Potassium and Water-Deficient Conditions Influence the Growth, Yield and Quality of Ratoon Sugarcane (Saccharum officinarum L.) in a Semi-Arid Agroecosystem
Abstract
1. Introduction
2. Material and Methods
2.1. Experiment Location and Inherent Soil Fertility
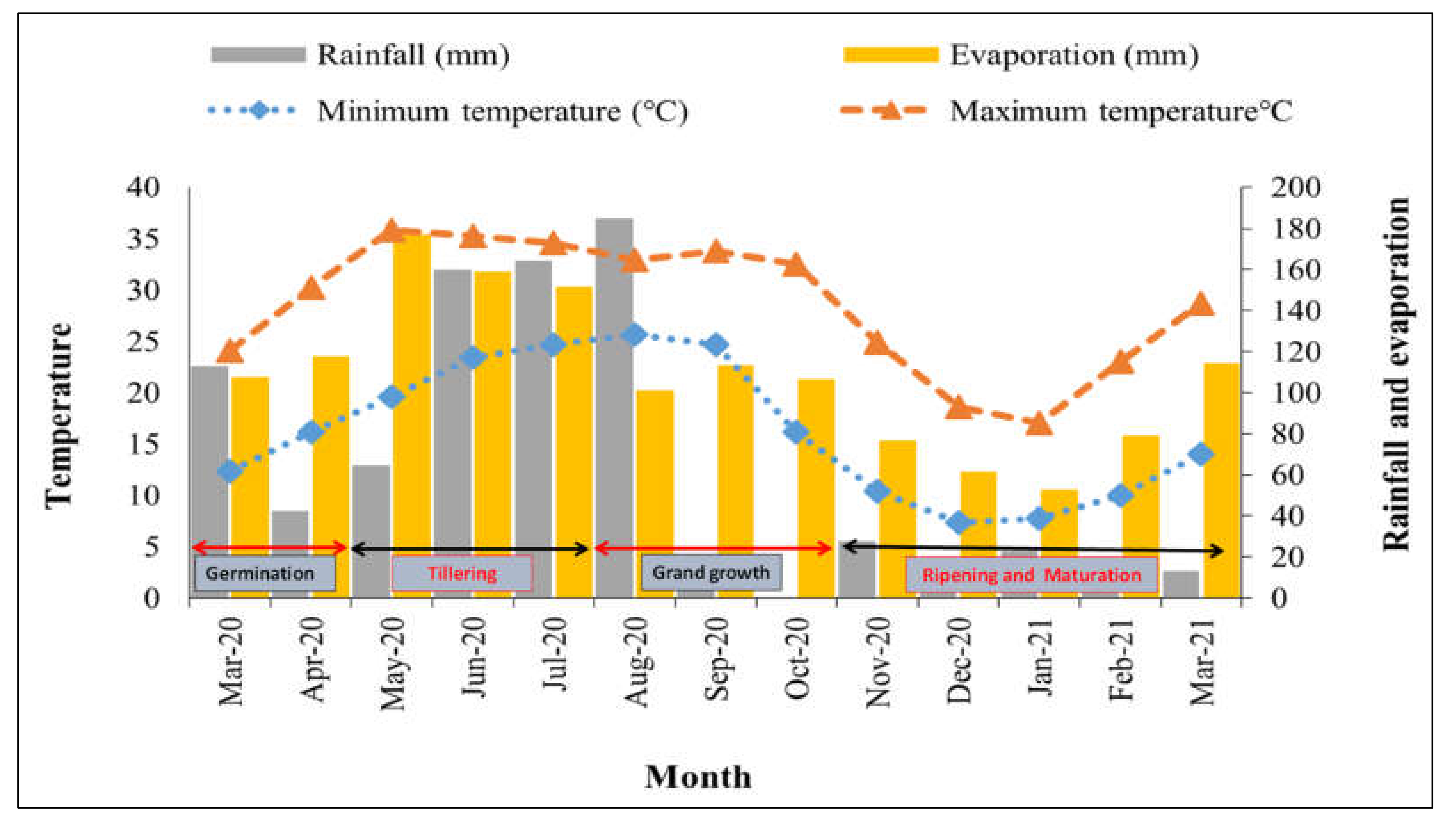
2.2. Weather during the Experiment
2.3. Experimental Treatments and Recorded Observations
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Ratoon Crop Productivity
3.2. Insect Pest Occurrence
3.3. Ratoon Crop Quality
3.4. Correlations between Quality Parameters
3.5. Economic Analysis
4. Discussion
4.1. Ratoon Sugarcane Performance under Irrigation
4.2. Ratoon Sugarcane Performance under Potassium Fertilizer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhatt, R.; Singh, P.; Hussain, A.; Tamsina, J. Rice-wheat system in the north-west Indo-Gangetic Plains of South Asia: Issues and technological interventions for increasing productivity and sustainability. Paddy Water Environ. 2021, 1–21, Epub ahead of print. [Google Scholar] [CrossRef]
- Bhatt, R.; Arora, S. Soil matric potential based irrigation using tensiometers for conserving irrigation water. Curr. Sci. 2021, 121, 197–200. [Google Scholar]
- Bhatt, R. Resources Management for Sustainable Sugarcane Production. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, S.R., Jhariya, K.M., Eds.; Springer: Singapore, 2020; pp. 650–685. [Google Scholar]
- Nishiyama, R.; Watanabe, Y.; Leyva-Gonzalez, M.A.; VanHa, C.; Fujita, Y.; Tanaka, M.; Sekid, M.; Shinozakie, K.Y.; Shinozakif, K.; Estrellab, K.H.; et al. Arabidopsis AHP2, AHP3, and AHP5histidinephos-photransfer proteins function as redundant negative regulators of drought stress response. Proc. Natl. Acad. Sci. USA 2013, 110, 4840–4845. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.V.; Leyva-Gonzalez, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanakae, M.; Sekie, M.; Yamaguchif, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 581–856. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, K.; Osakabe, Y. Plantlightstress. In Encyclopaedia of Life Sciences; Robinson, S.A., Ed.; Nature Publishing Group: London, UK, 2012. [Google Scholar]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, A.; Abo, M.; et al. Osmotic stress responses and plant growthc ontrolled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef]
- Osakabe, Y.; Shinozaki, Y.; Shinozaki, K.; PhanTran, L.S. Sensing the environment: Key roles of membrane-localized kinases in plant perception and response to abiotic stress. J. Exp. Bot. 2013, 64, 445–458. [Google Scholar] [CrossRef]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G. SLAC1 is required for plant guard cell S-typeanionchan- nel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef]
