The Biological Function and Roles in Phytohormone Signaling of the F-Box Protein in Plants
Abstract
:1. Introduction
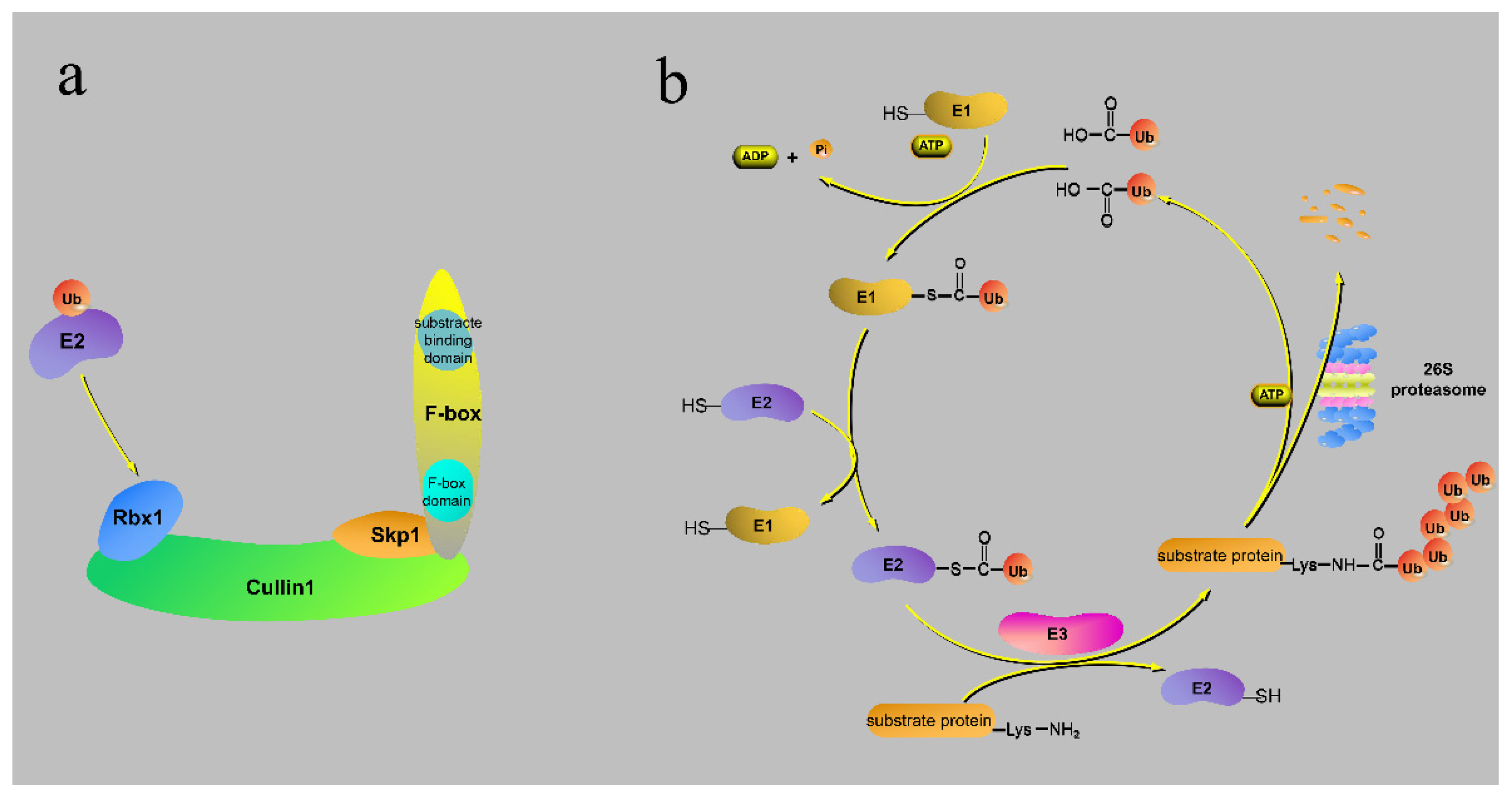
2. The Structure and Action Processes of the F-Box Protein
3. The Function of F-Box Proteins in Plant
3.1. The F-Box Proteins and Plant Development
3.1.1. Root Development
3.1.2. Leaf and Stem Development
3.1.3. Flower and Fruit Development
4. The F-Box Proteins and Biotic Stress
5. The F-Box Proteins and Abiotic Stress
6. F-Box Proteins and Phytohormone Signaling
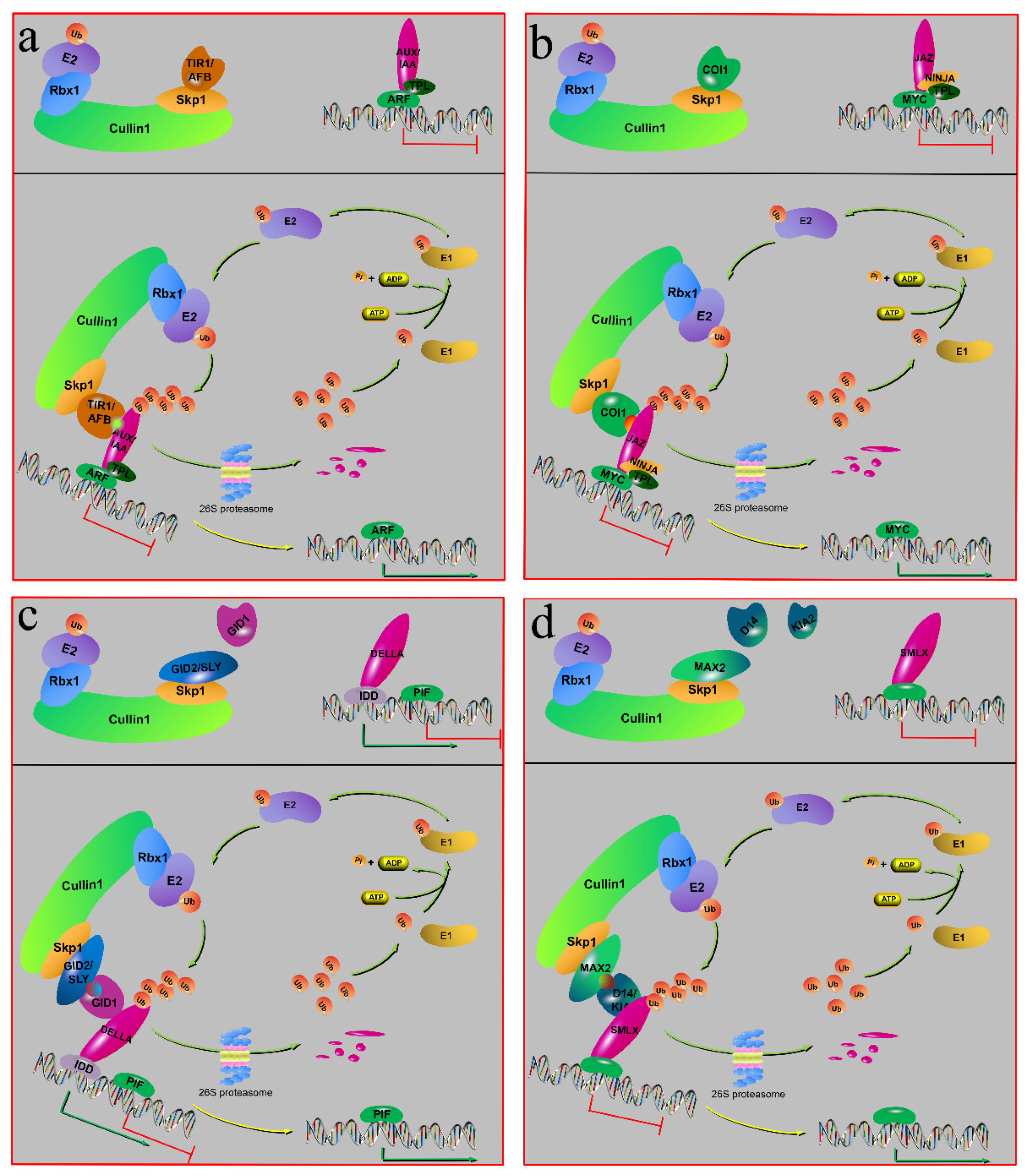
6.1. Auxin Signaling
6.2. JA Signaling
6.3. Gibberellic Acid Signaling
6.4. Strigolactones and Karrikin Signaling
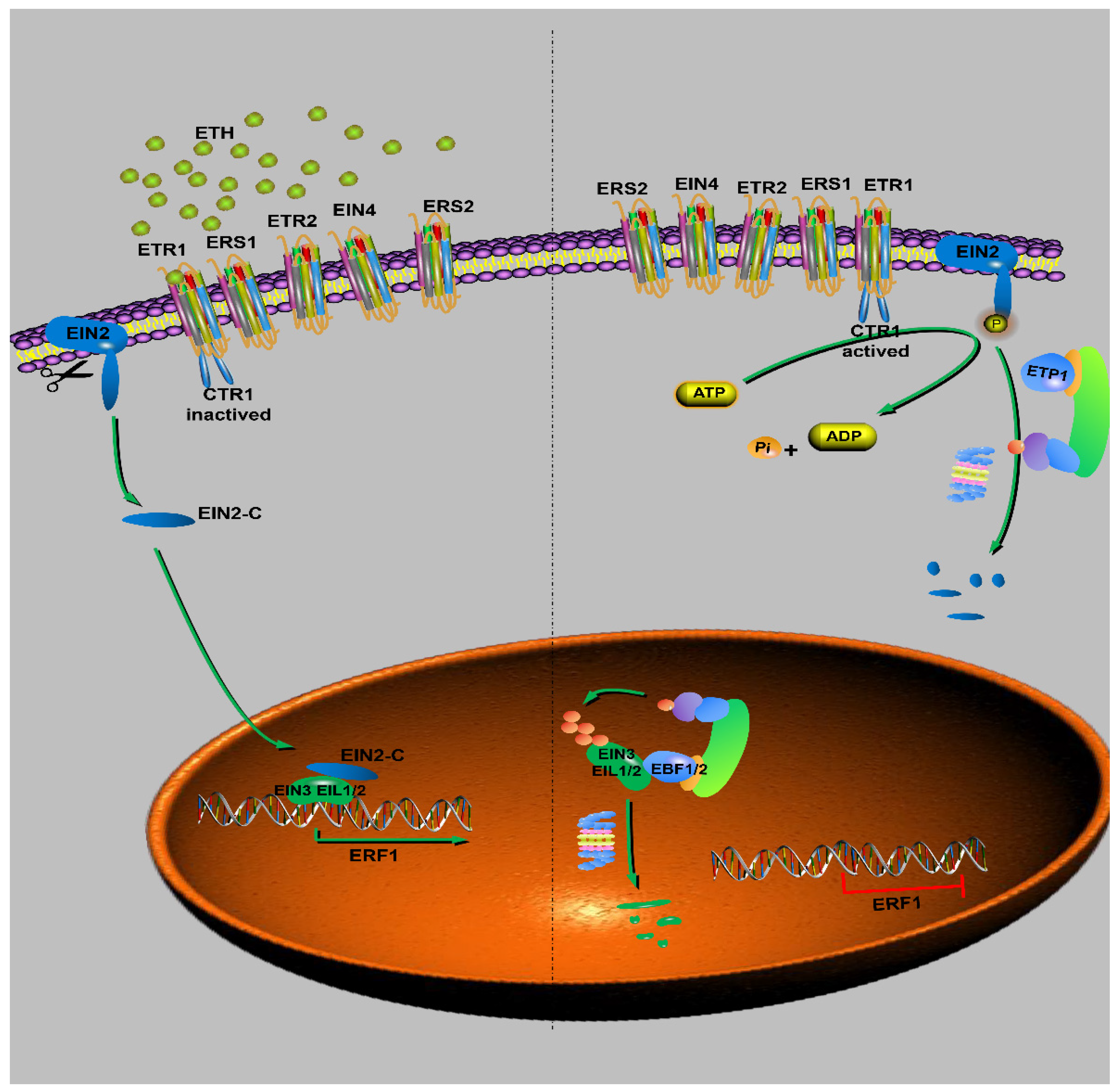
6.5. Ethylene Signaling
6.6. Other Phytohormone Signaling
7. Future Issues
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gagne, J.M.; Downes, B.P.; Shiu, S.H.; Durski, A.M.; Vierstra, R.D. The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 11519–11524. [Google Scholar] [CrossRef] [Green Version]
- Callis, J.; Vierstra, R.D. Protein degradation in signaling. Curr. Opin. Plan. Biol. 2000, 3, 381–386. [Google Scholar] [CrossRef]
- Yen, H.S.; Xu, Q.; Chou, D.M.; Zhao, Z.; Elledge, S.J. Global Protein Stability Profiling in Mammalian Cells. Science 2008, 322, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaar, J.R.; Pagan, J.K.; Pagano, M. Mechanisms and function of substrate recruitment by F-box proteins. Nat. Rev. Mol. Cell Biol. 2013, 14, 369–381. [Google Scholar] [CrossRef]
- Morreale, F.E.; Walden, H. Types of ubiquitin ligases. Cell 2016, 165, 248. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Zhou, Q.; Wang, Z.; Wei, W. Recent advances in SCF ubiquitin ligase complex: Clinical implications. Biochim. Et Biophys. Acta 2016, 1866, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Tian, Z.; Li, H.; Guo, Y.; Zhang, Y.; Roberts, J.A.; Zhang, X.; Miao, Y. Genome—wide analysis and characterization of F-box gene family in Gossypium hirsutum L. BMC Genom. 2019, 20, 1–16. [Google Scholar] [CrossRef]
- Kumar, A.; Paietta, J.V. The sulfur controller-2 negative regulatory gene of Neurospora crassa encodes a protein with beta-transducin repeats. Proc. Natl. Acad. Sci. USA 1995, 92, 3343–3347. [Google Scholar] [CrossRef] [Green Version]
- Bai, C.; Sen, P.; Hofmann, K.; Ma, L.; Goebl, M.; Harper, J.W.; Elledge, S.J. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 1996, 86, 263–274. [Google Scholar] [CrossRef] [Green Version]
- Kuroda, H.; Takahashi, N.; Shimada, H.; Seki, M.; Shinozaki, K.; Matsui, M. Classification and expression analysis of Arabidopsis F-box-containing protein genes. Plant Cell Physiol. 2002, 43, 1073–1085. [Google Scholar] [CrossRef] [Green Version]
- Schwager, K.M.; Calderon-Villalobos, L.I.A.; Dohmann, E.M.; Willige, B.C.; Knierer, S.; Nill, C.; Schwechheimer, C. Characterization of the VIER F-BOX PROTEINE genes from Arabidopsis reveals their importance for plant growth and development. Plant Cell 2007, 19, 1163–1178. [Google Scholar] [CrossRef] [Green Version]
- Song, J.B.; Wang, Y.X.; Li, H.B.; Li, B.W.; Zhou, Z.S.; Gao, S.; Yang, Z.M. The F-box family genes as key elements in response to salt, heavy mental, and drought stresses in Medicago truncatula. Funct. Integr. Genom. 2015, 15, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nijhawan, A.; Arora, R.; Agarwal, P.; Ray, S.; Sharma, P.; Kapoor, S.; Tyagi, A.K.; Khurana, J.P. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007, 143, 1467–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Wei, C.; Meng, Y.; Fan, R.; Zhao, W.; Wang, X.; Yu, X.; Laroche, A.; Kang, Z.; Liu, D. Identification and expression analysis of some wheat F-box subfamilies during plant development and infection by Puccinia triticina. Plant Physiol. Biochem. 2020, 155, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Xiao, Z.-X.; Wong, F.-L.; Sun, S.; Liang, K.-J.; Lam, H.-M. Genome-wide analyses of the soybean F-box gene family in response to salt stress. Int. J. Mol. Sci. 2017, 18, 818. [Google Scholar] [CrossRef] [Green Version]
