Development and Applications of an In Situ Probe for Multi-Element High-Resolution Measurement at Soil/Sediment-Water Interface and Rice Rhizosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Materials, and Solutions
2.2. Laboratory Evaluation of DGT Performance
2.2.1. Synthesis and Characterization of LDHs
2.2.2. Probe Gel Preparation
2.2.3. Chemical Analysis and Concentration Calculation
2.2.4. Diffusion Coefficients in the Probe’s Diffusive Layer
2.2.5. Selectivity of LDHs-DGT Probe to Sulfide and Arsenate
2.2.6. Effects of Ionic Strength and pH on Probe Measurement
2.2.7. Capacity of the Probe
2.2.8. Competition Effect among Different Elements
2.3. Co-Measurement of Multi-Elements Using LDHS-DGT Probe in Water
2.4. High-Resolution Measurement of Multi-Element Distribution at Soil/Sediment-Water Interface
2.5. Application of LDHs-DGT Probe in Rice Rhizosphere for Imaging of the Dynamic Distributions of Mult-Elements
3. Results and Discussion
3.1. Laboratorial Characterization of the Performance of the LDHs-DGT Probe
3.1.1. Morphology and Structural Characterization of LDHS
3.1.2. Diffusion Coefficient of Multi-Elements in LDHS-DGT Diffusive Layer
3.1.3. Selectivity of LDHS-DGT Probe for Sulfide and Arsenate
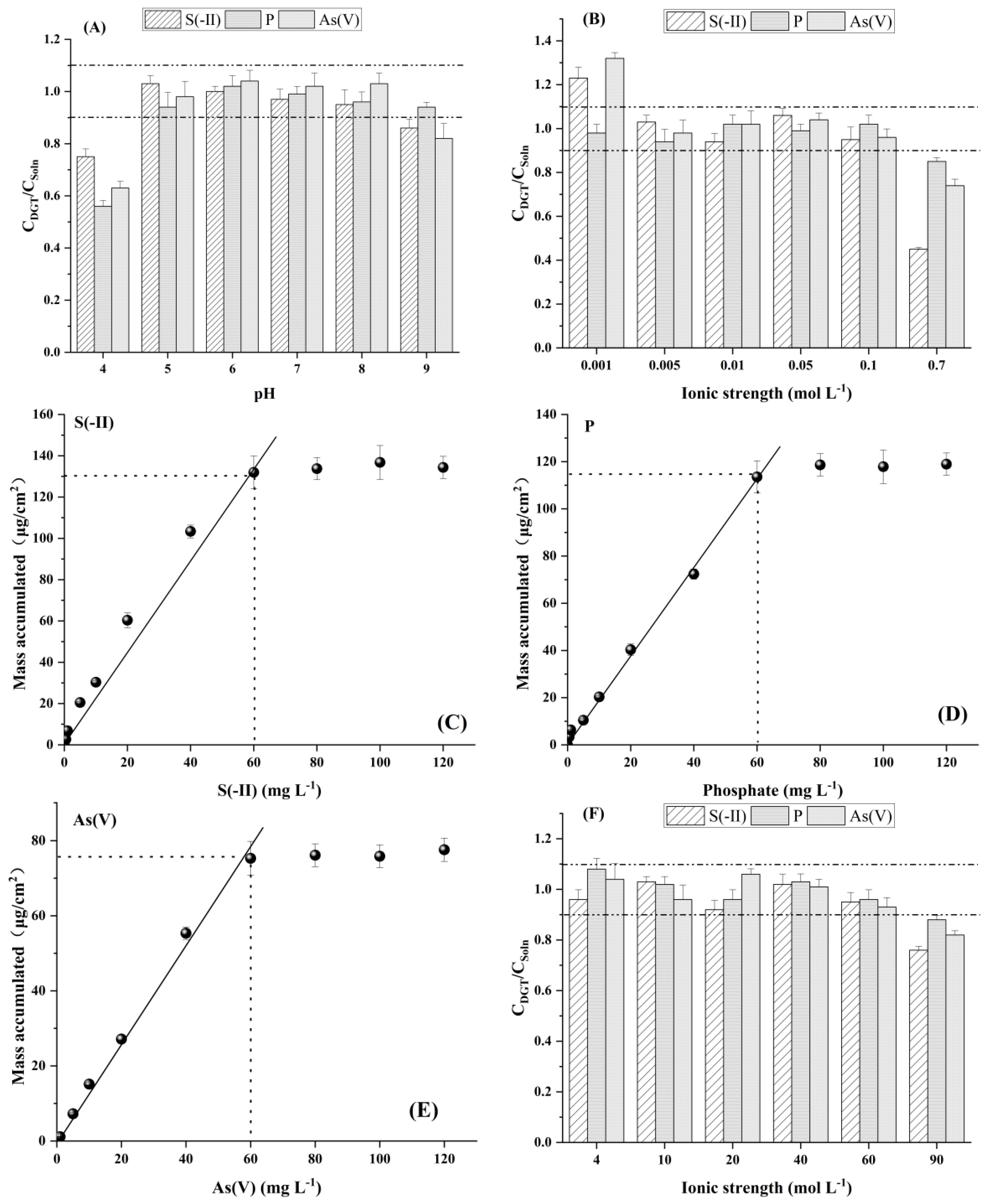
3.1.4. Effects of pH and Ionic Strength on the Measurement of LDHS-DGT Probe
3.1.5. Capacity of LDHs-DGT Probe for Measurement of Sulfide, Phosphate and Arsenate and Their Competition Effects
3.2. Measurement of Labile Concentrations of Multi-Elements in Field Waters Using LDHS-DGT Probe
3.3. Application of LDHs-DGT Probe in Soil/Sediment-Water Interface for Measurement of Multi-Elements at 1D Centimeter Scale
3.4. Measurement of the Dynamic Distribution of Multi-Elements at Rice Rhizosphere at 2D Millimeter Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, X.Z.; Guang, H.D.; Weng, J.Z.; Ya, G.; Hong, B.J.; Wei, R.; Ying, J.; Dai, C.W. Remediation of arsenic-contaminated paddy soil: Effects of elemental sulfur and gypsum fertilizer application. Ecotoxicol. Environ. Saf. 2021, 223, 112606. [Google Scholar]
- Qin, W.; Gu, Y.; Wang, G.; Wu, T.; Zhang, H.; Tang, X.; Zhang, Y.; Zhao, H. Zirconium metal organic frameworks-based DGT technique for in situ measurement of dissolved reactive phosphorus in waters. Water Res. 2018, 147, 223–232. [Google Scholar] [CrossRef]