- Priya, S.R.K.; Bajpai, P.K.; Suresh, K.K. Stochastic models for sugarcane yield forecasting. Ind. J. Sugarcane Technol. 2015, 30, 1–5. [Google Scholar]
- Shukla, S.K.; Yadav, R.L.; Singh, P.N.; Singh, I. Potassium nutrition for improving stubble bud sprouting, dry matter partitioning, nutrient uptake, and winter initiated sugarcane (Saccharum spp. hybrid complex) ratoon yield. Eur. J. Agron. 2009, 30, 27–33. [Google Scholar] [CrossRef]
- Bhatt, R.; Oliveira, M.W.; da Silva, V.S.G. Sugarcane nutrition for food and environmental security. Braz. J. Dev. 2021, 6, 64431–64467. [Google Scholar] [CrossRef]
- PAU. Package of Practices for crops of Punjab-Kharif; Punjab Agricultural University: Ludhiana, India, 2021; pp. 55–66. [Google Scholar]
- Bhatt, R.; Singh, P. Sugarcane response to irrigation and potash levels in subtropics. Agric. Res. J. PAU 2021, in press. [Google Scholar]
- Bhatt, R.; Sharma, M. Potassium Scenario—A case study in the Kapurthala district of Punjab, India. Agric. Res. J. 2011, 48, 24–27. [Google Scholar]
- Cakmak, I. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J. Plant Nutr. Soil Sci. 2005, 168, 521–530. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Inter. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsagermoller, I.; White, P. Function of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 135–189. [Google Scholar]
- Mengel, K. Potassium. In Handbook of Plant Nutrition; Barker, A.V., Pilbeam, D.J., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 91–120. [Google Scholar]
- Haeder, H.E.; Mengel, K. Translocation and respiration of assimilates in tomato plants as influenced by K nutrition. Z. Pflanz. Bodenkd. 1972, 131, 139–148. [Google Scholar] [CrossRef]
- Mengel, K.; Viro, M. Effect of Potassium Supply on the Transport of Photosynthates to the Fruits of Tomatoes (Lycopersicon esculentum). Physiol. Planta 1974, 30, 295–300. [Google Scholar] [CrossRef]
- Hartt, C.E. Effect of potassium deficiency upon translocation of 14C in attached blades and entire plants of sugarcane. Plant Physiol. 1969, 44, 1461–1469. [Google Scholar] [CrossRef][Green Version]
- Hartt, C.E. Effect of potassium deficiency upon translocation of 14C in detached blades of sugarcane. Plant Physiol. 1970, 45, 183–187. [Google Scholar] [CrossRef]
- Brunt, V.; Sultenfuss, J.H. Better crops with plant food. Potassium Funct. Potassium 1998, 82, 4–5. [Google Scholar]
- Berg, W.K.; Cunningham, S.M.; Brouder, S.M.; Joern, B.C.; Johnson, K.D.; Volence, J.J. Influence of phosphorus and potassium on alfalfa yield, taproot C and N pools, and transcript levels of key genes after defoliation. Crop Sci. 2009, 49, 974–982. [Google Scholar] [CrossRef]