- Jia, F.; Wu, B.; Li, H.; Huang, J.; Zheng, C. Genome-wide identification and characterisation of F-box family in maize. Mol. Genet. Genom. 2013, 288, 559–577. [Google Scholar] [CrossRef]
- Gupta, S.; Garg, V.; Kant, C.; Bhatia, S. Genome-wide survey and expression analysis of F-box genes in chickpea. BMC Genom. 2015, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.-R.; Zhang, Z.-R.; Lv, W.; Xu, J.-N.; Wang, X.-Y. Genome-wide characterization and analysis of F-box protein-encoding genes in the Malus domestica genome. Mol. Genet. Genom. 2015, 290, 1435–1446. [Google Scholar] [CrossRef]
- Wang, G.; Yin, H.; Qiao, X.; Tan, X.; Gu, C.; Wang, B.; Cheng, R.; Wang, Y.; Zhang, S. F-box genes: Genome-wide expansion, evolution and their contribution to pollen growth in pear (Pyrus bretschneideri). Plant Sci. 2016, 253, 164–175. [Google Scholar] [CrossRef]
- Kipreos, E.T.; Pagano, M. The F-box protein family. Genome Biol. 2000, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Carbonnel, S.; Das, D.; Varshney, K.; Kolodziej, M.C.; Villaécija-Aguilar, J.A.; Gutjahr, C. The karrikin signaling regulator SMAX1 controls Lotus japonicus root and root hair development by suppressing ethylene biosynthesis. Proc. Natl. Acad. Sci. USA 2020, 117, 21757–21765. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Williams, J.S.; Li, S.; Wu, L.; Wasi, A.K. S-Locus F-Box Proteins Are Solely Responsible for Pollen Function in S-RNase-Based Self-Incompatibility of Petunia. Plant Cell 2018, 30, 2959–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Xu, Y.; Luo, W.; Li, W.; Chen, N.; Zhang, D.; Chong, K. The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects leaf senescence and seed size in rice. Plant Physiol. 2013, 60, 75–83. [Google Scholar]
- Zhang, Y.; Zhang, J.; Guo, J.; Zhou, F.; Singh, S.; Xu, X.; Xie, Q.; Yang, Z.; Huang, C.-F. F-box protein RAE1 regulates the stability of the aluminum-resistance transcription factor STOP1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 319–327. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, Y.; Liu, N.; Wang, P.; Hou, Y. Enhanced resistance to Verticillium dahliae mediated by an F-box protein GhACIF1 from Gossypium hirsutum. Plant Sci. 2019, 284, 127–134. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Inuzuka, H.; Wei, W. Roles of F-box proteins in cancer. Nat. Rev. Cancer 2014, 14, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Yumimoto, K.; Yamauchi, Y.; Nakayama, K.I. F-Box Proteins and Cancer. Cancers 2020, 12, 1249. [Google Scholar] [CrossRef]
- Parry, G.; Calderon-Villalobos, L.; Prigge, M.; Peret, B.; Dharmasiri, S.; Itoh, H.; Lechner, E.; Gray, W.; Bennett, M.; Estelle, M. Complex regulation of the TIR1/AFB family of auxin receptors. Proc. Natl. Acad. Sci. USA 2009, 106, 22540–22545. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Yao, R.; Chen, L.; Li, S.; Gu, M.; Nan, F.; Xie, D. Dynamic perception of jasmonates by the F-box protein COI1. Mol. Plant 2018, 11, 1237–1247. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, X.; Su, D.; Feng, Y.; Liu, M. Comprehensive Profiling of Tubby-Like Protein Expression Uncovers Ripening-Related TLP Genes in Tomato (Solanum lycopersicum). Int. J. Mol. Sci. 2020, 21, 1000. [Google Scholar] [CrossRef] [Green Version]
- Thomas, B. Light signals and flowering. J. Exp. Bot. 2006, 57, 3387–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feke, A.M.; Gendron, J.M. Functional domain studies uncover novel roles for the ZTL Kelch repeat domain in clock function. PLoS ONE 2020, 16, e0235938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gou, M.; Liu, C.J. Arabidopsis Kelch Repeat F-Box Proteins Regulate Phenylpropanoid Biosynthesis via Controlling the Turnover of Phenylalanine Ammonia-Lyase. Plant Cell 2013, 25, 4994–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Zhao, X.; Zhao, Y.; Shanklin, J.; Zhao, Q.; Liu, C.J. Arabidopsis SnRK1 negatively regulates phenylpropanoid metabolism via Kelch domain-containing F-box proteins. New Phytol. 2020, 229, 3345–3359. [Google Scholar] [CrossRef]
