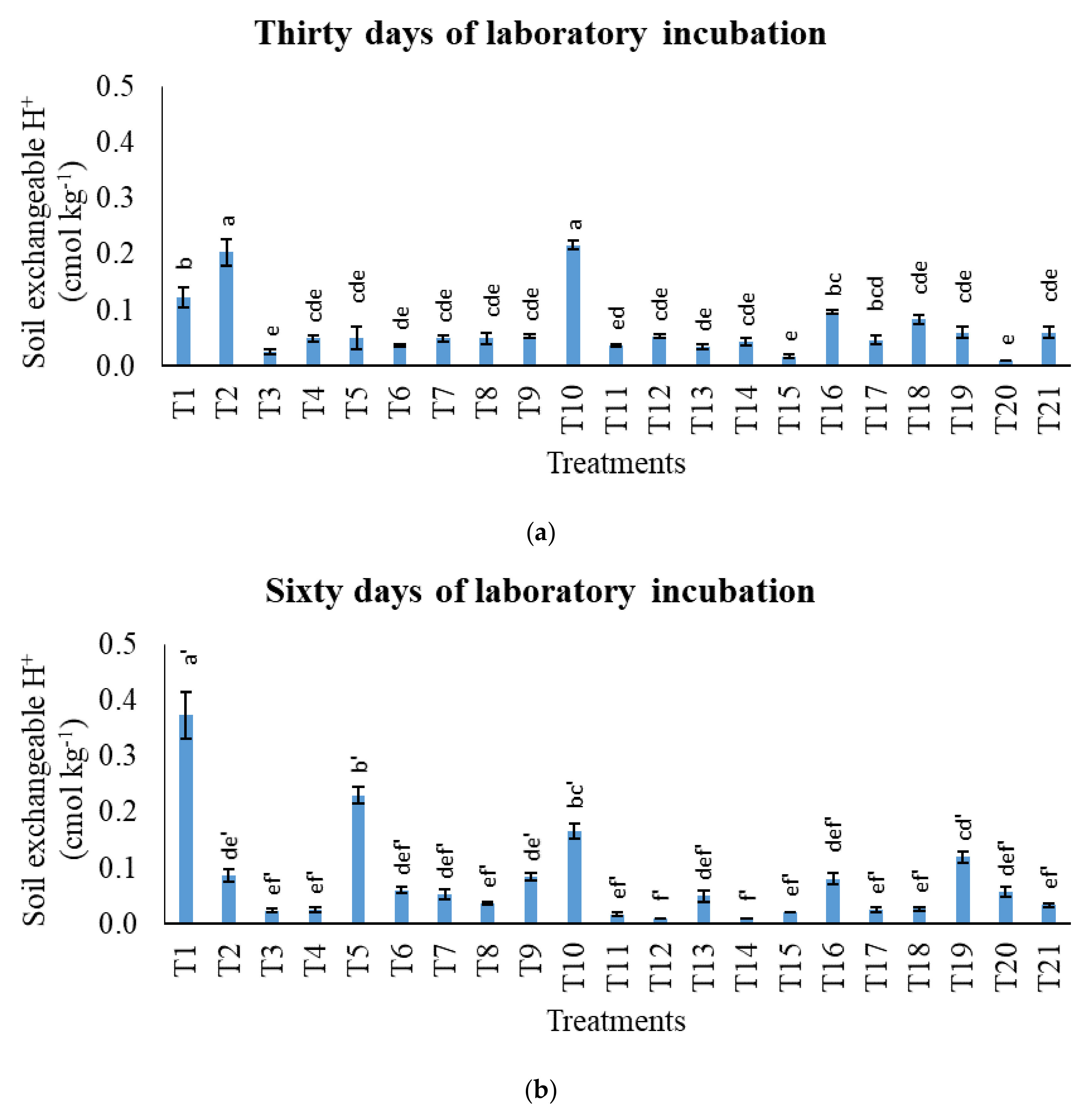
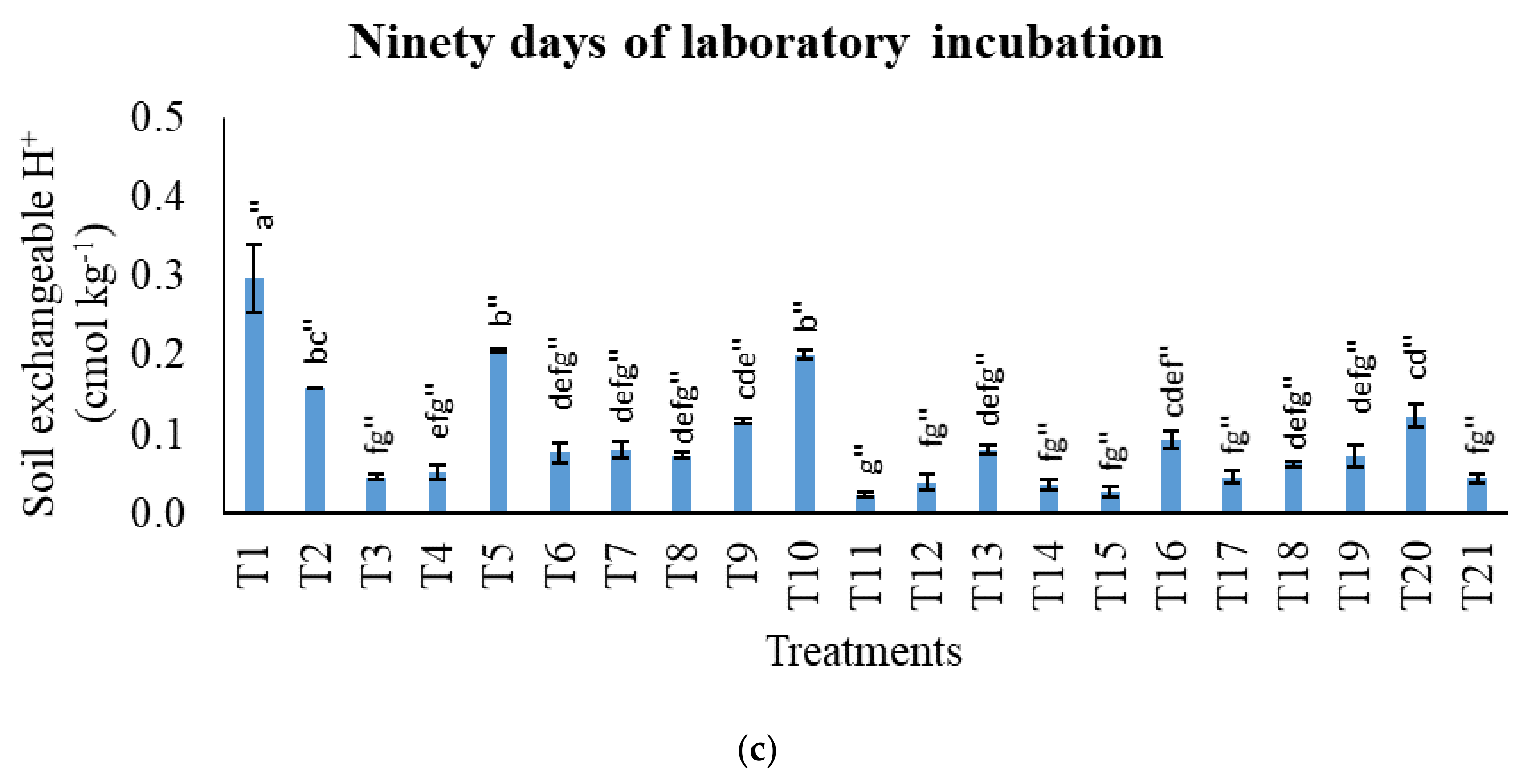
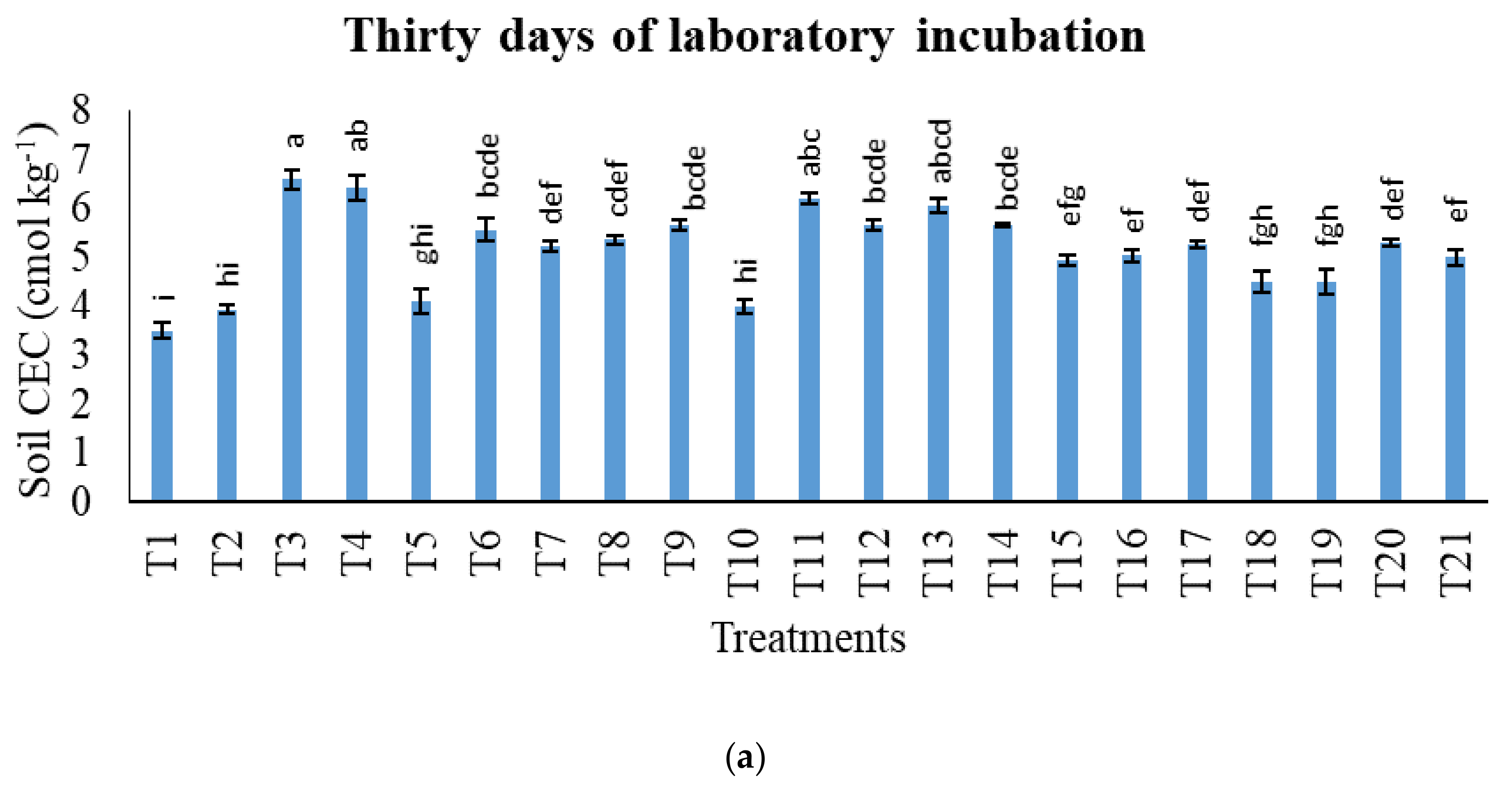
3.1. Treatments on pH in Potassium Chloride, Total Carbon, Cation Exchange Capacity, Exchangeable Acidity, Exchangeable Aluminium, and Exchangeable Hydrogen
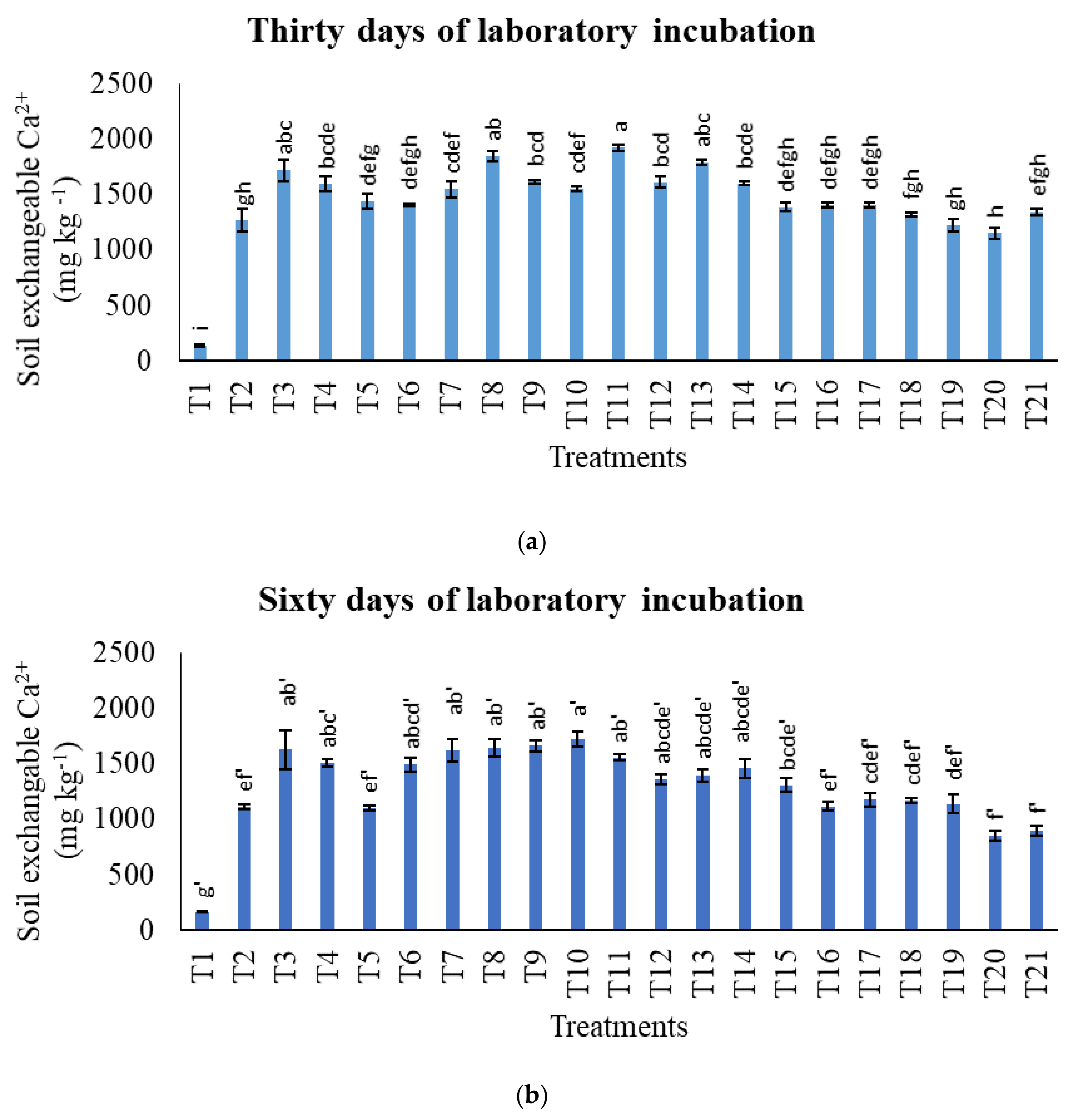
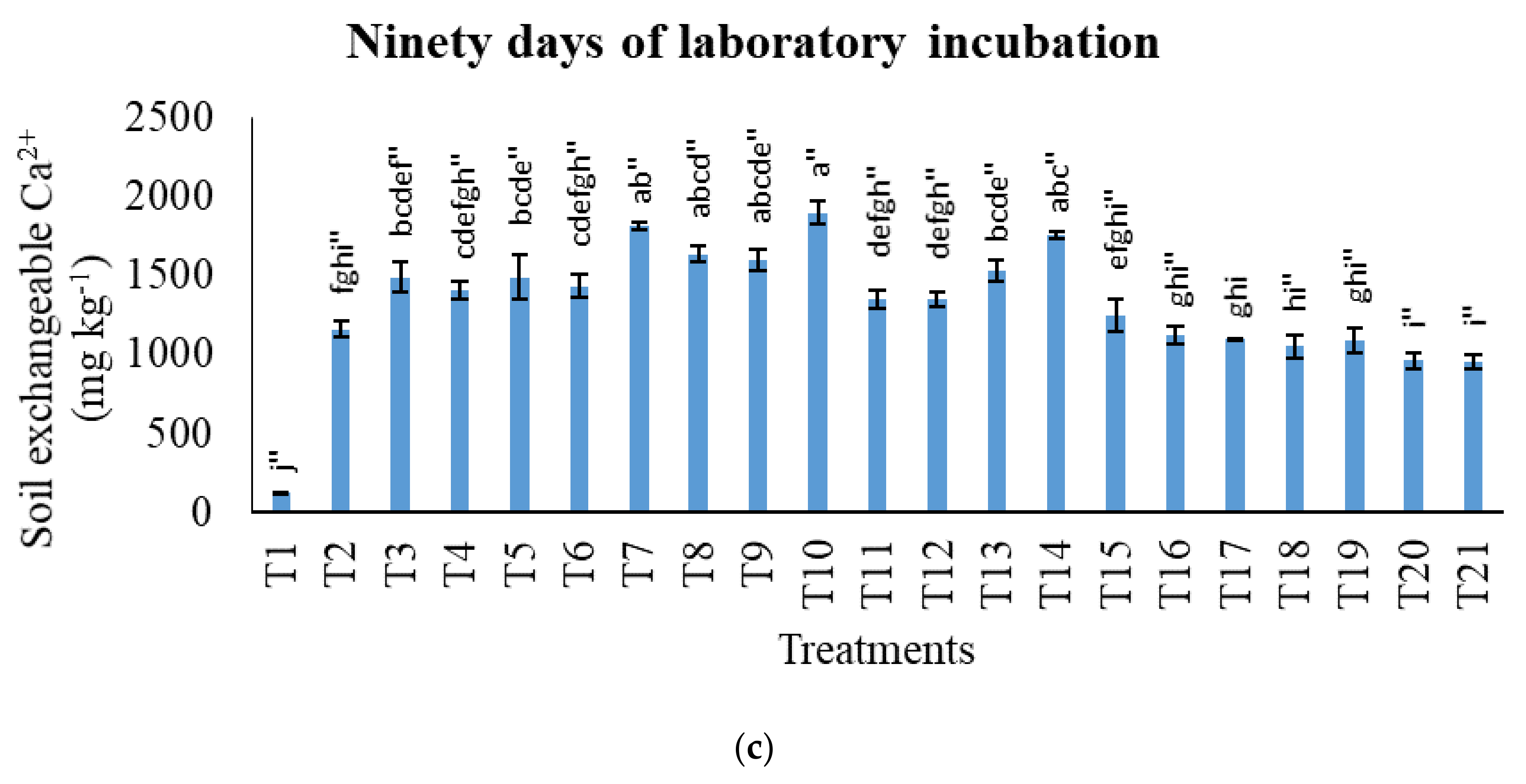
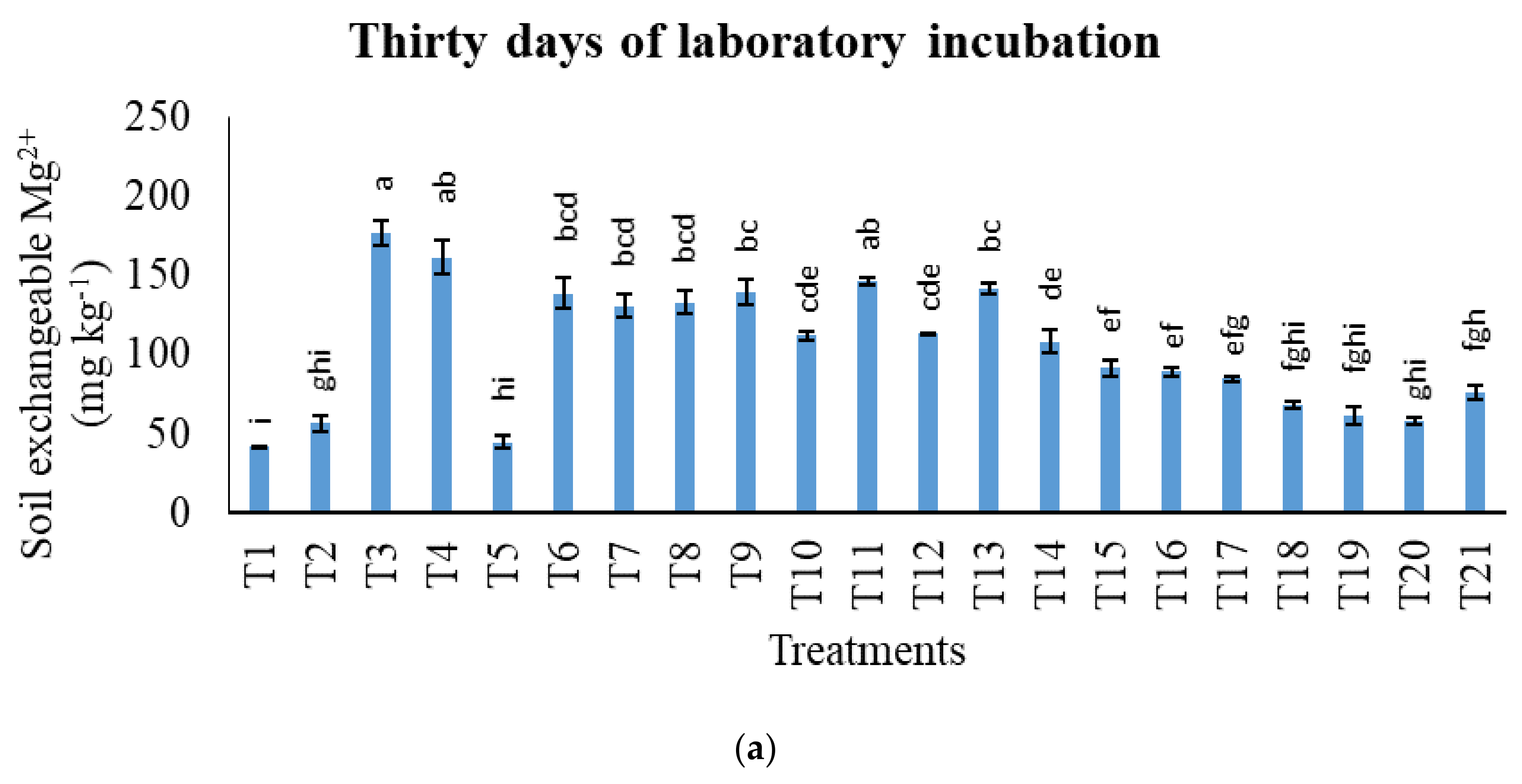
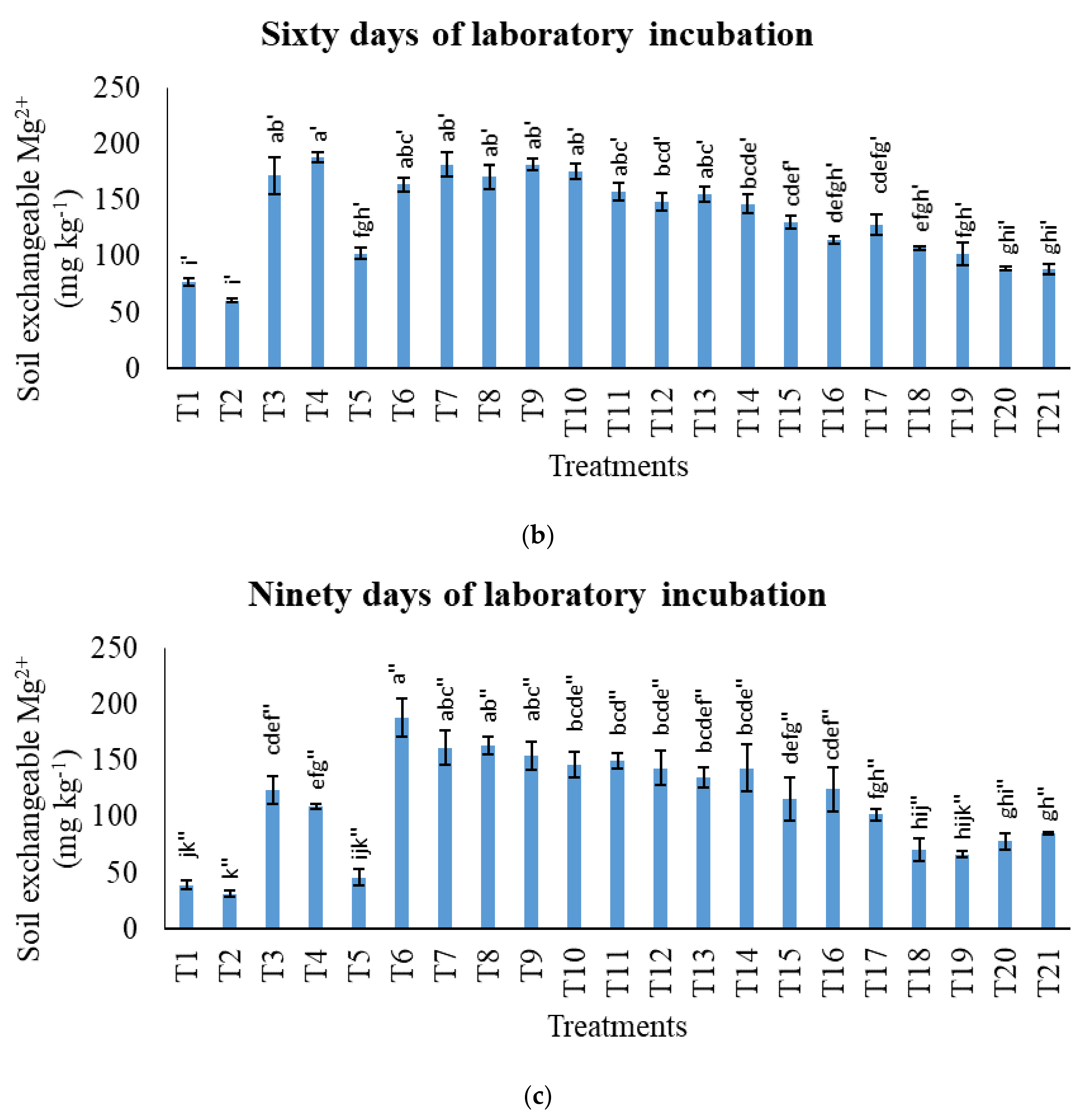
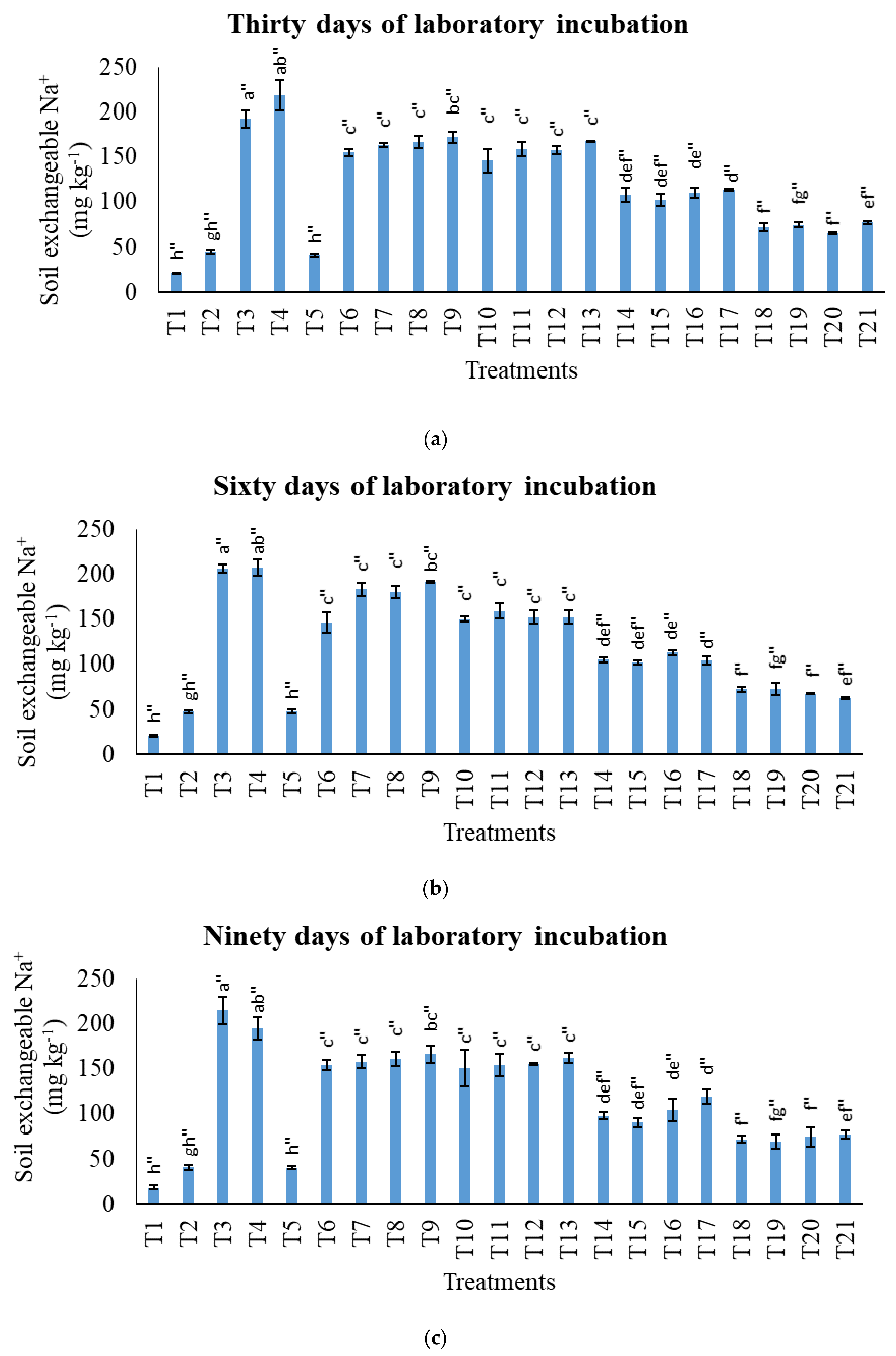
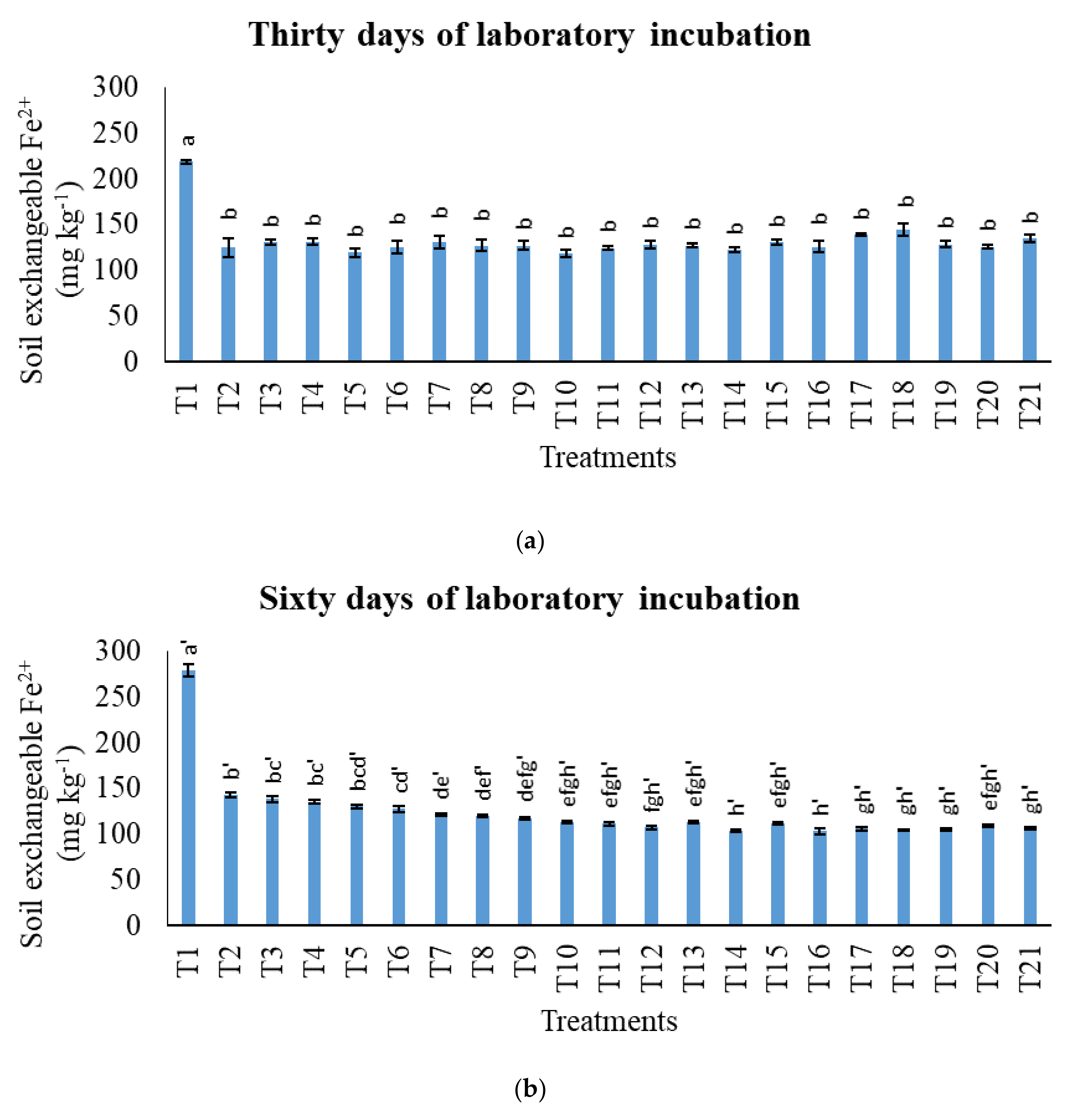
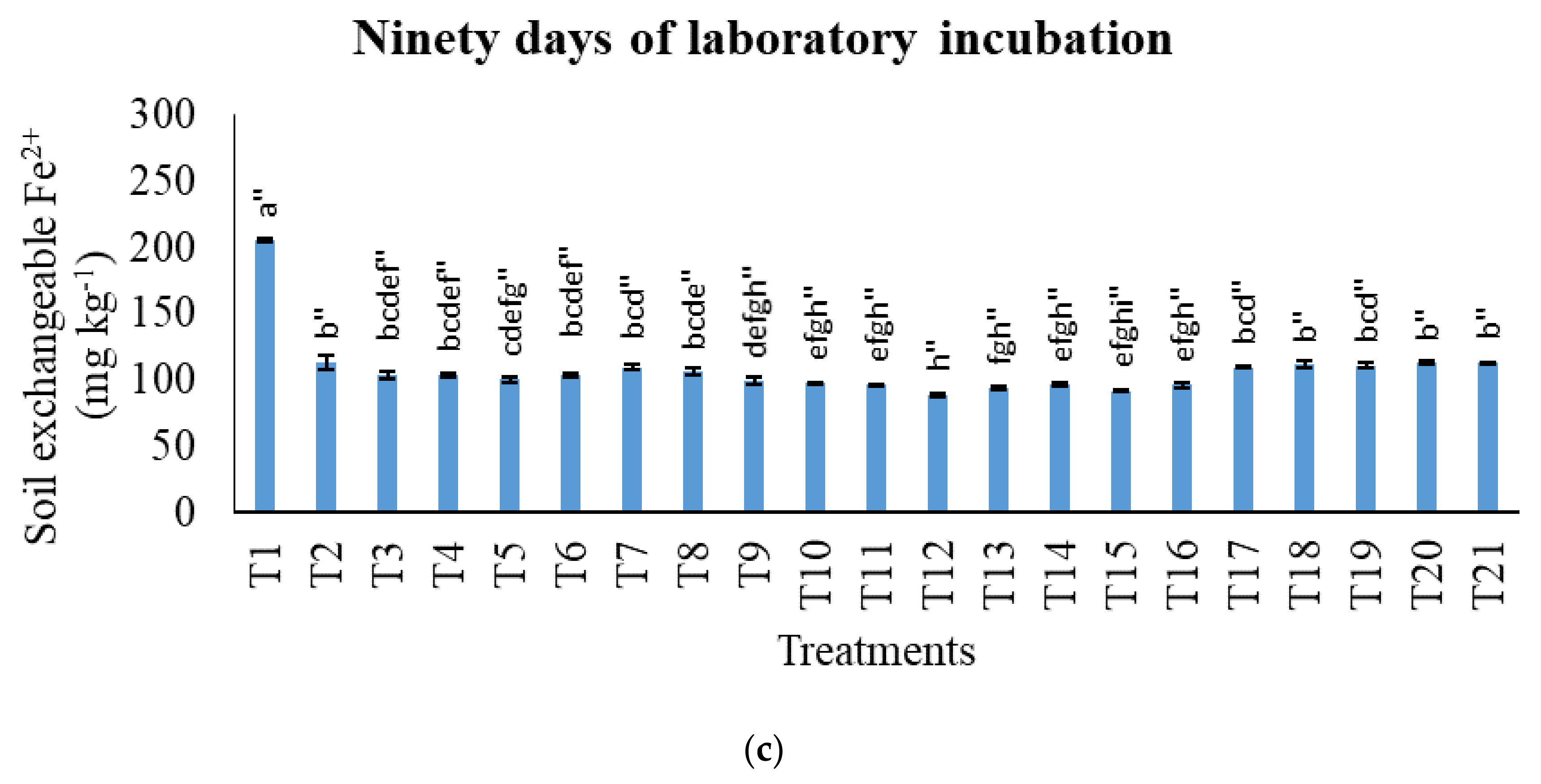
3.2. Treatments on Soil-Exchangeable Calcium, Exchangeable Magnesium, Exchangeable Sodium, and Exchangeable Iron
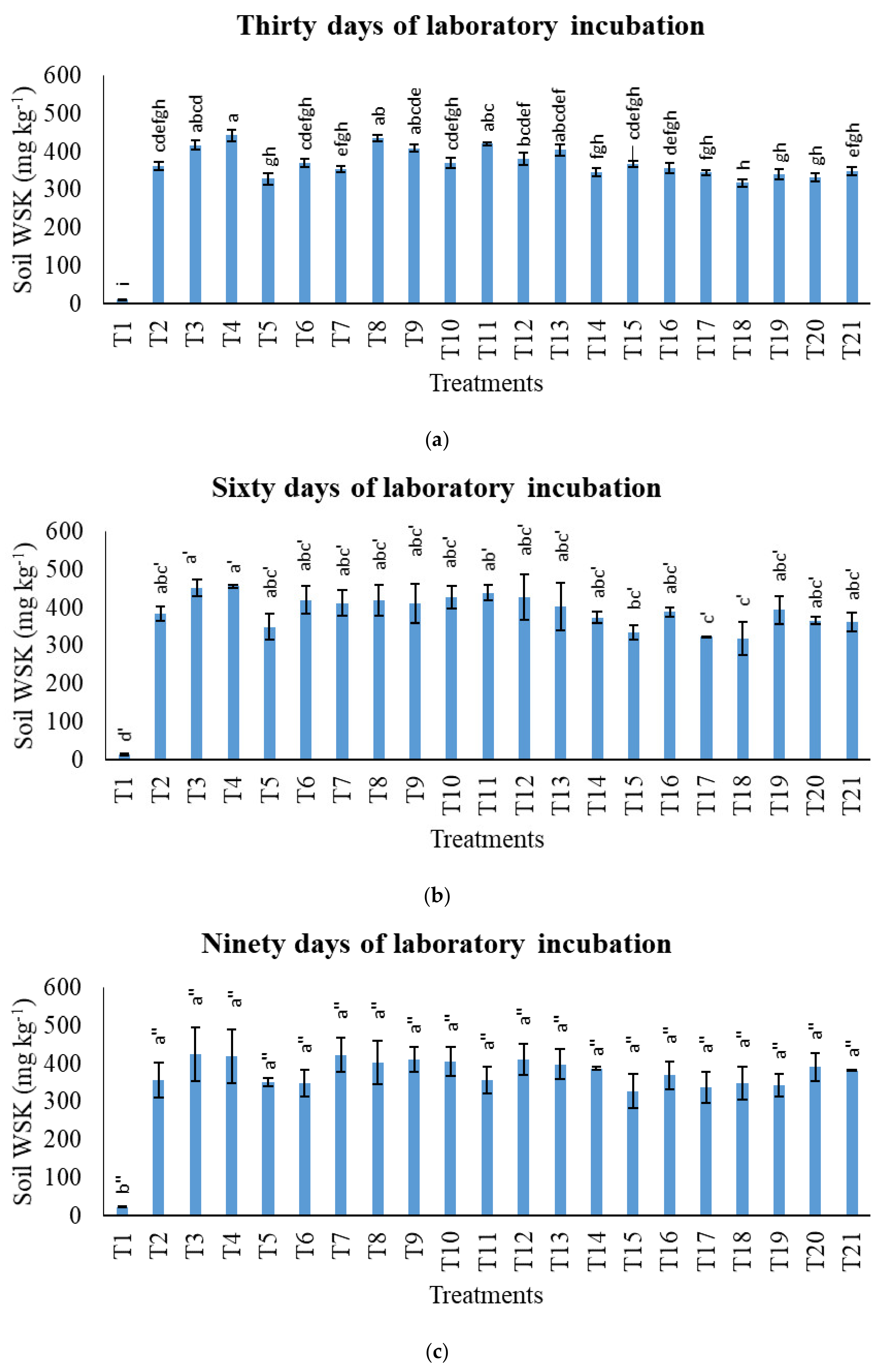
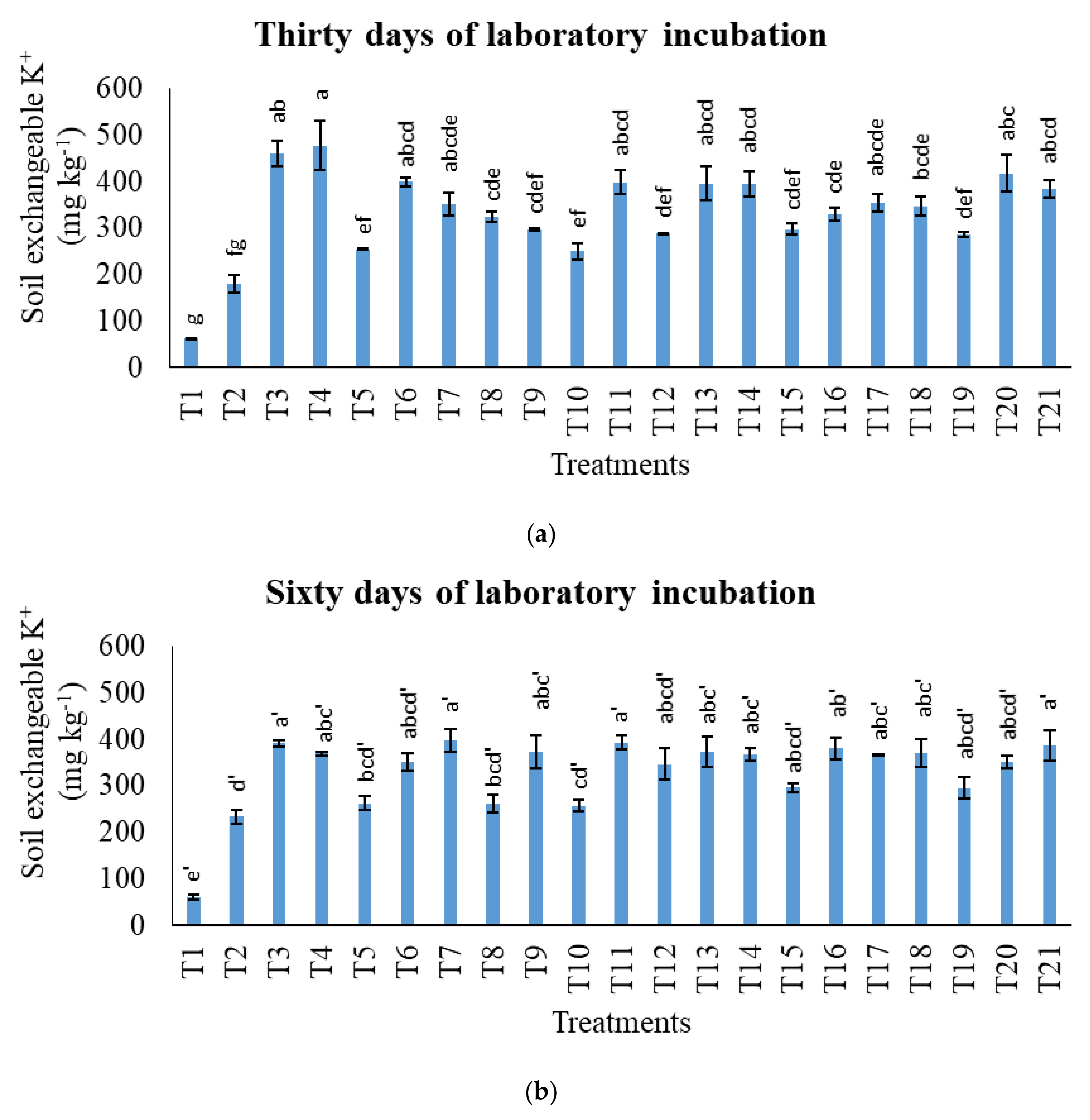