- Filho, J.O. Potassium nutrition of sugarcane. In Potassium in Agriculture; Munson, R.D., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1985; pp. 1045–1062. [Google Scholar]
- Kwong, K.F. The effects of potassium on growth, development, yield, and quality of sugarcane. In Potassium for Sustainable Crop Production and Food Security, Proccedings of the First National Potash Symposium, Dar es Salaam, Tanzania, 28–29 July 2015; International Potash Institute: Zug, Switzerland, 2002; pp. 430–444. [Google Scholar]
- Kumar, A.; Babar, L.; Mohan, N.; Bansal, S.K. Effect of Potassium Application on Yield, Nutrient Uptake and Quality of Sugarcane and Soil Health. Ind. J. Fert. 2019, 15, 782–786. [Google Scholar]
- Marshner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: Cambridge, MA, USA, 1995; p. 889. [Google Scholar]
- Sidhu, P.S. Mineralogy of potassium in soils of Punjab, Haryana, Himachal Pradesh, and Jammu and Kashmir. In Mineralogy of Soil Potassium; Potash Research Institute of India: Gurgaon, India, 1982; pp. 6–14. [Google Scholar]
- Grewal, J.S.; Kanwar, J.S. Potassium and Ammonium Fixation in Indian Soils (A Review); Indian Council of Agricultural Research: New Delhi, India, 1973; p. 75. [Google Scholar]
- Vasudeo, R.; Naidu, R.; Lakshmikantham, M. Ratooning sugarcane in Madras. Madras Agric. J. 1946, 4, 3–12. [Google Scholar]
- Verma, R.S. Sugarcane Ratoon Management; International Book Distributing Company: Lucknow, India, 2002; p. 102. [Google Scholar]
- Choudhary, H.R.; Singh, R.K. Effect of sequential application of herbicides on weeds and productivity of spring-planted sugarcane (Saccharum officinarum L.). Bioscan 2016, 11, 687–690. [Google Scholar]
- Sundara, B.; Tripathi, B.K. Available N changes and N balance under multi-ratooning of sugarcane varieties in a tropical vertisol. In Proceedings of the ISSCT XXIII (II), New Delhi, India, 22–26 February 1999; pp. 80–88. [Google Scholar]
- Dissanayake, N.; Hoy, J.W. Organic material soil amendment effects on root rot and sugarcane growth and characterization of the materials. Plant Dis. 1999, 83, 1039–1046. [Google Scholar] [CrossRef]
- Kanwar, R.; Kaur, S.H. Further studies on improving the productivity of stubble crops in low temperate area of north India. In Proceedings of the National Seminar on Ratoon Management, Lucknow, India, 14–15 March 1981; pp. 27–34. [Google Scholar]
- Verma, R.S.; Yadav, R.L. Intercropping in sugarcane for improving stubble sprouting under low temperature conditions in subtropical India. Bhartiya Sugar 1988, 13, 45–48. [Google Scholar]
- Jagtap, S.M.; Jadhav, M.B.; Kulkarm, R.V. Effect of levels of NPK on yield and quality of sugarcane (cv. Co. 7527). Ind. Sugar 2006, 56, 35–40. [Google Scholar]