- Callis, J. The ubiquitination machinery of the ubiquitin system. Arab. Book/Am. Soc. Plant Biol. 2014, 12, e0174. [Google Scholar] [CrossRef] [Green Version]
- Hegde, A.N. Proteolysis, synaptic plasticity and memory. Neurobiol. Learn. Mem. 2017, 138, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Su, T.; Yang, M.; Wang, P.; Zhao, Y.; Ma, C. Interplay between the Ubiquitin Proteasome System and Ubiquitin-Mediated Autophagy in Plants. Cells 2020, 9, 2219. [Google Scholar] [CrossRef]
- Li, W.; Ye, Y. Polyubiquitin chains: Functions, structures, and mechanisms. Cell. Mol. Life Sci. 2008, 65, 2397–2406. [Google Scholar] [CrossRef] [Green Version]
- Boughton, A.J.; Krueger, S.; Fushman, D. Branching via K11 and K48 Bestows Ubiquitin Chains with a Unique Interdomain Interface and Enhanced Affinity for Proteasomal Subunit Rpn1. Structure 2020, 28, 29–43. [Google Scholar] [CrossRef]
- Pickart, C.M. Ubiquitin in chains. Trends Biochem. Sci. 2000, 25, 544–548. [Google Scholar] [CrossRef]
- Pickart, C.M.; Fushman, D. Polyubiquitin chains: Polymeric protein signals. Curr. Opin. Chem. Biol. 2004, 8, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Kulathu, Y.; Komander, D. Atypical ubiquitylation—the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat. Rev. Mol. Cell Biol. 2012, 13, 508–523. [Google Scholar] [CrossRef]
- Swarbreck, S.M.; Guerringue, Y.; Matthus, E.; Jamieson, F.J.; Davies, J.M. Impairment in karrikin but not strigolactone sensing enhances root skewing in Arabidopsis thaliana. Plant J. 2019, 98, 607–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyzenga, W.J.; Booth, J.K.; Stone, S.L. The Arabidopsis RING-type E3 ligase XBAT32 mediates the proteasomal degradation of the ethylene biosynthetic enzyme, 1-aminocyclopropane-1-carboxylate synthase 7. Plant J. 2012, 71, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, L.; Zhang, Y.e.; Zhang, Y.; Deng, X.; Xue, Y. An auxin-inducible F-box protein CEGENDUO negatively regulates auxin-mediated lateral root formation in Arabidopsis. Plant Mol. Biol. 2006, 60, 599–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Miller, N.D.; Lewis, D.R.; Christians, M.J.; Lee, K.-H. AUXIN UP-REGULATED F-BOX PROTEIN1 Regulates the Cross Talk between Auxin Transport and Cytokinin Signaling during Plant Root Growth. Plant Physiol. 2011, 156, 1878–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Hamid, N.A.; Ahmad-Fauzi, M.I.; Zainal, Z.; Ismail, I. Diverse and dynamic roles of F-box proteins in plant biology. Planta 2020, 251, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Coates, J.C.; Laplaze, L.; Haseloff, J. Armadillo-related proteins promote lateral root development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Joke, B.; Stefanie, P.; Jolien, D.B.; Jonas, B.; Mieke, V.L.; Dirk, I. F-Box Protein FBX92 Affects Leaf Size in Arabidopsis thaliana. Plant Cell Physiol. 2017, 58, 962–975. [Google Scholar]
- Lin, L.-B.; Xue, H.-W. Rice miR394 suppresses leaf inclination through targeting an F-box gene, LEAF INCLINATION 4. J. Integr. Plant Biol. 2018, 61, 406–416. [Google Scholar]
- Gonzalez-Carranza, Z.H.; Rompa, U.; Peters, J.L.; Bhatt, A.M.; Wagstaff, C.; Stead, A.D.; Roberts, J.A. Hawaiian skirt: An F-box gene that regulates organ fusion and growth in arabidopsis. Plant Physiol. 2007, 144, 1370–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damayanti, F.; Lombardo, F.; Masuda, J.-i.; Shinozaki, Y.; Ichino, T.; Hoshikawa, K.; Okabe, Y.; Wang, N.; Fukuda, N.; Ariizumi, T. Functional disruption of the tomato putative ortholog of HAWAIIAN SKIRT results in facultative parthenocarpy, reduced fertility and leaf morphological defects. Front. Plant Sci. 2019, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.A.; Park, J.H.; Lee, G.I.; Paek, K.H.; Park, S.K.; Hong, G.N. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 2010, 12, 527–535. [Google Scholar] [CrossRef]