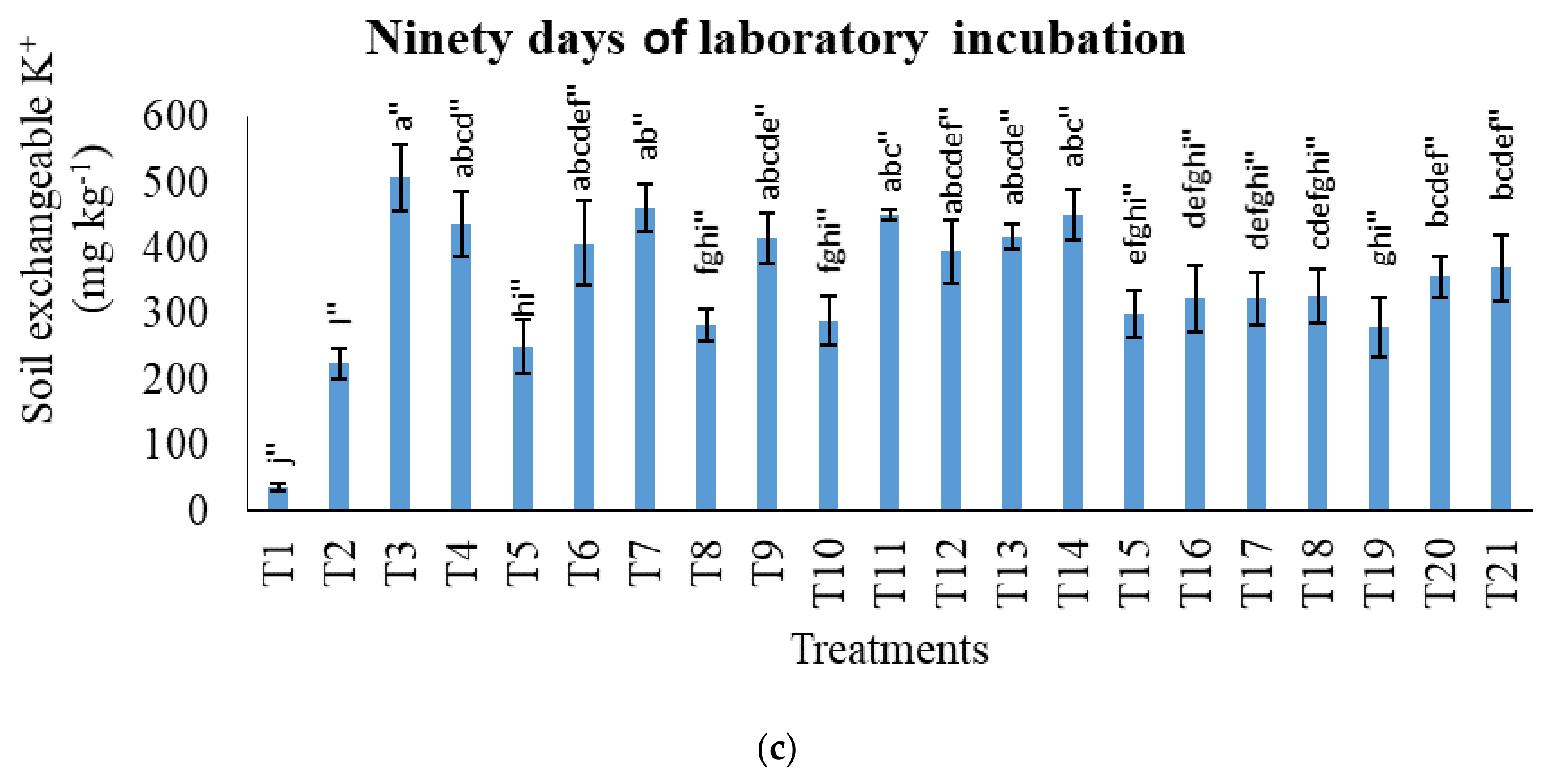
3.3. Treatments on Soil Total Potassium, Exchangeable Potassium, and Water-Soluble Potassium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Commoidty Trade Statistics Database. UN Comtrade. 2019. Commodity Trade Statistics Database. 2019. Available online: http://data.un.org/Data.aspx?d=ComTrade&f=_l1Code%3A11 (accessed on 13 April 2021).
- United States Department of Agriculture 2018. Grain Feed Annual. Available online: https://gain.fas.usda.gov/Recent%20GAIN%20Publications/Grain%20and%20Feed%20Annual_Kuala%20Lumpur_Malaysia_4-12-2018.pdf (accessed on 20 April 2021).
- Wang, H.Y.; Zhou, J.M.; Du, C.W.; Chen, X.Q. Potassium fractions in soils as affected by monocalcium phosphate, ammonium sulfate, and potassium chloride application. Pedosphere 2010, 20, 368–377. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparks, D.L. Bioavailability of soil potassium. In Handbook of Soil Science; Sumner, M.E., Ed.; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Jaiswal, D.K.; Verma, J.P.; Prakash, S.; Meena, V.S.; Meena, R.S. Potassium as an important plant nutrient in sustainable agriculture: A state of the art. Potassium Solubilizing Microorg. Sustain. Agric. 2016, 21–29. [Google Scholar] [CrossRef]
- Sumner, M.E. Handbook of Soil Science; CRC Press LLC: Boca Raton, FL, USA, 2000. [Google Scholar]
- Darunsontaya, T.; Suddhiprakarn, A.; Kheoruenromne, I.; Prakongkep, N.; Gilkes, R.J. The forms and availability to plants of soil potassium as related to mineralogy for upland Oxisols and Ultisols from Thailand. Geoderma 2012, 170, 11–24. [Google Scholar] [CrossRef]
- Goh, K.J.; Chew, P.S. Workshop on direct application of phosphate rocks and appropriate technology fertilisers in Asia: What hinder acceptance and growth. In Direct Application of Phosphates to Plantation Tree Crops in Malaysia; IFDC: Kandy, Sri Lanka, 1995; pp. 59–76. [Google Scholar]
- Sallade, Y.E.; Sims, J.T. Phosphorus transformations in the sediments of Delaware’s agricultural dranageways: II. Effect of reducing conditions on phosphorus release. J. Environ. Qual. 1997, 26, 1579–1588. [Google Scholar] [CrossRef]
- Khatri, N.; Tyagi, S. Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front. Life Sci. 2015, 8, 23–39. [Google Scholar] [CrossRef]
