Soil Health Check-Up of Conservation Agriculture Farming Systems in Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Agro-Ecoregions, Croplands and Within-Field Yield Environments
2.2. Sampling Strategies for Soil Enzyme, Physicochemical Soil Analysis, Soil DNA Characterization and Crop Yield
2.3. Statistical Analysis
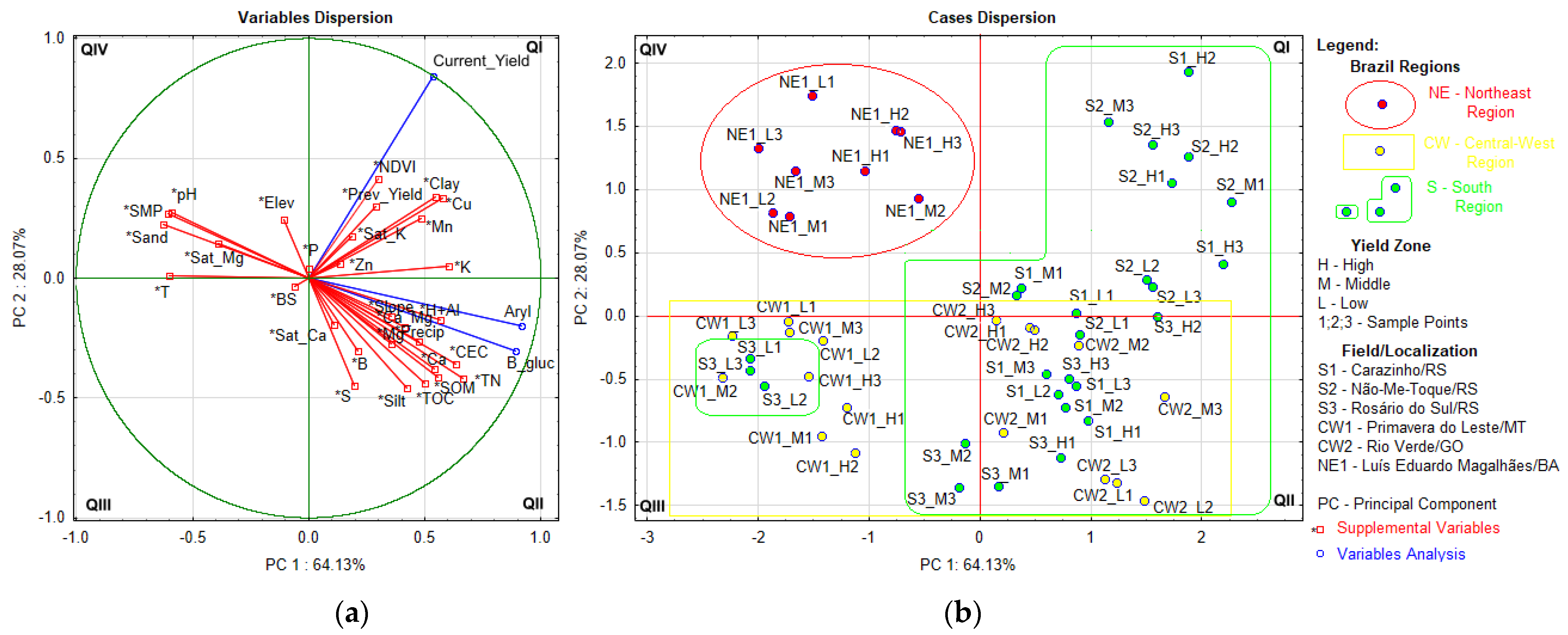