- Woo, H.R.; Chung, K.M.; Park, J.H.; Oh, S.A.; Ahn, T.; Hong, S.H.; Nam, J.H.G. ORE9, an F-Box Protein That Regulates Leaf Senescence in Arabidopsis. Plant Cell 2001, 13, 1779–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Jian, L.; Chai, J.; Da, X. Mitogen-activated protein kinase 6 mediates nuclear translocation of ORE3 to promote ORE9 gene expression in methyl jasmonate-induced leaf senescence. J. Exp. Bot. 2016, 67, 83–94. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, X.; Chen, G.; Zhu, Z.; Yin, W. Silencing SlGID2, a putative F-box protein gene, generates a dwarf plant and dark-green leaves in tomato. Plant Physiol. Biochem. 2016, 109, 491–501. [Google Scholar] [CrossRef]
- Wang, Z.; Li, N.; Jiang, S.; Gonzalez, N.; Li, Y. SCFSAP controls organ size by targeting PPD proteins for degradation in Arabidopsis thaliana. Nat. Commun. 2016, 7, 11192. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Ma, Q.; Wang, H.; Feng, D.; Wang, X.; Pei, Y.; Wen, J.; Tadege, M.; Niu, L.; Lin, H. SMALL LEAF AND BUSHY1 controls organ size and lateral branching by modulating the stability of BIG SEEDS1 in Medicago truncatula. New Phytol. 2020, 226, 1399–1412. [Google Scholar] [CrossRef] [Green Version]
- Levin, J.Z.; Meyerowitz, E.M. UFO: An Arabidopsis gene involved in both floral meristem and floral organ development. Plant Cell 1995, 7, 529–548. [Google Scholar]
- Zhao, D.; Yu, Q.; Chen, M.; Ma, H. The ASK1 gene regulates B function gene expression in cooperation with UFO and LEAFY in Arabidopsis. Development 2001, 128, 2735. [Google Scholar] [CrossRef]
- Sharma, B.; Meaders, C.; Wolfe, D.; Holappa, L.; Kramer, E.M. Homologs of LEAFY and UNUSUAL FLORAL ORGANS Promote the Transition From Inflorescence to Floral Meristem Identity in the Cymose Aquilegia coerulea. Front. Plant Sci. 2019, 10, 1218. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, S.; Chen, Z.; Zheng, L.; Diao, Z. Dwarf and deformed flower 1, encoding an F-box protein, is critical for vegetative and floral development in rice (Oryza sativa L.). Plant J. 2012, 72, 829–842. [Google Scholar] [CrossRef]
- He, R.; Li, X.; Zhong, M.; Yan, J.; Ji, R. A photo-responsive F-box protein FOF2 regulates floral initiation by promoting FLC expression in Arabidopsis. Plant J. Cell Mol. Biol. 2017, 91, 788–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Carranza, Z.H.; Zhang, X.; Peters, J.L.; Boltz, V.; Szecsi, J.; Bendahmane, M.; Roberts, J.A. HAWAIIAN SKIRT controls size and floral organ number by modulating CUC1 and CUC2 expression. PLoS ONE 2017, 12, e0185106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; Hee, S.-Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [Green Version]
- Piisil, M.; Keceli, M.A.; Brader, G.; Jakobson, L.; Jesaar, I.; Sipari, N.; Kollist, H.; Palva, E.T.; Kariola, T. The F-box protein MAX2 contributes to resistance to bacterial phytopathogens in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 53. [Google Scholar] [CrossRef] [Green Version]
- Lannoo, N.; Damme, E.J.M.V. Nucleocytoplasmic plant lectins. Biochim. Et Biophys. Acta 2010, 1800, 190–201. [Google Scholar] [CrossRef]
- Stefanowicz, K.; Lannoo, N.; Zhao, Y.; Eggermont, L.; Hove, J.V.; Atalah, B.A.; Damme, E.J.V. Glycan-binding F-box protein from Arabidopsis thaliana protects plants from Pseudomonas syringae infection. BMC Plant Biol. 2016, 16, 213. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Yang, Y.; Dayong Li, H.Z.; Zhong, Z.; Song, F. Overexpression of a rice defense-related F-box protein gene OsDRF1 in tobacco improves disease resistance through potentiation of defense gene expression. Physiol. Plant 2008, 134, 440–452. [Google Scholar] [CrossRef]