- Bhatt, R.; Singh, P.; Kumar, R.; Kashyp, L.; Bansal, S.K. Sugarcane growth, yield and quality parameters as affected by different irrigation and potash levels in Punjab, India. In Proceedings of the International Conference Canecon-2021 Entitled, “Sugarcane for Sugar and Beyond”, Coimbatore, India, 19–22 June 2021; Palaniswami, C., Hemaprabha, G., Viswanathan, R., Karuppaiyan, R., Mohanraj, K., Mahadeva Swamy, H.K., Ram, B., Eds.; pp. 336–340, ISBN 978-93-85267-30-7. [Google Scholar]
- Hunsigi, G. Sugarcane in Agriculture and Industry; Prism Books Pvt. Ltd.: Bangalore, India, 2001; pp. 125–138, 207. [Google Scholar]
- Wood, A.W.; Schroeder, B.L. Potassium: A critical role in sugarcane production, particularly in drought conditions. In Proceedings of the Australian Society of Sugarcane Technologists, Brisbane, Australia, 4–7 May 2004; Available online: http://www.cabdirect.org/abstracts/20043079912.html (accessed on 7 May 2020).
- Wood, R.A. The roles of nitrogen, phosphorus and potassium in the production of sugarcane in South Africa. Fertil. Res. 1990, 26, 87–98. [Google Scholar] [CrossRef]
- Cotlear, C.B.G. Sugarcane Juice Extraction and Preservation, and Long-Term Lime Pretreatment of Bagasse. Ph.D. Dissertation, Office of Graduate Studies of Texas A&M University, College Station, TX, USA, 2004. [Google Scholar]
- Bhatt, R.; Singh, P.; Kumar, R. Assessment of Potash in Improving Yield and Quality of Sugarcane under Water Stressed and Unstressed Conditions; Final project report submitted to Indian Potash Limited (IPL): Gurgaon, India, 2021. [Google Scholar]
- Ghaffar, A.; Saleem, M.F.; Fiaz, N.; Nadeem, M.A.; Wains, G.M. Yield and quality of sugarcane as influenced by different doses of potash and its time of application. Pak. J. Agric. Sci. 2013, 50, 345–350. [Google Scholar]
- Bansal, S.K.; Imas, P.; Nachmansohn, J. The Impact of Potassium Fertilization on Sugarcane Yields: A Comprehensive Experiment of Pairwise Demonstration Plots in Uttar Pradesh, India. e-ifc. Int. Potash Inst. 2018, 54, 13–20. Available online: https://www.ipipotash.org/uploads/udocs/e-ifc-54-sep-2018-india-sugarcane.pdf (accessed on 9 October 2021).
- Meade, G.P.; Chen, J.C.P. Can Sugar Handbook, 10th ed.; Wiley-Inter Science Publication: New York, NY, USA, 1977; p. 405. [Google Scholar]
- Waraich, E.A.; Ahmad, R.; Hur, R.G.M.; Ahmad, A.; Mahmood, N. Response of foliar application of KNO3 on yield, yield components and lint quality of cotton (Gossypium hirsutum L.). Afr. J. Agric. Res. 2011, 6, 5457–5463. [Google Scholar]
- Medeiros, D.B.; da Silva, E.C.; Mansur Custodio Nogueira, R.J.; Teixeira, M.M.; Buckeridge, M.S. Physiological limitations in two sugarcane varieties under water suppression and after recovering. Theor. Exp. Plant Physiol. 2013, 25, 213–222. [Google Scholar] [CrossRef]