- Pi, K.; Wang, Y.; Xie, X.; Ma, T.; Su, C.; Liu, Y. Role of sulfur redox cycling on arsenic mobilization in aquifers of Datong Basin, northern China. Appl. Geochem. 2017, 77, 31–43. [Google Scholar] [CrossRef]
- Strawn, D.G. Review of interactions between phosphorus and arsenic in soils from four case studies. Geochem. Transactions 2018, 19, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva‚, M.; Da, S.; Abate‚, G.; Masini‚, J. Spectrophotometric determination of acid volatile sulfide in river sediments by sequential injection analysis exploiting the methylene blue reaction. Talanta 2001, 53, 843–850. [Google Scholar] [CrossRef]
- Kirk, M.F.; Roden, E.E.; Crossey, L.J.; Brealey, A.J.; Spilde, M.N. Experimental analysis of arsenic precipitation during microbial sulfate and iron reduction in model aquifer sediment reactors. Geochim. Cosmochim. Acta 2010, 74, 2538–2555. [Google Scholar] [CrossRef]
- Kocar, B.; Borch, T.; Fendorf, S. Arsenic repartitioning during biogenic sulfidization and transformation of ferrihydrite. Geochim. Cosmochim. Acta 2010, 74, 980–994. [Google Scholar] [CrossRef]
- Davlson, W.; Zhang, H. In situspeciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef] [Green Version]
- Kreuzeder, A.; Santner, J.; Prohaska, T.; Wenzel, W.W. Gel for simultaneous chemical imaging of anionic and cationic solutes using diffusive gradients in thin films. Anal. Chem. 2013, 85, 12028–12036. [Google Scholar] [CrossRef]
- Anonymous, J.; Broney, C. Environmental Chemistry Study Findings on Environmental Chemistry Are Outlined in Reports from A; Stockdale and Colleagues: Atlanta, GA, USA, 2008; p. 371. [Google Scholar]
- Guan, D.X. Diffusive Gradients in Thin-Films (DGT): An Effective and Simple Tool for Assessing Contaminant Bioavailability in Waters, Soils and Sediments. Encycl. Environ. Health 2019, 1025, 111–124. [Google Scholar]
- Zhang, H.; Davison, W. Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal. Chem. 1995, 67, 3391. [Google Scholar] [CrossRef]
- Zhang, H.; Davison, W.; Knight, B.; McGrath, S. In situ measurements of solution concentrations and fluxes of trace metals in soils using DGT. Environ. Sci. Technol. 1998, 32, 704–710. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.J.; Sun, B.; Davison, W.; McGrath, S.P. A new method to measure effective soil solution concentration predicts copper availability to plants. Environ. Sci. Technol. 2001, 35, 2602–2607. [Google Scholar] [CrossRef]