- Motesharezadeh, B.; Valizadeh-Rad, K.; Dadrasnia, A.; Amir-Mokri, H. Trend of fertilizer application during the last three decades (Case study: America, Australia, Iran and Malaysia). J. Plant. Nutr. 2017, 40, 532–542. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils; Prentice Hall: Upper Saddle River, NJ, USA, 2008; pp. 662–710. [Google Scholar]
- Jusop, S.; Ishak, C.F. Weathered Tropical Soils the Ultisols and Oxisols; Universiti Putra Malaysia Press: Serdang, Malaysia, 2010. [Google Scholar]
- Troeh, F.R.; Thompson, L.M. Soils and Soil Fertility; Blackwell: New York, NY, USA, 2005. [Google Scholar]
- Girijaveni, V.; Reddy, K.S.; Sharma, K.L.; Moulika, G. Zeolites are emerging soil amendments for improving soil physical and chemical properties in agriculture: A Review. Int. J. Agric. Environ. Biotechnol. 2018, 11, 841–849. [Google Scholar] [CrossRef]
- Galinato, S.P.; Yoder, J.K.; Granatstein, D. The economic value of biochar in crop production and carbon sequestration. Energy Policy 2011, 39, 6344–6350. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Enhancing nitrogen availability from urea using clinoptilolite zeolite. Geoderma 2017, 306, 152–159. [Google Scholar] [CrossRef]
- Hoi, W.K. Wood waste utilisation in Malaysia. In Proceedings of the Fourth Conference on Forestry and Forest Products Research, Forest Research Institute Malaysia, Kepong, Selangor, Malaysia, 2–4 October 1997. [Google Scholar]
- Forest Department Sarawak, Annual Report. 2011. Available online: https://forestry.sarawak.gov.my/modules/web/pages.php?mod=download&sub=download_show&id=73 (accessed on 23 April 2021).