- Wang, J.; Yao, W.; Wang, L.; Ma, F.; Tong, W.; Wang, C.; Bao, R.; Jiang, C.; Yang, Y.; Zhang, J. Overexpression of VpEIFP1, a novel F-box/Kelch-repeat protein from wild Chinese Vitis pseudoreticulata, confers higher tolerance to powdery mildew by inducing thioredoxin z proteolysis. Plant Sci. 2017, 263, 142–155. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.-Y.; Li, J.; Wang, P.-Y.; Qin, F. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, B.; Kukreja, S.; Goutam, U. Milestones achieved in response to drought stress through reverse genetic approaches. F1000Research 2018, 7, 1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Zhang, J.; Liu, L.; Wang, Z.; Li, Y.; Guo, L.; Li, Y.; Zhang, X.; Ren, S.; Zhao, B. SlTLFP8 reduces water loss to improve water-use efficiency by modulating cell size and stomatal density via endoreduplication. Plant Cell Environ. 2020, 43, 2666–2679. [Google Scholar] [CrossRef] [PubMed]
- An, J.-P.; Li, R.; Qu, F.-J.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. Apple F-box protein MdMAX2 regulates plant photomorphogenesis and stress response. Front. Plant Sci. 2016, 7, 1685. [Google Scholar] [CrossRef] [Green Version]
- An, J.; Li, Q.; Yang, J.; Zhang, G.; Wang, W. Wheat F-box Protein TaFBA1 Positively Regulates Plant Drought Tolerance but Negatively Regulates Stomatal Closure. Front. Plant Sci. 2019, 10, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Sun, X.; Yin, S.; Kong, X.; Zhou, S.; Xu, Y.; Luo, Y.; Wang, W. The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol. Biochem. 2014, 84, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, G.; Zhou, S.; Ren, Y.; Wang, W. The improvement of salt tolerance in transgenic tobacco by overexpression of wheat F-box gene TaFBA1. Plant Sci. 2017, 259, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Shu-Mei, Z.; Xiang-Zhu, K.; Han-Han, K.; Xiu-Dong, S.; Wei, W.; Pandey, G.K. The Involvement of Wheat F-Box Protein Gene TaFBA1 in the Oxidative Stress Tolerance of Plants. PLoS ONE 2015, 10, e0122117. [Google Scholar]
- Qinxue, L.; Wenqiang, W.; Wenlong, W.; Guangqiang, Z.; Yang, L.; Yong, W.; Wei, W. Wheat F-Box Protein Gene TaFBA1 Is Involved in Plant Tolerance to Heat Stress. Front. Plant Sci. 2018, 9, 521. [Google Scholar]
- Liu, W.; Xu, F.; Lv, T.; Zhou, W.; Chen, Y.; Jin, C.; Lu, L.; Lin, X. Spatial responses of antioxidative system to aluminum stress in roots of wheat (Triticum aestivum L.) plants. Sci. Total. Environ. 2018, 627, 462–469. [Google Scholar] [CrossRef]
- Zhang, M.; Lu, X.; Li, C.-L.; Zhang, B.; Zhang, C.; Zhang, S.; Zhang, X.; Ding, Z. Auxin Efflux Carrier ZmPGP1 Mediates Root Growth Inhibition under Aluminum Stress. Plant Physiol. 2018, 177, 819–832. [Google Scholar] [CrossRef] [Green Version]
- Iuchi, S.; Koyama, H.; Iuchi, A.; Kobayashi, Y.; Kitabayashi, S.; Kobayashi, Y.; Ikka, T.; Hirayama, T.; Shinozaki, K.; Kobayashi, M. Zinc finger protein STOP1 is critical for proton tolerance in Arabidopsis and coregulates a key gene in aluminum tolerance. Proc. Natl. Acad. Sci. USA 2007, 104, 9900–9905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, G.; Cui, Y.; Wang, M.; Li, M.; Yin, X.; Xia, X. OsMsr9, a novel putative rice F-box containing protein, confers enhanced salt tolerance in transgenic rice and Arabidopsis. Mol. Breed. 2014, 34, 1055–1064. [Google Scholar] [CrossRef]
- Venkatesh, J.; Kang, M.-Y.; Liu, L.; Kwon, J.-K.; Kang, B.-C. F-Box Family Genes, LTSF1 and LTSF2, Regulate Low-Temperature Stress Tolerance in Pepper (Capsicum chinense). Plants 2020, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Guo, W.; Yin, Y.; Gong, Z.H. A Novel F-Box Protein CaF-Box Is Involved in Responses to Plant Hormones and Abiotic Stress in Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2014, 15, 2413–2430. [Google Scholar] [CrossRef]