- Zhao, M.; Qian, E.; Zhang, F.; Liu, R.; Liu, X.; Zhao, Y.; Liang, X. Spatiotemporal dynamics of labile Cd in soil during rice growth. Sci. Total Environ. 2020, 738, 139832. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, H.; Santner, J.; Davison, W. Performance characteristics of diffusive gradients in thin films equipped with a binding gel layer containing precipitated ferrihydrite for measuring arsenic (V), selenium (VI), vanadium (V), and antimony (V). Anal. Chem. 2010, 82, 8903–8909. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, J.; Rauch, S.; Morrison, G. Estimation of Measurement Uncertainties for the DGT Passive Sampler Used for Determination of Copper in Water. Int. J. Anal. Chem. 2014, 389125, 1–7. [Google Scholar] [CrossRef]
- Guan, D.X.; Williams, P.N.; Luo, J.; Zheng, J.L.; Xu, H.C.; Cai, C.; Ma, L.Q. Novel precipitated zirconia-based DGT technique for high-resolution imaging of oxyanions in waters and sediments. Environ. Sci. Technol. 2015, 49, 3653–3661. [Google Scholar] [CrossRef]
- Ding, S.; Xu, D.; Wang, Y.; Wang, Y.; Li, Y.; Gong, M.; Zhang, C. Simultaneous Measurements of Eight Oxyanions Using High-Capacity Diffusive Gradients in Thin Films (Zr-Oxide DGT) with a High-Efficiency Elution Procedure. Environ. Sci. Technol. 2016, 50, 7572–7580. [Google Scholar] [CrossRef]
- Österlund, H.; Chlot, S.; Faarinen, M.; Widerlund, A.; Rodushkin, I.; Ingri, J.; Baxter, D.C. Simultaneous measurements of As, Mo, Sb, V and W using a ferrihydrite diffusive gradients in thin films (DGT) device. Anal. Chim. Acta 2010, 682, 59–65. [Google Scholar] [CrossRef]
- Fan, X.; Ding, S.; Chen, M.; Gao, S.; Fu, Z.; Gong, M.; Tsang, D.; Wang, Y.; Zhang, C. Peak Chromium Pollution in Summer and Winter Caused by High Mobility of Chromium in Sediment of a Eutrophic Lake: In Situ Evidence from High Spatiotemporal Sampling. Environ. Sci. Technol. 2019, 53, 4755–4764. [Google Scholar] [CrossRef]
- Fan, X.; Ding, S.; Chen, M.; Gao, S.; Fu, Z.; Gong, M.; Wang, Y.; Zhang, C. Mobility of chromium in sediments dominated by macrophytes and cyanobacteria in different zones of Lake Taihu. Sci. Total Environ. 2019, 666, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Panther, J.G.; Stewart, R.R.; Teasdale, P.R.; Bennett, W.W.; Welsh, D.T.; Zhao, H. Titanium dioxide-based DGT for measuring dissolved As (V), V (V), Sb (V), Mo (VI) and W (VI) in water. Talanta 2013, 105, 80–86. [Google Scholar] [CrossRef]