- Shafie, S.M.; Othman, Z.; Hami, N. Potential utilisation of wood residue in Kedah: A preliminary study. J. Technol. Oper. Manag. 2017, 60–69. [Google Scholar] [CrossRef]
- Bujang, K. Potentials of bioenergy from the sago industries in Malaysia. In EOLSS-Encyclopedia of Life Support Systems; Doelle, H.W., Rokem, S., Berovic, M., Eds.; EOLSS Publisher: Oxford, UK, 2006; pp. 124–131. [Google Scholar]
- Singhal, R.S.; Kennedy, J.F.; Gopalakrishnan, S.M.; Kaczmarek, A.; Knill, C.J.; Akmar, P.F. Industrial production, processing, and utilization of sago palm-derived products. Carbohydr. Polym. 2008, 72, 1–20. [Google Scholar] [CrossRef]
- Awg-Adeni, D.S.; Abd-Aziz, S.; Bujang, K.; Hassan, M.A. Bioconversion of sago residue into value added products. Afr. J. Biotechnol. 2010, 9, 2016–2021. [Google Scholar]
- Vikineswary, S.; Shim, Y.L.; Thambirajah, J.J.; Blakebrough, N. Possible microbial utilization of sago processing wastes. Resour. Conserv. Recycl. 1994, 11, 289–296. [Google Scholar] [CrossRef]
- Ngaini, Z.; Noh, F.; Wahi, R. Esterified sago waste for engine oil removal in aqueous environment. Environ. Technol. 2014, 35, 2761–2766. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Sabli, N.; Izhar, S.; Yoshida, H. Sago Wastes and Its Applications. Pertanika J. Sci. Technol 2019, 27, 1841–1862. [Google Scholar]
- Van Laer, T.; De Smedt, P.; Ronsse, F.; Ruysschaert, G.; Boeckx, P.; Verstraete, W.; Lavrysen, L.J. Legal constraints and opportunities for biochar: A case analysis of EU law. GCB Bioenergy 2015, 7, 14–24. [Google Scholar] [CrossRef]
- Nweke, I.A.; Mbah, C.N.; Oweremadu, E.O.; Dambaba, N.; Orji, E.C.; Ekesiobi, A.I.; Nnabuife, E.L.C. Soil pH, available P of an ultisol and castor performance as influenced by contrasting tillage methods and wood ash. Afr. J. Agric. Res. 2017, 12, 606–616. [Google Scholar] [CrossRef] [Green Version]
- Nabeela, F.; Murad, W.; Khan, I.; Mian, I.A.; Rehman, H.; Adnan, M.; Azizullah, A. Effect of wood ash application on the morphological, physiological and biochemical parameters of Brassica napus L. Plant. Physiol. Biochem. 2015, 95, 15–25. [Google Scholar] [CrossRef]
- Kopecky, M.J.; Meyers, N.L.; Wasko, W. Using industrial wood ash as a soil amendment. Magnes. Res. 1995, 1, 240–440. [Google Scholar]
- Jacobson, S.; Gustafsson, L. Effects on ground vegetation of the application of wood ash to a Swedish Scots pine stand. Basic Appl. Ecol. 2001, 2, 233–241. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, N.V.; Halim, M.A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef] [Green Version]
- Boateng, A.A. Characterization and thermal conversion of charcoal derived from fluidized-bed fast pyrolysis oil production of switchgrass. Ind. Eng. Chem. Res. 2007, 46, 8857–8862. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Qiu, Y.; Cheng, H.; Xu, C.; Sheng, G.D. Surface characteristics of crop-residue-derived black carbon and lead (II) adsorption. Water Res. 2008, 42, 567–574. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Paramananthan, S. Soil of Malaysia: Their Characteristics and Identification; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2000; Volume 1, pp. 11–125. [Google Scholar]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Ndor, E.; Dauda, S.N.; Azagaku, E.D. Response of maize varieties (Zea mays) to biochar amended soil in Lafia, Nigeria. J. Exp. Agric. Int. 2015, 5, 525–531. [Google Scholar] [CrossRef]
- Mandre, M.; Pärn, H.; Ots, K. Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For. Ecol. Manag. 2006, 223, 349–357. [Google Scholar] [CrossRef]
- Ozolincius, R.; Buozyte, R.; Varnagiryte, K.I. Wood ash and nitrogen influence on ground vegetation cover and chemical composition. Biomass Bioenergy 2007, 31, 710–716. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Malaysian Agricultural Research and Development Institute (MARDI). Jagung Manis Baru, Masmadu [New Sweet Corn, Masmadu]; Malaysian Agricultural Research and Development: Serdang, Malaysia, 1993; pp. 3–5. [Google Scholar]
- Peech, H.M. Hydrogen-ion Activity. In Methods of Soil Analysis Chemical and Microbiological Properties; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 914–926. [Google Scholar]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analyses of soils 1. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Chefetz, B.; Hatcher, P.H.; Hadar, Y.; Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Dixon, R.K.; Wisniewski, J. Global Forest systems: An uncertain response to atmospheric pollutants and global climate change? Water Air Soil Pollut. 1995, 85, 101–110. [Google Scholar] [CrossRef]
- Cottenie, A. Soil testing and plant testing as a basis of fertilizer recommendation. FAO Soils Bull. 1980, 38, 70–73. [Google Scholar]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis: Part 2; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.F., Dinauer, R.C., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Bernas, B. New method for decomposition and comprehensive analysis of silicates by atomic absorption spectrometry. Anal. Chem. 1968, 40, 1682–1686. [Google Scholar] [CrossRef]
- Rowell, D.L. Soil Science: Methods and Application; Longman Publishers Ltd.: London, UK, 1994; p. 169. [Google Scholar]
- Saarsalmi, A.; Mälkönen, E.; Kukkola, M. Effect of wood ash fertilization on soil chemical properties and stand nutrient status and growth of some coniferous stands in Finland. Scand. J. For. Res. 2004, 19, 217–233. [Google Scholar] [CrossRef]
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, A.R. Abiotic and microbial oxidation of laboratory-produced black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1295–1301. [Google Scholar] [CrossRef]
- Naramabuye, F.X.; Haynes, R.J. Short-term effects of three animal manures on soil pH and Al solubility. Soil Res. 2006, 44, 515–521. [Google Scholar] [CrossRef]
- Haynes, R.J.; Mokolobate, M.S. Amelioration of Al toxicity and P deficiency in acid soils by additions of organic residues: A critical review of the phenomenon and the mechanisms involved. Nutr. Cycl. Agroecosystems 2001, 59, 47–63. [Google Scholar] [CrossRef]
- Belay, A.; Claassens, A.; Wehner, F.C. Effect of direct nitrogen and potassium and residual phosphorus fertilizers on soil chemical properties, microbial components and maize yield under long-term crop rotation. Biol. Fertil. Soils 2002, 35, 420–427. [Google Scholar]
- Motavalli, P.; Miles, R. Soil phosphorus fractions after 111 years of animal manure and fertilizer applications. Biol. Fertil. Soils 2002, 36, 35–42. [Google Scholar]
- Bonfim-Silva, E.M.; Nonato, J.J.; Simeon, B.G.; Alves, R.D.D.S.; da Silva, M.I.P.; Silva, T.J.A.D. Mung bean shoot and root growth under wood ash as a soil acidity neutralizer and fertilizer. Int. J. Veg. Sci. 2020, 27, 303–314. [Google Scholar] [CrossRef]
- Haraldsen, T.K.; Pedersen, P.A.; Gronlund, A. Mixtures of bottom wood ash and meat and bone meal as NPK fertilizer. In Recycling of Biomass Ashes; Insam, H., Knapp, B.A., Eds.; Springer: Berlin, Germany, 2011; pp. 33–44. [Google Scholar]