- Waadt, R. Phytohormone signaling mechanisms and genetic methods for their modulation and detection. Curr. Opin. Plan. Biol. 2020, 57, 31–40. [Google Scholar] [CrossRef]
- Guilfoyle, T.; Hagen, G.; Larrieu, A.; Vernoux, T. Comparison of plant hormone signalling systems. Essays Biochem. 2015, 58, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Weijers, D.; Nemhauser, J.; Yang, Z. Auxin: Small molecule, big impact. J. Exp. Bot. 2018, 69, 133–136. [Google Scholar] [CrossRef]
- Quint, M.; Gray, W.M. Auxin signaling. Curr. Opin. Plant Biol. 2006, 9, 448–453. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Nelson, D.C.; Weijers, D. Evolution of plant hormone response pathways. Annu. Rev. Plant Biol. 2020, 71, 327–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.B. The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription. Plant Cell 2003, 15, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, A.M.; Gaj, M.D. miR393 contributes to the embryogenic transition induced in vitro in Arabidopsis via the modification of the tissue sensitivity to auxin treatment. Planta 2016, 244, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasternack, C. Jasmonates: An update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef] [Green Version]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Huang, H.; Liu, B.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef] [Green Version]
- Han, G.-Z. Evolution of jasmonate biosynthesis and signaling mechanisms. J. Exp. Bot. 2017, 68, 1323–1331. [Google Scholar] [CrossRef]
- Chini, A.; Cimmino, A.; Masi, M.; Reveglia, P.; Nocera, P.; Solano, R.; Evidente, A. The fungal phytotoxin lasiojasmonate A activates the plant jasmonic acid pathway. J. Exp. Bot. 2018, 69, 3095–3102. [Google Scholar] [CrossRef] [Green Version]
- Miricescu, A.; Goslin, K.; Graciet, E. Ubiquitylation in plants: Signaling hub for the integration of environmental signals. J. Exp. Bot. 2018, 69, 4511–4527. [Google Scholar] [CrossRef]
- Major, I.T.; Yoshida, Y.; Campos, M.L.; Kapali, G.; Xin, X.F.; Sugimoto, K.; de Oliveira Ferreira, D.; He, S.Y.; Howe, G.A. Regulation of growth–defense balance by the JASMONATE ZIM-DOMAIN (JAZ)-MYC transcriptional module. New Phytol. 2017, 215, 1533–1547. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Yao, J.; Ke, J.; Zhang, L.; Lam, V.Q.; Xin, X.-F.; Zhou, X.E.; Chen, J.; Brunzelle, J.; Griffin, P.R. Structural basis of JAZ repression of MYC transcription factors in jasmonate signalling. Nature 2015, 525, 269–273. [Google Scholar] [CrossRef] [Green Version]
- Marquis, V.; Smirnova, E.; Poirier, L.; Zumsteg, J.; Schweizer, F.; Reymond, P.; Heitz, T. Stress-and pathway-specific impacts of impaired jasmonoyl-isoleucine (JA-Ile) catabolism on defense signalling and biotic stress resistance. Plant Cell Environ. 2020, 43, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Daviere, J.M.; Achard, P. Gibberellin signaling in plants. Development 2013, 140, 1147–1151. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 2008, 59, 225–251. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, P.; Kumar, P.P. Regulation of Seed Germination:The Involvement of Multiple Forces Exerted via Gibberellic Acid Signaling. Mol Plant 2019, 12, 24–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, B.; Zhao, Y.; Zhang, J.; Li, Z. Auxin and GA signaling play important roles in the maize response to phosphate deficiency. Plant Sci. 2019, 283, 177–188. [Google Scholar] [CrossRef]
- Blázquez, M.A.; Green, R.; Nilsson, O.; Sussman, M.R.; Weigel, D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 1998, 10, 791–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedden, P. The genes of the Green Revolution. TRENDS Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Asano, K.; Hirano, K.; Ueguchi-Tanaka, M.; Angeles-Shim, R.B.; Komura, T.; Satoh, H.; Kitano, H.; Matsuoka, M.; Ashikari, M. Isolation and characterization of dominant dwarf mutants, Slr1-d, in rice. Mol. Genet. Genom. 2009, 281, 223–231. [Google Scholar] [CrossRef]