- Wu, P. Anionic Clay Intercalation Construction and Environmental Remediation Technology; Science Press: Beijing, China, 2016. [Google Scholar]
- Chen, T.; Zhan, X.; Fan, M.; Chen, G.; Sun, J. Intercalation of pyrophosphate in the structure of anionic clay for instant synthesis. Miner. Rock 2005, 76, 105–108. [Google Scholar]
- Türk, T.; Alp, İ.; Deveci, H. Adsorption of As (V) from water using Mg–Fe-based hydrotalcite (FeHT). J. Hazard. Mater. 2009, 171, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yan, L.G.; Yang, Y.M.; Yu, S.J.; Shan, R.R.; Yu, H.Q.; Zhu, B.C.; Du, B. Adsorptive removal of phosphate by Mg–Al and Zn–Al layered double hydroxides: Kinetics, isotherms and mechanisms. Sep. Purif. Technol. 2014, 124, 36–42. [Google Scholar] [CrossRef]
- Liang, X.F.; Hou, W.G.; Xu, Y.M.; Sun, G.H.; Wang, L.; Sun, Y.; Qin, X. Sorption of lead ion by layered double hydroxide intercalated with diethylenetriaminepentaacetic acid. Colloids Surf. a-Physicochem. Eng. Asp. 2010, 366, 50–57. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, S.; Ren, M.; Li, C.; Xu, S.; Sun, Q.; Xu, L. Enhanced DGT capability for measurements of multiple types of analytes using synergistic effects among different binding agents. Sci. Total Environ. 2019, 657, 446–456. [Google Scholar] [CrossRef]
- Hou, H.; Cui, W.; Xu, Q.; Tao, Z.; Guo, Y.; Deng, T. Arsenic Species Analysis at Trace Level by High Performance Liquid Chromatography with Inductively Coupled Plasma Mass Spectrometry. Int. J. Anal. Chem. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Komorowicz, I.; Sajnóg, A.; Barałkiewicz, D. Total arsenic and arsenic species determination in freshwater fish by ICP-DRC-MS and HPLC/ICP-DRC-MS techniques. Molecules 2019, 24, 3. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.A.; de Graterol, L.S.; Mesa, J. Determination of chloride, sulfate and nitrate in groundwater samples by ion chromatography. J. Chromatogr. A 2000, 884, 185–190. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, H.; Tang, Z.; Zhao, T.; Qin, N.; Li, H.; Wu, F.; Giesy, J. Amendment of water quality standards in China: Viewpoint on strategic considerations. Environ. Sci. Pollut. Res. 2018, 25, 3078–3092. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, S.; Gong, M.; Xu, S.; Xu, W.; Zhang, C. Diffusion characteristics of agarose hydrogel used in diffusive gradients in thin films for measurements of cations and anions. Anal. Chim. Acta 2016, 945, 47–56. [Google Scholar] [CrossRef]