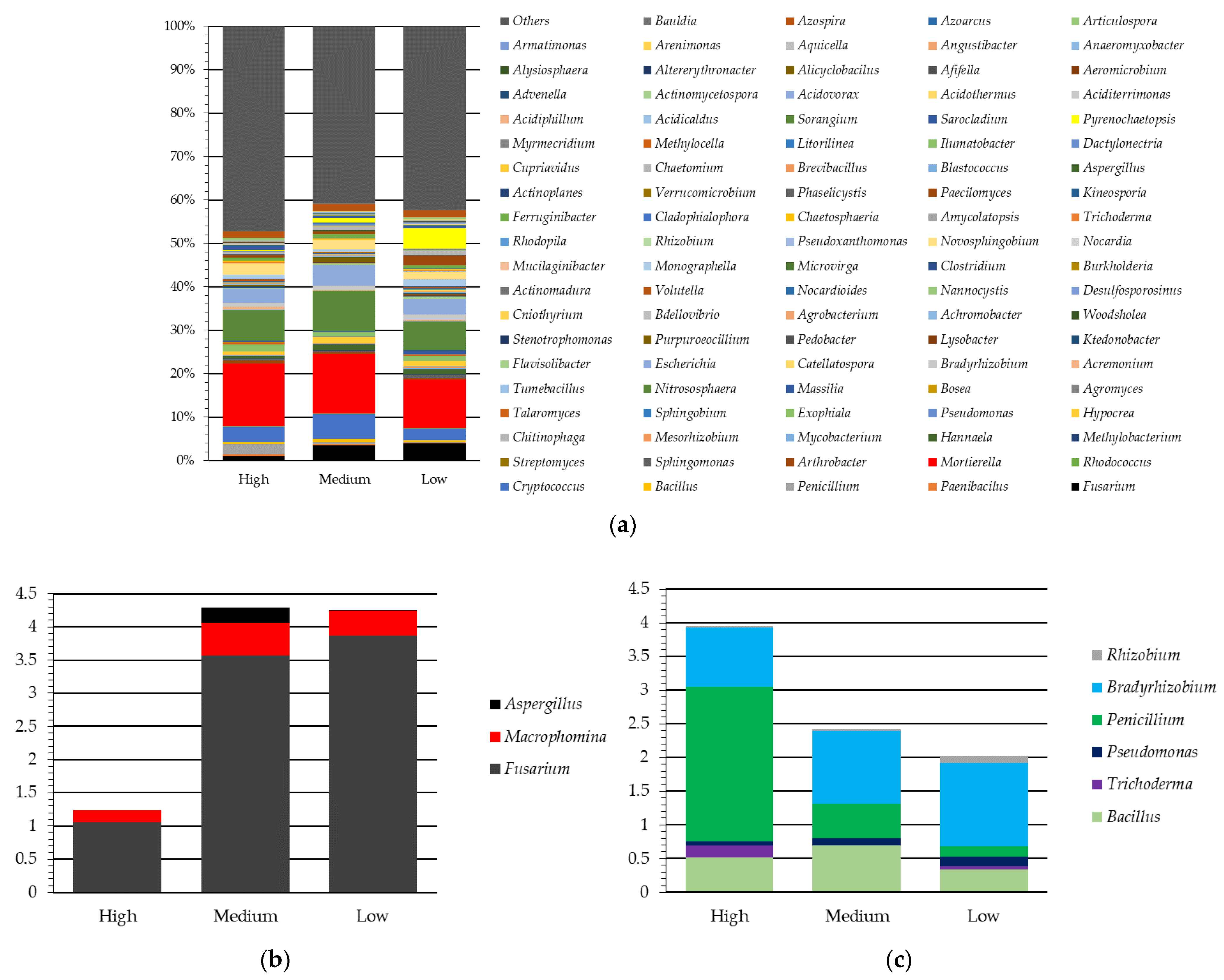
3. Results and Discussion
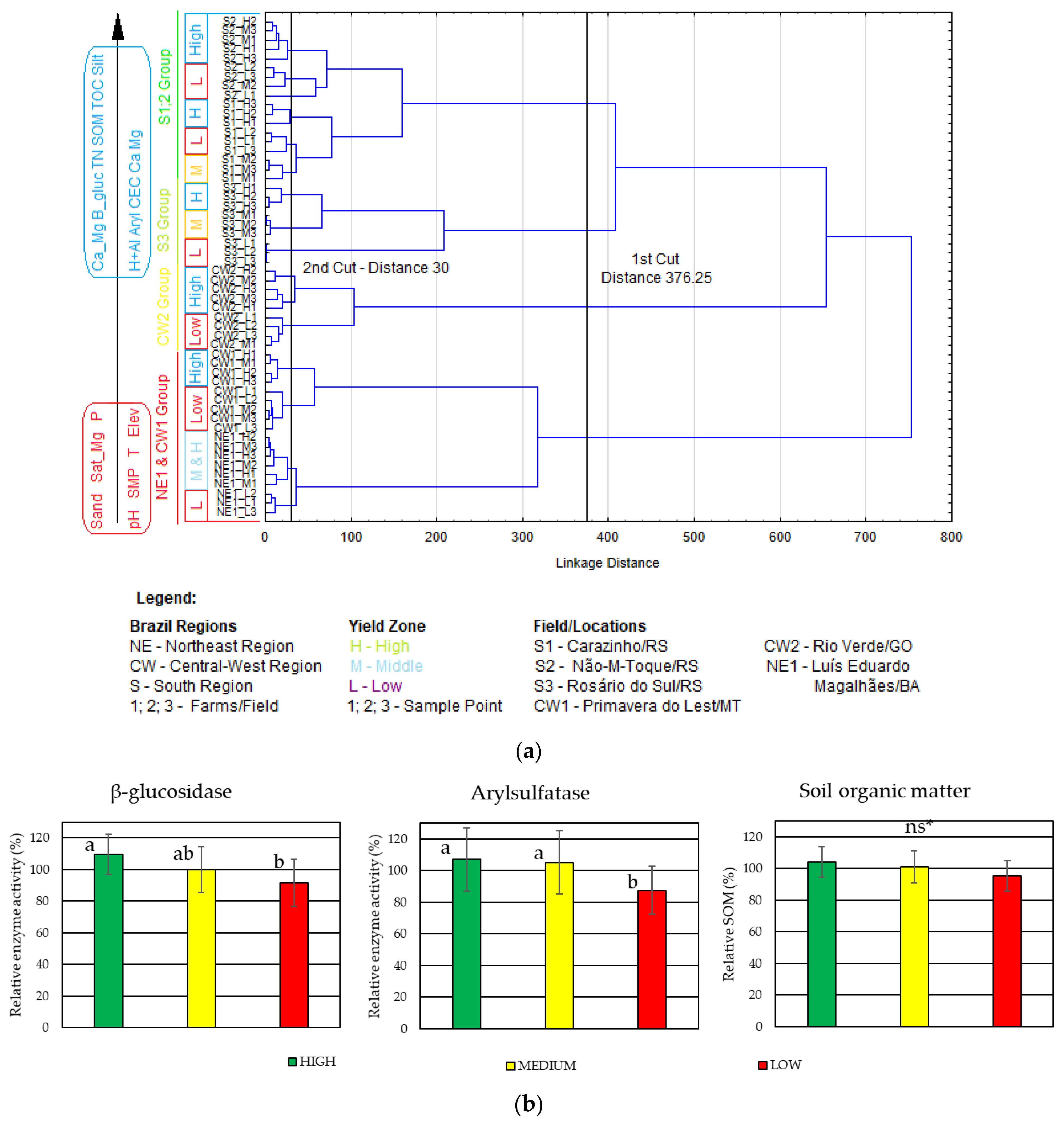
3.1. Characterization of Soil Attributes, Crop Yield and Enzyme