- Saarsalmi, A.; Smolander, A.; Kukkola, M.; Arola, M. Effect of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes, and stand growth in two coniferous stands in Finland. Plant. Soil 2010, 331, 329–340. [Google Scholar] [CrossRef]
- Kochian, L.V.; Pineros, M.A.; Hoekenga, O.A. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant. Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Qian, L.; Chen, B.; Hu, D. Effective alleviation of aluminum phytotoxicity by manure-derived biochar. Environ. Sci. Technol. 2013, 47, 2737–2745. [Google Scholar] [CrossRef]
- Bramyrd, T.; Fransman, B. Silvicultural use of wood ash. Effect of nutrients and heavy metal balance in a pine (Pinus sylvestris L.). For. Soil Air Soil Pollut. 1995, 85, 1039–1044. [Google Scholar] [CrossRef]
- Herrmann, S.; Oelmuller, R.; Buscot, F. Manipulation of the onset of ectomycorrhiza formation by indole-2-acetic acid, activated charcoal or relative humidity in the association between oak micocuttings and Piloderma croceum: Influence on plant development and photosynthesis. J. Plant. Physiol. 2004, 161, 509–517. [Google Scholar] [CrossRef] [Green Version]
- Topoliantz, S.; Ponge, J.F.; Ballof, S. Manioc peel and charcoal: A potential organic amendment for sustainable soil fertility in the tropics. Biol. Fertil. Soils 2005, 41, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Rutto, K.L.; Mizutani, F. Effect of mycorrhizal inoculation and activated charcoal on growth and nutrition in peach (Prunus persica Batsch) seedlings treated with peach root-bark extracts. J. Jpn. Soc. Hortic. Sci. 2006, 75, 463–468. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil: Concepts and mechanisms. Plant. Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Asap, A.; Haruna, A.O.; Ab Majid, N.M.; Ali, M. Amending triple superphosphate with chicken litter biochar improves phosphorus availability. Eurasian J. Soil Sci. 2018, 7, 121–132. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Chang. Biol. 2010, 16, 1366e1379. [Google Scholar] [CrossRef]
- Khanna, P.K.; Raison, R.; Falkiner, R. Chemical properties of ash derived from Eucalyptus litter and its effects on forest soils. For. Ecol. Manag. 1994, 66, 107–125. [Google Scholar] [CrossRef]
- Ludwig, B.; Rumpf, S.; Mindrup, M.; Meiwes, K.J.; Khanna, P.K. Effects of lime and wood ash on soil-solution chemistry, soil chemistry and nutritional status of a pine stand in northern Germany. Scand. J. For. Res. 2002, 17, 225–237. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Mälkönen, E.; Piirainen, S. Effects of wood ash fertilization on forest soil chemical properties. Silva. Fenn. 2001, 35, 355–368. [Google Scholar] [CrossRef] [Green Version]
- Mbah, C.N.; Njoku, C.; Oludare, C. Use of ash to improve the nutrient content of an ultisol and its effect on maize (Zea mays L.) Growth and dry matter yield. Int. J. For. Soil Eros. (IJFSE) 2013, 3, 118–121. [Google Scholar]
- Nguyen, B.T.; Lehmann, J.; Kinyangi, J.; Smernik, R.; Riha, S.J.; Engelhard, M.H. Long-term black carbon dynamics in cultivated soil. Biogeochemistry 2009, 92, 163–176. [Google Scholar] [CrossRef]
- DeLuca, T.H.; Aplet, G.H. Charcoal and carbon storage in forest soils of the Rocky Mountain West. Front. Ecol. Environ. 2008, 6, 18–24. [Google Scholar] [CrossRef]
- Unger, Y.L.; Fernandez, I.J. The short-term effects of wood-ash amendment on forest soils. Water Air Soil Pollut. 1990, 49, 299–314. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal–a review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Vu, D.T.; Tang, C.; Armstrong, R.D. Changes and availability of P fractions following 65 years of P application to a calcareous soil in a Mediterranean climate. Plant. Soil 2008, 304, 21–33. [Google Scholar] [CrossRef]
- Paterson, E.; Goodman, B.A.; Farmer, V.C. The chemistry of aluminium, iron and manganese oxides in acid soils. Soil Acidity 1991, 97–124. [Google Scholar] [CrossRef]
- Kundu, M.C.; Hazra, G.C.; Biswas, P.K.; Mondal, S.; Ghosh, G.K. Forms and distribution of potassium in some soils of Hooghly district of West Bengal. Crucible 2014, 2, 4. [Google Scholar]







| Property | Soil | Charcoal | Sago Bark Ash |
|---|---|---|---|
| pH (water) | 3.95 | 7.74 | 9.99 |
| pH (KCl) | 4.61 | 7.31 | 9.66 |
| EC (µS cm−1) | 35.10 | 269.33 | 5753.00 |
| Bulk density (g m−3) | 1.25 | nd | nd |
| (%) | |||
| Total carbon | 2.16 | nd | nd |
| Total N | 0.08 | nd | nd |
| (mg kg−1) | |||
| Total P | 22.25 | nd | nd |
| Total K | 101.27 | nd | nd |
| (cmol kg−1) | |||
| Cation exchange capacity | 4.67 | nd | 13.13 |
| Exchangeable acidity | 1.15 | 0.10 | nd |
| Exchangeable Al3+ | 0.13 | 0.047 | nd |
| Exchangeable H+ | 1.02 | 0.05 | nd |
| (mg kg−1) | |||
| Exchangeable K+ | 0.06 | 1435.20 | 9120.00 |
| Exchangeable Ca2+ | 0.02 | 2346.67 | 3361.20 |
| Exchangeable Mg2+ | 0.22 | 409.07 | 433.73 |
| Exchangeable Na+ | 0.03 | 99.38 | 348.00 |
| Exchangeable Fe2+ | 1.09 | 41.90 | 8.43 |
| Sand (%) | 71.90 | nd | nd |
| Silt (%) | 13.50 | nd | nd |
| Clay (%) | 14.60 | nd | nd |
| Texture (USDA) | Sandy loam | nd | nd |
| Treatment | Soil (kg) | MOP (g) | Charcoal (g) | Sago Bark Ash (g) |
|---|---|---|---|---|
| g Container−1 | ||||
| T1 | 1 | - | - | - |
| T2 | 1 | 2.41 | - | - |
| T3 | 1 | 2.41 | 51.4 | 25.7 |
| T4 | 1 | 2.41 | - | 25.7 |
| T5 | 1 | 2.41 | 51.4 | - |
| T6 | 1 | 2.41 | 41.1 | 20.6 |
| T7 | 1 | 2.41 | 30.8 | 20.6 |
| T8 | 1 | 2.41 | 20.6 | 20.6 |
| T9 | 1 | 2.41 | 10.3 | 20.6 |
| T10 | 1 | 2.41 | 41.1 | 15.4 |
| T11 | 1 | 2.41 | 30.8 | 15.4 |
| T12 | 1 | 2.41 | 20.6 | 15.4 |
| T13 | 1 | 2.41 | 10.3 | 15.4 |
| T14 | 1 | 2.41 | 41.1 | 10.3 |
| T15 | 1 | 2.41 | 30.8 | 10.3 |
| T16 | 1 | 2.41 | 20.6 | 10.3 |
| T17 | 1 | 2.41 | 10.3 | 10.3 |
| T18 | 1 | 2.41 | 41.1 | 5.1 |
| T19 | 1 | 2.41 | 30.8 | 5.1 |
| T20 | 1 | 2.41 | 20.6 | 5.1 |
| T21 | 1 | 2.41 | 10.3 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramisparam, P.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Maru, A.; Johan, P.D. Amending Potassic Fertilizer with Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Potassium Availability in a Tropical Acid Soil. Agronomy 2021, 11, 2222. https://doi.org/10.3390/agronomy11112222
Paramisparam P, Ahmed OH, Omar L, Ch’ng HY, Maru A, Johan PD. Amending Potassic Fertilizer with Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Potassium Availability in a Tropical Acid Soil. Agronomy. 2021; 11(11):2222. https://doi.org/10.3390/agronomy11112222
Chicago/Turabian StyleParamisparam, Puvan, Osumanu Haruna Ahmed, Latifah Omar, Huck Ywih Ch’ng, Ali Maru, and Prisca Divra Johan. 2021. "Amending Potassic Fertilizer with Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Potassium Availability in a Tropical Acid Soil" Agronomy 11, no. 11: 2222. https://doi.org/10.3390/agronomy11112222
APA StyleParamisparam, P., Ahmed, O. H., Omar, L., Ch’ng, H. Y., Maru, A., & Johan, P. D. (2021). Amending Potassic Fertilizer with Charcoal and Sago (Metroxylon sagu) Bark Ash to Improve Potassium Availability in a Tropical Acid Soil. Agronomy, 11(11), 2222. https://doi.org/10.3390/agronomy11112222