- Guan, D.X.; He, S.X.; Li, G.; Teng, H.H.; Ma, L.Q. Application of diffusive gradients in thin-films technique for speciation, bioavailability, modeling and mapping of nutrients and contaminants in soils. Crit. Rev. Environ. Sci. Technol. 2021, 1, 1–45. [Google Scholar] [CrossRef]
- He, S.; Wang, X.; Wu, X.; Yin, Y.; Ma, L.Q. Using rice as a remediating plant to deplete bioavailable arsenic from paddy soils. Environ. Int. 2020, 141, 105799. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Sun, Q.; Xu, D.; Jia, F.; He, X.; Zhang, C. High-resolution simultaneous measurements of dissolved reactive phosphorus and dissolved sulfide: The first observation of their simultaneous release in sediments. Environ. Sci. Technol. 2012, 46, 8297–8304. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, P.R.; Hayward, S.; Davison, W. In situ, High-Resolution Measurement of Dissolved Sulfide Using Diffusive Gradients in Thin Films with Computer-Imaging Densitometry. Anal. Chem. 1999, 71, 2186–2191. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.X.; Williams, P.N.; Xu, H.C.; Li, G.; Luo, J.; Ma, L.Q. High-resolution measurement and mapping of tungstate in waters, soils and sediments using the low-disturbance DGT sampling technique. J. Hazard. Mater. 2016, 316, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Xu, Y.; Wang, L.; Sun, Y.; Lin, D.; Sun, Y.; Qin, X.; Wan, Q. Sorption of Pb2+ on mercapto functionalized sepiolite. Chemosphere 2013, 90, 548–555. [Google Scholar] [CrossRef]
- Criscenti, L.; Sverjensky, D. The role of electrolyte anions (ClO4−, NO3−, and Cl−) in divalent metal (M2+) adsorption on oxide and hydroxide surfaces in salt solutions. Am. J. Sci. 1999, 299, 828–899. [Google Scholar] [CrossRef]