- Basnayake, J.; Jackson, P.A.N.; Inman-Bamber, G.; Lakshmanan, P. Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. J. Exp. Bot. 2015, 66, 3945–3958. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, P.; Robinson, N. Stress physiology: Abiotic stresses. In Sugarcane: Physiology, Biochemistry, and Functional Biology; Moore, P.H., Botha, F.C., Eds.; John Wiley & Sons, Inc.: Chichester, UK, 2014; pp. 411–434. [Google Scholar]
- Basnayake, J.; Jackson, P.A.N.; Inman-Bamber, G.; Lakshmanan, P. Sugarcane for water-limited environments. Genetic variation in cane yield and sugar content in response to water stress. J. Exp. Bot. 2012, 63, 6023–6033. [Google Scholar] [CrossRef]
- Gentile, A.; Dias, L.I.; Mattos, R.S.; Ferreira, T.H.; Menossi, M. MicroRNAs and drought responses in sugarcane. Front. Plant Sci. 2015, 6, 58. [Google Scholar] [CrossRef]
- Inman-Bamber, N.; Smith, D. Water relations in sugarcane and response to water deficits. Field Crops Res. 2005, 92, 185–202. [Google Scholar] [CrossRef]
- Machado, R.; Ribeiro, R.; Marchiori, P.; Machado, D.; Machado, E.; Landell, M. Biometric and physiological responses to water deficit in sugarcane at different phenological stages. Pesqui. Agropecuária Bras. 2009, 44, 1575–1582. [Google Scholar] [CrossRef]
- Inman-Bamber, N.; Lakshmanan, P.; Park, S. Sugarcane for water-limited environments: Theoretical assessment of suitable traits. Field Crops Res. 2012, 134, 95–104. [Google Scholar] [CrossRef]
- Inman-Bamber, N.; Bonnett, G.; Spillman, M.; Hewitt, M.; Jackson, J. Increasing sucrose accumulation in sugarcane by manipulating leaf extension and photosynthesis with irrigation. Aust. J. Agric. Res. 2008, 59, 13–26. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Oliveira, L.A. O potássio na cultura da cana-de-açúcar. In Potássio na Agricultura Brasileira; Yamada, T., Roberts, T.L., Eds.; Esalq/USP: Piracicaba, Brazil, 2005. [Google Scholar]
- Schultz, N.; Lima, E.; Pereira, M.G.; Zonta, E. Residual effects of nitrogen, potassium and vinasse, fertilization on cane plant and ratoon harvested with and without straw burning. Rev. Bras. Ciência Solo 2010, 34, 811–820. [Google Scholar] [CrossRef]
- Weber, H.; Daros, E.; Zambon, J.L.C.; Ido, O.T.; Barela, J.D. Ratoon sugarcane productivity recovering with NPK fertilization. Sci. Agrar. 2002, 2, 73–77. [Google Scholar]
- Mengel, K.; Seçer, M.; Koch, K. Potassium effect on protein formation and amino acid turnover in developing wheat grain. Agron. J. 1981, 73, 74–78. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Hussain, F.; Akhter, J.; Gul, A.; Ross, M.; Ebert, G. Effect of different sources and rates of nitrogen and supra optimal level of potassium fertilization on growth, yield and nutrient uptake by sugarcane. Grown under saline conditions. Pak. J. Bot. 2008, 40, 1521–1531. [Google Scholar]