- Blanco-Touriñán, N.; Legris, M.; Minguet, E.G.; Costigliolo-Rojas, C.; Nohales, M.A.; Iniesto, E.; Garcίa-Leόn, M.; Pacίn, M.; Heucken, N.; Blomeier, T. COP1 destabilizes DELLA proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 17, 13792–13799. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Teramura, H.; Murakoshi, S.; Nasuno, K.; Nishida, N.; Ito, T.; Yoshida, M.; Kamiya, Y.; Yamaguchi, S.; Takahashi, Y. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 2014, 26, 2920–2938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.-p. The molecular mechanism and evolution of the GA–GID1–DELLA signaling module in plants. Curr. Biol. 2011, 21, R338–R345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, K.; Hirano, Y.; Sun, T.-p.; Hakoshima, T. Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 2008, 456, 459–463. [Google Scholar] [CrossRef]
- Duan, J.; Yu, H.; Yuan, K.; Liao, Z.; Meng, X.; Jing, Y.; Liu, G.; Chu, J.; Li, J. Strigolactone promotes cytokinin degradation through transcriptional activation of CYTOKININ OXIDASE/DEHYDROGENASE 9 in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 14319–14324. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Li, G.; Chen, X.; Xing, L.; An, N. Role of Cytokinin, Strigolactone, and Auxin Export on Outgrowth of Axillary Buds in Apple Front. Plant Sci. 2019, 10, 616. [Google Scholar]
- Ueda, H.; Kusaba, M. Strigolactone regulates leaf senescence in concert with ethylene in Arabidopsis. Plant Physiol. 2015, 169, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Seto, Y.; Yamaguchi, S. Strigolactone biosynthesis, transport and perception. Plant J. 2020, 105, 335–350. [Google Scholar] [CrossRef]
- Seto, Y.; Yamaguchi, S. Strigolactone biosynthesis and perception. Curr. Opin. Plant Biol. 2014, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Marzec, M.; Brewer, P. Binding or hydrolysis? How does the strigolactone receptor work? Trends Plant Sci. 2019, 24, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Mach, J. Strigolactones regulate plant growth in arabidopsis via degradation of the DWARF53-like proteins SMXL6, 7, and 8. Plant Cell 2015, 27, 3022–3023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, R.; Ma, X.; Wang, H. SMXL6/7/8: Dual-Function Transcriptional Repressors of Strigolactone Signaling. Mol. Plant 2020, 13, 1244–1246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shang, L.; Yu, H.; Zeng, L.; Hu, J.; Ni, S.; Rao, Y.; Li, S.; Chu, J.; Meng, X. A strigolactones biosynthesis gene contributed to the Green Revolution in rice. Mol. Plant 2020, 13, 923–932. [Google Scholar] [CrossRef]
- Stanga, J.P.; Morffy, N.; Nelson, D.C. Functional redundancy in the control of seedling growth by the karrikin signaling pathway. Planta 2016, 243, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-f.; Qi, Y.; Meng, Y.; Shuai, H.; Chen, F.; Yang, W.; Shu, K. Current understanding of signaling transduction pathway and biological functions of Karrikins. Yi Chuan= Hered. 2016, 38, 52–61. [Google Scholar]
- Morffy, N.; Faure, L.; Nelson, D.C. Smoke and hormone mirrors: Action and evolution of karrikin and strigolactone signaling. Trends Genet. 2016, 32, 176–188. [Google Scholar] [CrossRef]
- Waters, M.T.; Nelson, D.C.; Scaffidi, A.; Flematti, G.R.; Sun, Y.K.; Dixon, K.W.; Smith, S.M. Specialisation within the DWARF14 protein family confers distinct responses to karrikins and strigolactones in Arabidopsis. Development 2012, 139, 1285–1295. [Google Scholar] [CrossRef] [Green Version]
- Kagiyama, M.; Hirano, Y.; Mori, T.; Kim, S.Y.; Kyozuka, J.; Seto, Y.; Yamaguchi, S.; Hakoshima, T. Structures of D 14 and D 14 L in the strigolactone and karrikin signaling pathways. Genes Cells 2013, 18, 147–160. [Google Scholar] [CrossRef]