- Bennett, W.W.; Teasdale, P.R.; Panther, J.G.; Welsh, D.T.; Jolley, D.F. New diffusive gradients in a thin film technique for measuring inorganic arsenic and selenium(IV) using a titanium dioxide based adsorbent. Anal. Chem. 2010, 82, 7401–7407. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, S.; Shi, L.; Gong, M.; Xu, S.; Zhang, C. Simultaneous measurements of cations and anions using diffusive gradients in thin films with a ZrO-Chelex mixed binding layer. Anal. Chim. Acta 2017, 972, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Coyte, R.M.; Vengosh, A. Factors controlling the risks of co-occurrence of the redox-Sensitive elements of arsenic, chromium, vanadium, and uranium in groundwater from the eastern united states. Environ. Sci. Technol. 2020, 54, 4367–4375. [Google Scholar] [CrossRef] [PubMed]
- Puca, C.; Moldovan, M.; Silaghi-Dumitrescu, L.; Ungureanu, L.; Silaghi-Dumitrescu, R. On the apparent redox reactivity of “oxygen-enriched water”. Biol. Trace Elem. Res. 2020, 198, 350–358. [Google Scholar]
- Yan, Y.; Qian, Y.; Wang, Z.Y.; Yang, X.Y.; Wang, H.W. Ecological risk assessment from the viewpoint of surface water pollution in Xiamen City, China. Int. J. Sustain. Dev. World Ecol. 2018, 25, 403–410. [Google Scholar] [CrossRef]
- Coffin, M.; Courtenay, S.C.; Pater, C.C.; Heuvel, M. An empirical model using dissolved oxygen as an indicator for eutrophication at a regional scale. Mar. Pollut. Bull. 2018, 133, 261–270. [Google Scholar] [CrossRef]
- Jiang, M.; Sheng, Y.; Liu, Q.; Wang, W.; Liu, X. Conversion mechanisms between organic sulfur and inorganic sulfur in surface sediments in coastal rivers. Sci. Total Environ. 2020, 752, 141829. [Google Scholar] [CrossRef]
- Wu, B.; Liu, F.; Fang, W.; Yang, T.; Wang, S. Microbial Sulfur metabolism and environmental implications. Sci. Total Environ. 2021, 778, 146085. [Google Scholar] [CrossRef]
- Armstrong, J.; Armstrong, W. Rice: Sulfide-induced barriers to root radial oxygen loss, fesup2plus/sup and water uptake, and lateral root emergence. Ann. Bot. 2005, 96, 625–638. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Chen, L.; Chen, S.; Chun, G.; Wang, D.; Zhang, X. Effects of rhizosphere oxygen concentration on root physiological characteristics and anatomical structure at the tillering stage of rice. Ann. Appl. Biol. 2020, 177, 61–73. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhang, C.H.; Yan, J.L.; Yue, Q.; Ge, Y. Effects of sulfur supply and hydrogen peroxide pretreatment on the responses by rice under cadmium stress. Plant Growth Regul. 2015, 77, 299–306. [Google Scholar] [CrossRef]