- Olivot, T.; Dal’Col Lúcio, A. Metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
| Treatments | Cane Height (cm) at DAH | |||||
|---|---|---|---|---|---|---|
| 116 | 155 | 178 | 200 | 277 | 312 | |
| Irrigation treatment | ||||||
| I1 | 68.9 a | 155.5 a | 206.6 a | 221.7 a | 248.0 a | 261.5 a |
| I0 | 60.9 b | 137.3 b | 183.1 a | 198.3 a | 228.2 b | 233.1 a |
| Significance level (p ≤ 0.05) | ** | ** | SS | SS | ** | NS |
| CV (%) | 6.7 | 3.2 | 9.2 | 9.3 | 2.5 | 10.0 |
| Potassium fertilizer treatment | ||||||
| M1 | 62.0 a | 142.0 a | 186.5 a | 208.8 b | 215.3 c | 217.5 c |
| M2 | 64.9 a | 145.8 a | 194.3 a | 227.5 ab | 233.3 bc | 234.7 bc |
| M3 | 65.5 a | 147.8 a | 197.0 a | 239.5 a | 248.0 ab | 254.2 ab |
| M4 | 66.7 a | 150.0 a | 201.5 a | 246.7 a | 260.3 a | 260.0 a |
| Significance level (p ≤ 0.05) | SS | SS | SS | ** | ** | ** |
| CV (%) | 7.9 | 9.9 | 7.7 | 6.6 | 8.9 | 7.4 |
| I × M | SS | SS | SS | SS | SS | SS |
| Treatments | Cane Diameter (cm) at DAH | Leaves per Plant at DAH | ||||
|---|---|---|---|---|---|---|
| 200 | 237 | 277 | 312 | 200 | 237 | |
| Irrigation treatment | ||||||
| I1 | 28.6 a | 28.1 a | 28.9 a | 28.5 a | 9.6 a | 16.7 a |
| I0 | 28.5 a | 27.9 b | 28.2 a | 27.6 a | 9.4 a | 15.6 a |
| F-test (p ≤ 0.05) | SS | ** | SS | SS | SS | SS |
| CV (%) | 10.6 | 5.6 | 3.1 | 5.4 | 9.4 | 10.4 |
| Potassium fertilizer treatment | ||||||
| M1 | 27.8 a | 26.6 c | 27.4 - | 26.5 b | 9.3 a | 15.3 a |
| M2 | 28.5 a | 27.5 bc | 28.3 ab | 27.7 ab | 9.4 a | 15.6 a |
| M3 | 28.8 a | 28.4 ab | 29.2 a | 28.4 ab | 9.6 a | 16.5 a |
| M4 | 29.3 a | 29.4 a | 29.6 a | 29.5 a | 9.8 a | 16.9 a |
| F-test (p ≤ 0.05) | SS | ** | ** | ** | SS | ** |
| CV (%) | 5.5 | 4.4 | 4.4 | 5.5 | 8.0 | 5.3 |
| I × M | SS | SS | SS | SS | SS | SS |
| Treatments | Internodes per Plant at DAH | Brix at DAH | ||||
|---|---|---|---|---|---|---|
| 200 | 237 | 277 | 312 | 277 | 312 | |
| Irrigation treatment | ||||||
| I1 | 9.5 a | 12.8 a | 10.7 a | 13.9 a | 20.5 a | 20.8 a |
| Io | 10.8 a | 13.5 a | 10.7 a | 14.7 a | 20.3 a | 19.5 a |
| F-test (p ≤ 0.05) | SS | SS | SS | SS | SS | SS |
| CV (%) | 7.2 | 11.2 | 8.7 | 3.9 | 8.3 | 7.7 |
| Potassium fertilizer treatment | ||||||
| M1 | 9.6 b | 12.5 b | 10.1 a | 12.7 a | 19.5 a | 18.1 a |
| M2 | 9.9 b | 12.7 b | 10.5 a | 14.5 a | 20.0 a | 19.5 a |
| M3 | 10.3 b | 13.5 ab | 10.9 a | 14.8 a | 20.7 a | 21.0 a |
| M4 | 10.8 a | 14.0 a | 11.4 a | 15.2 a | 21.2 a | 21.9 a |
| F-test (p ≤ 0.05) | ** | ** | SS | ** | NS | NS |
| CV (%) | 6.6 | 6.5 | 8.5 | 5.6 | 14.7 | 11.7 |
| I × M | SS | SS | SS | SS | SS | SS |
| Treatments | Resprouted Ratoon 35 DAH (%) | NMC (000/ha) | Biomass Yield (t ha−1) | Cane Yield (t ha−1) |
|---|---|---|---|---|
| Irrigation treatment | ||||
| I1 | 40.1 a | 55.4 a | 10.53 a | 57.1 a |