- Dubois, M.; van den Broeck, L.; Inzé, D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Wang, X.; Wang, X.; Ahmed, S.; Hussain, S.; Zhang, N.; Ma, Y.; Wang, S. Integration of RACK1 and ethylene signaling regulates plant growth and development in Arabidopsis. Plant Sci. 2019, 280, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.S.; Giovannoni, J.J. Ripening in the tomato Green-ripe mutant is inhibited by ectopic expression of a protein that disrupts ethylene signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 7923–7928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmann, A.; Minges, A.; Groth, G. Interfering Peptides Targeting Protein–Protein Interactions in the Ethylene Plant Hormone Signaling Pathway as Tools to Delay Plant Senescence. Methods Mol. Biol. 2021, 71–85. [Google Scholar]
- Ju, C.; Chang, C. Mechanistic insights in ethylene perception and signal transduction. Plant Physiol. 2015, 169, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Chen, Y.; Chen, Y.; Shin, J.H.; Mila, I.; Audran, C.; Zouine, M.; Pirrello, J.; Bouzayen, M. The tomato Ethylene Response Factor Sl-ERF. B3 integrates ethylene and auxin signaling via direct regulation of Sl-Aux/IAA 27. New Phytol. 2018, 219, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Binder, B.M. Ethylene signaling in plants. J. Biol. Chem. 2020, 295, 7710–7725. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhang, Y.; Tu, Y.; Wang, Y.; Cheng, W.; Yang, Y. Overexpression of an EIN3-binding F-box protein2-like gene caused elongated fruit shape and delayed fruit development and ripening in tomato. Plant Sci. 2018, 272, 131–141. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Gou, M.; Shi, Z.; Zhu, Y.; Bao, Z.; Wang, G.; Hua, J. The F-box protein CPR1/CPR30 negatively regulates R protein SNC1 accumulation. Plant J. Cell Mol. Biol. 2012, 69, 411–420. [Google Scholar] [CrossRef]
- Gou, M.; Nan, S.; Zheng, J.; Huai, J.; Wang, G. An F-box gene, CPR30, functions as a negative regulator of the defense response in Arabidopsis. Plant J. 2010, 60, 757–770. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; He, J.; Zhang, S.; Han, H.; Wang, Z.; Liu, W.C.; Liang, Y.K.; Gao, Z. RIN13-mediated disease resistance depends on SNC1-EDS1/PAD4 signaling pathway in Arabidopsis. J. Exp. Bot. 2020, 71, 7393–7404. [Google Scholar] [CrossRef] [PubMed]
- Garner, C.M.; Spears, B.J.; Su, J.; Cseke, L.; Gassmann, W. Opposing functions of the plant TOPLESS gene family during SNC1-mediated autoimmunity. PLoS Genet. 2020, 17, e1009026. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Zhang, N.; Qiu, Y.; Meng, L.; Wang, A. Molecular Characterization, Gene Evolution and Expression Analysis of the F-Box Gene Family in Tomato (Solanum lycopersicum). Genes 2021, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Lin, Q.; Zhu, L.; Ren, Y.; Zhou, K.; Shabek, N.; Wu, F.; Mao, H.; Dong, W.; Gan, L.; et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature 2013, 504, 406–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Wu, N.; Yao, W.; Li, X.; Zhou, Y.; Li, H. The Biological Function and Roles in Phytohormone Signaling of the F-Box Protein in Plants. Agronomy 2021, 11, 2360. https://doi.org/10.3390/agronomy11112360
Xu K, Wu N, Yao W, Li X, Zhou Y, Li H. The Biological Function and Roles in Phytohormone Signaling of the F-Box Protein in Plants. Agronomy. 2021; 11(11):2360. https://doi.org/10.3390/agronomy11112360
Chicago/Turabian StyleXu, Keheng, Nan Wu, Wenbo Yao, Xiaowei Li, Yonggang Zhou, and Haiyan Li. 2021. "The Biological Function and Roles in Phytohormone Signaling of the F-Box Protein in Plants" Agronomy 11, no. 11: 2360. https://doi.org/10.3390/agronomy11112360
APA StyleXu, K., Wu, N., Yao, W., Li, X., Zhou, Y., & Li, H. (2021). The Biological Function and Roles in Phytohormone Signaling of the F-Box Protein in Plants. Agronomy, 11(11), 2360. https://doi.org/10.3390/agronomy11112360