- Zhang, D.; Du, G.; Chen, D.; Shi, G.; Rao, W.; Li, X.; Jiang, Y.; Liu, S.; Wang, D. Effect of elemental sulfur and gypsum application on the bioavailability and redistribution of cadmium during rice growth. Sci. Total Environ. 2019, 657, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
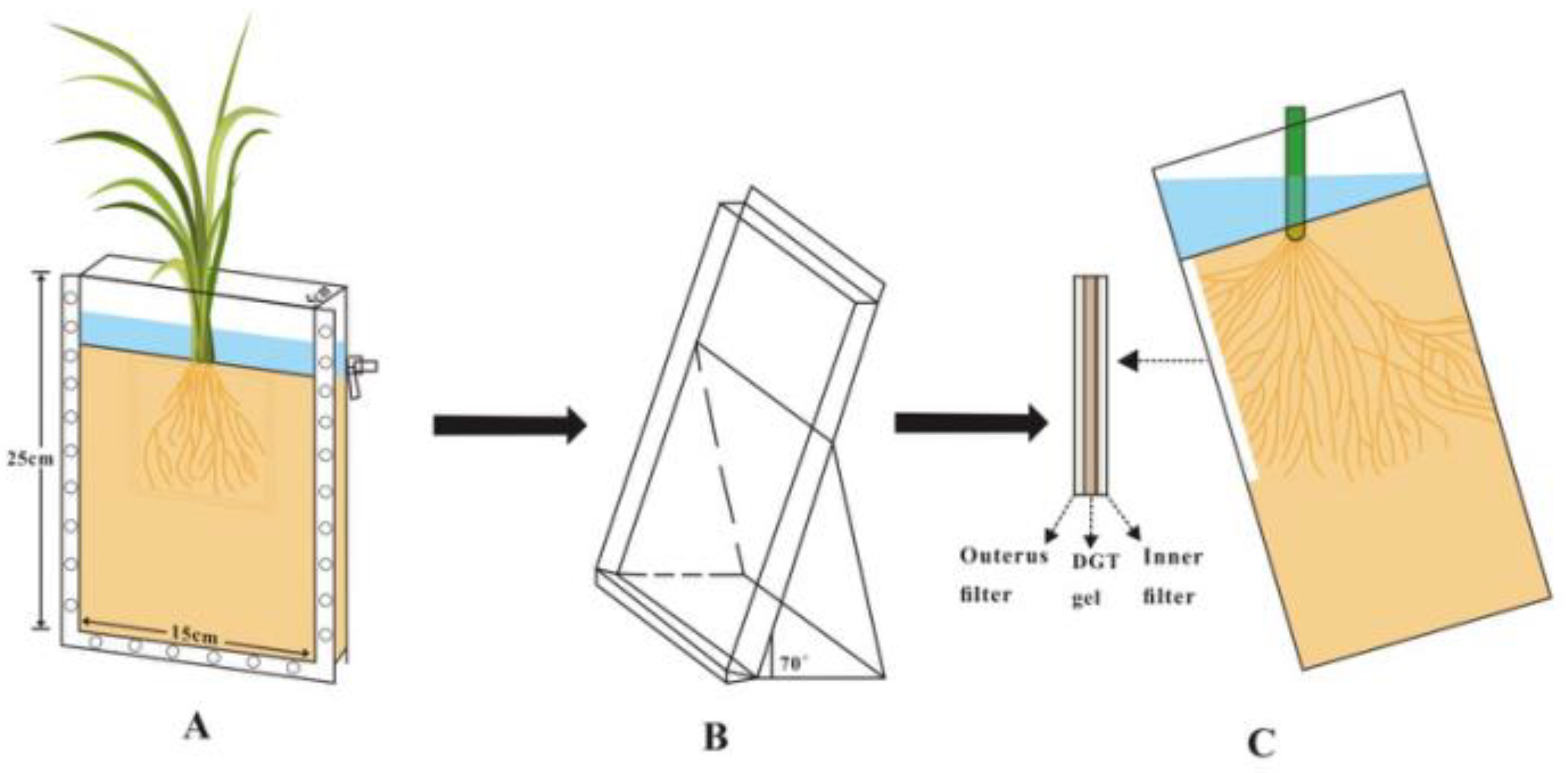


| Analyte | S(-II) | SO4(II) | Phosphate | As(III) | As(V) |
|---|---|---|---|---|---|
| DDGT | 5.99 ± 0.12 | 7.50 ± 0.28 | 4.57 ± 0.14 | 3.73 ± 0.15 | 3.41 ± 0.10 |
| Water | W1 | W2 | W3 |
|---|---|---|---|
| pH | 6.81 ± 0.07 | 5.93 ± 0.06 | 7.26 ± 0.07 |
| S(-II)-Cwater (μg L−1) | 15.50 ± 0.62 | 58.46 ± 4.09 | 10.26 ± 0.51 |
| Phosphate-Cwater (μg L−1) | 24.62 ± 0.49 | 74.52 ± 2.24 | 19.45 ± 0.58 |
| As(V)-Cwaterl (μg L−1) | 8.53 ± 0.17 | 4.34 ± 0.09 | 12.22 ± 0.49 |
| S(-II)-CDGT (μg L−1) | 14.11 ± 0.42 | 54.92 ± 1.65 | 9.23 ± 0.28 |
| Phosphate-CDGT (μg L−1) | 22.90 ± 0.69 | 72.28 ± 2.89 | 17.89 ± 0.54 |
| As(V)-CDGT (μg L−1) | 8.10 ± 0.32 | 4.04 ± 0.08 | 11.36 ± 0.34 |
| a RS(-II) | 0.91 | 0.94 | 0.90 |
| a Rphosphate | 0.93 | 0.97 | 0.92 |
| a RAs(V) | 0.95 | 0.94 | 0.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Liu, J.; Zhang, C.; Liang, X.; E, Q.; Liu, R.; Zhao, Y.; Liu, X. Development and Applications of an In Situ Probe for Multi-Element High-Resolution Measurement at Soil/Sediment-Water Interface and Rice Rhizosphere. Agronomy 2021, 11, 2383. https://doi.org/10.3390/agronomy11122383
Zhao M, Liu J, Zhang C, Liang X, E Q, Liu R, Zhao Y, Liu X. Development and Applications of an In Situ Probe for Multi-Element High-Resolution Measurement at Soil/Sediment-Water Interface and Rice Rhizosphere. Agronomy. 2021; 11(12):2383. https://doi.org/10.3390/agronomy11122383
Chicago/Turabian StyleZhao, Meng, Jiang Liu, Chuangchuang Zhang, Xuefeng Liang, Qian E, Rongle Liu, Yujie Zhao, and Xiaowei Liu. 2021. "Development and Applications of an In Situ Probe for Multi-Element High-Resolution Measurement at Soil/Sediment-Water Interface and Rice Rhizosphere" Agronomy 11, no. 12: 2383. https://doi.org/10.3390/agronomy11122383
APA StyleZhao, M., Liu, J., Zhang, C., Liang, X., E, Q., Liu, R., Zhao, Y., & Liu, X. (2021). Development and Applications of an In Situ Probe for Multi-Element High-Resolution Measurement at Soil/Sediment-Water Interface and Rice Rhizosphere. Agronomy, 11(12), 2383. https://doi.org/10.3390/agronomy11122383