| I0 | 37.1 a | 47.2 a | 9.79 a | 54.7 a |
| F-test (p ≤ 0.05) | SS | SS | SS | SS |
| CV (%) | 14.6 | 7.6 | 5.0 | 3.5 |
| Potassium fertilizer treatment | ||||
| M1 | 32.7 a | 47.9 a | 9.32 a | 50.8 b |
| M2 | 36.6 a | 48.4 a | 10.15 a | 53.8 b |
| M3 | 50.5 a | 59.9 a | 10.28 a | 58.1 a |
| M4 | 52.3 a | 60.0 a | 10.88 a | 61.0 a |
| F-test (p ≤ 0.05) | SS | SS | SS | ** |
| CV (%) | 10.6 | 8.7 | 8.8 | 6.2 |
| I × M | SS | SS | SS | SS |
| Treatments | Shoot Borer (%) | Top Borer (%) | Stalk Borer (%) |
|---|---|---|---|
| Irrigation treatment | |||
| I1 | 6.3 a | 7.1 b | 6.6 b |
| I0 | 7.7 a | 8.4 a | 8.5 a |
| F-test (p ≤ 0.05) | SS | ** | ** |
| CV (%) | 5.2 | 8.6 | 3.7 |
| Potassium fertilizer treatment | |||
| M1 | 7.7 a | 8.3 a | 8.2 a |
| M2 | 6.8 a | 7.7 ab | 7.2 a |
| M3 | 6.3 a | 7.0 b | 7.2 a |
| M4 | 7.2 a | 8.0 a | 7.6 a |
| F-test (p ≤ 0.05) | SS | ** | SS |
| CV (%) | 7.6 | 9.2 | 12.3 |
| I × M | SS | SS | SS |
| Treatments | Average Sugarcane Quality Parameters 10 Months After Harvesting | |||||
|---|---|---|---|---|---|---|
| Brix (o) | Pol (%) | Purity (%) | CCS (%) | Extraction (%) | CCS (Tonnes ha−1) | |
| Irrigation treatment | ||||||
| I1 | 18.8 a | 17.2 a | 91.7 a | 12.1 a | 53.5 a | 6.8 a |
| I0 | 18.0 a | 16.5 b | 89.7 a | 11.6 a | 52.8 a | 6.4 a |
| F-test (p ≤ 0.05) | SS | ** | SS | SS | SS | SS |
| CV (%) | 4.6 | 1.0 | 4.6 | 3.4 | 6.7 | 7.4 |
| Potassium fertilizer treatment | ||||||
| M1 | 17.5 a | 16.3 c | 89.2 a | 11.1 c | 49.6 b | 5.8 b |
| M2 | 18.2 a | 16.6 b | 91.4 a | 11.6 b | 53.2 ab | 6.3 b |
| M3 | 18.7 a | 17.1 a | 91.3 a | 12.0 ab | 55.0 a | 7.0 a |
| M4 | 19.1 a | 17.3 a | 90.8 a | 12.1 a | 56.7 a | 7.5 a |
| F-test (p ≤ 0.05) | SS | ** | SS | ** | ** | ** |
| CV (%) | 7.7 | 2.2 | 5.2 | 2.4 | 6.5 | 7.3 |
| I × M | SS | SS | SS | SS | SS | SS |
| Treatments | Average Sugarcane Quality Parameters 12 Months After Harvesting | |||||
|---|---|---|---|---|---|---|
| Brix(o) | Pol (%) | Purity (%) | CCS (%) | Extraction (%) | CCS (Tonnes ha−1) | |
| Irrigation treatment | ||||||
| I1 | 21.3 a | 19.6 a | 92.1 a | 13.8 a | 58.6 a | 7.9 a |
| I0 | 20.6 a | 18.9 a | 91.9 a | 13.3 a | 57.0 a | 7.3 a |
| F-test (p ≤ 0.05) | SS | SS | SS | SS | SS | SS |
| CV (%) | 7.3 | 9.0 | 1.6 | 9.6 | 7.0 | 12.3 |
| Potassium fertilizer treatment | ||||||
| M1 | 19.9 c | 18.3 c | 91.9 a | 12.9 b | 54.8 b | 6.5 c |
| M2 | 20.8 b | 19.1 b | 92.2 a | 13.5 ab | 56.6 b | 7.2 b |
| M3 | 21.4 ab | 19.6 ab | 91.5 a | 13.8 a | 58.8 ab | 8.0 a |
| M4 | 21.7 a | 20.1 a | 92.3 a | 14.2 a | 61.0 a | 8.6 a |
| F-test (p ≤ 0.05) | ** | ** | SS | ** | ** | ** |
| CV (%) | 3.1 | 2.4 | 3.4 | 3.5 | 6.0 | 7.3 |
| I × M | SS | SS | SS | 1.52 | SS | SS |
| 10 Months after Harvesting the Seed Crop | ||||||
|---|---|---|---|---|---|---|
| Brix (◦) | Pol (%) | Purity (%) | CCS (%) | Extraction (%) | CCS (Tonnes ha−1) | |
| Brix (◦) | 1 | 0.6 | −0.6 | 0.2 | 0.4 | 0.1 |
| Pol (%) | 0.6 | 1 | 0.2 | 0.9 | 0.5 | 0.5 |
| Purity (%) | −0.6 | 0.2 | 1 | 0.6 | 0.1 | 0.4 |
| CCS (%) | 0.2 | 0.9 | 0.6 | 1 | 0.5 | 0.6 |
| Extraction (%) | 0.4 | 0.5 | 0.1 | 0.5 | 1 | 0.5 |
| CCS (tonnes ha−1) | 0.1 | 0.5 | 0.4 | 0.6 | 0.5 | 1 |
| 12 Months after Harvesting the Seed Crop | ||||||
| Brix (◦) | Pol (%) | Purity (%) | CCS (%) | Extraction (%) | CCS (Tonnes ha−1) | |
| Brix (◦) | 1 | 0.8 | −0.1 | 0.7 | 0.3 | 0.7 |
| Pol (%) | 0.8 | 1 | 0.5 | 1.0 | 0.2 | 0.8 |
| Purity (%) | −0.1 | 0.5 | 1 | 0.6 | −0.2 | 0.3 |
| CCS (%) | 0.7 | 1.0 | 0.6 | 1 | 0.1 | 0.8 |
| Extraction (%) | 0.3 | 0.2 | −0.2 | 0.1 | 1 | 0.4 |
| CCS (tonnes ha−1) | 0.7 | 0.8 | 0.3 | 0.8 | 0.4 | 1 |
| Treatments | Fertilizer Cost (Rs ha−1) | Recorded Yield (Tonnes ha−1) | Reported Response | Additional Income due to Applied K (Rs ha−1) | Benefit-Cost Ratio | Overall Trend |
|---|---|---|---|---|---|---|
| I1M1 | 0 | 51.0 | -- | -- | -- | |
| I1M2 | 1273 | 54.1 | 3.1 | 9145 | 7.18 | 3.36 |
| I1M3 | 2546 | 59.4 | 8.4 | 24,780 | 9.73 | 4.20 |
| I1M4 | 3800 | 64.0 | 13.0 | 38,350 | 10.09 | 3.92 |
| I0M1 | 0 | 50.7 | -- | -- | -- | |
| I0M2 | 1273 | 53.4 | 2.7 | 7965 | 6.26 | |
| I0M3 | 2546 | 56.8 | 6.1 | 17,995 | 7.07 | |
| I0M4 | 3800 | 57.9 | 7.2 | 21,240 | 5.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, R.; Singh, J.; Laing, A.M.; Meena, R.S.; Alsanie, W.F.; Gaber, A.; Hossain, A. Potassium and Water-Deficient Conditions Influence the Growth, Yield and Quality of Ratoon Sugarcane (Saccharum officinarum L.) in a Semi-Arid Agroecosystem. Agronomy 2021, 11, 2257. https://doi.org/10.3390/agronomy11112257
Bhatt R, Singh J, Laing AM, Meena RS, Alsanie WF, Gaber A, Hossain A. Potassium and Water-Deficient Conditions Influence the Growth, Yield and Quality of Ratoon Sugarcane (Saccharum officinarum L.) in a Semi-Arid Agroecosystem. Agronomy. 2021; 11(11):2257. https://doi.org/10.3390/agronomy11112257
Chicago/Turabian StyleBhatt, Rajan, Jagdish Singh, Alison M. Laing, Ram Swaroop Meena, Walaa F. Alsanie, Ahmed Gaber, and Akbar Hossain. 2021. "Potassium and Water-Deficient Conditions Influence the Growth, Yield and Quality of Ratoon Sugarcane (Saccharum officinarum L.) in a Semi-Arid Agroecosystem" Agronomy 11, no. 11: 2257. https://doi.org/10.3390/agronomy11112257
APA StyleBhatt, R., Singh, J., Laing, A. M., Meena, R. S., Alsanie, W. F., Gaber, A., & Hossain, A. (2021). Potassium and Water-Deficient Conditions Influence the Growth, Yield and Quality of Ratoon Sugarcane (Saccharum officinarum L.) in a Semi-Arid Agroecosystem. Agronomy, 11(11), 2257. https://doi.org/10.3390/agronomy11112257