Activity by Yield Environments in Selected Fields
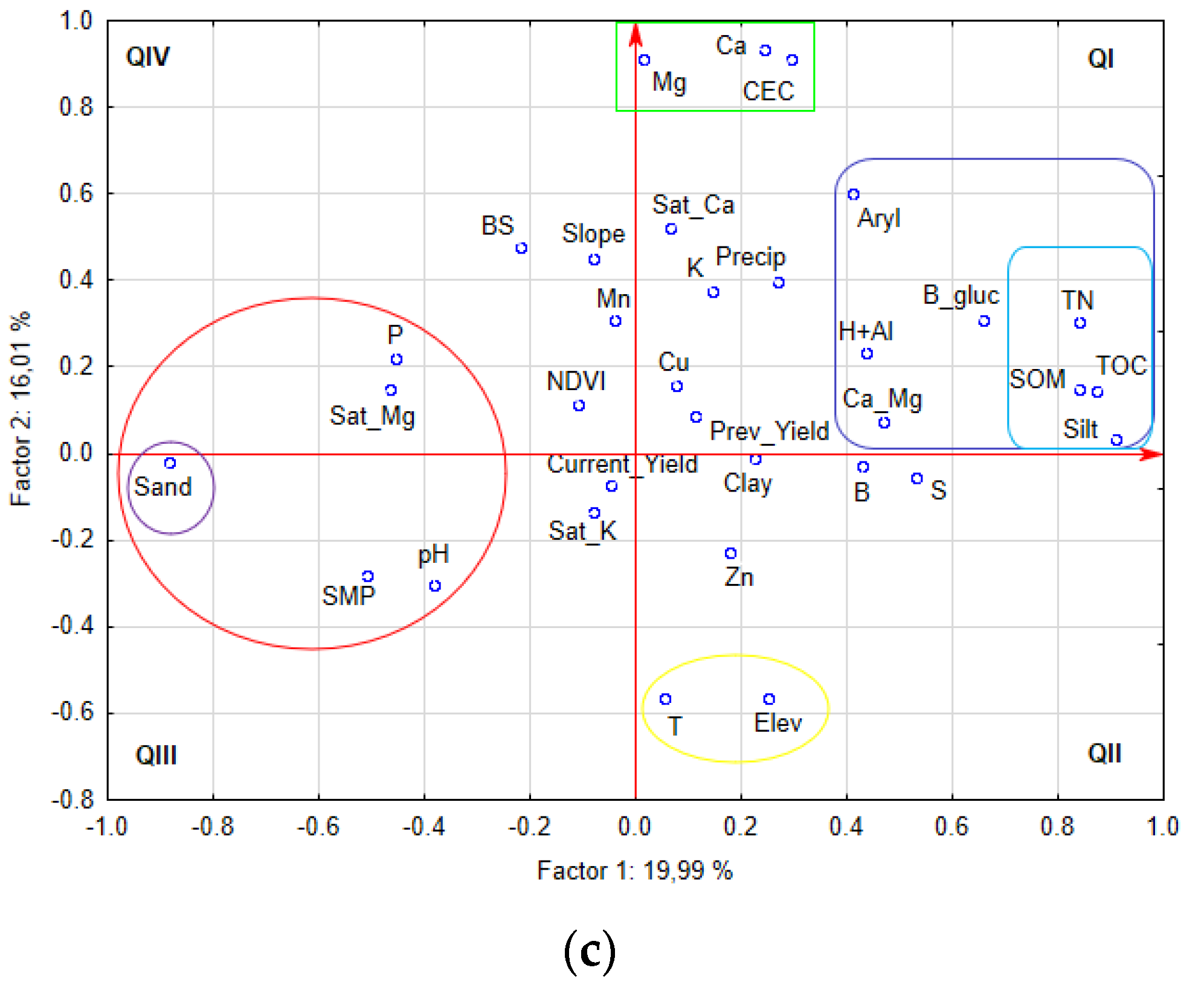
3.2. Soil Attributes by Fields in Agro-Ecoregion and Relationship with Soil Enzyme Activity
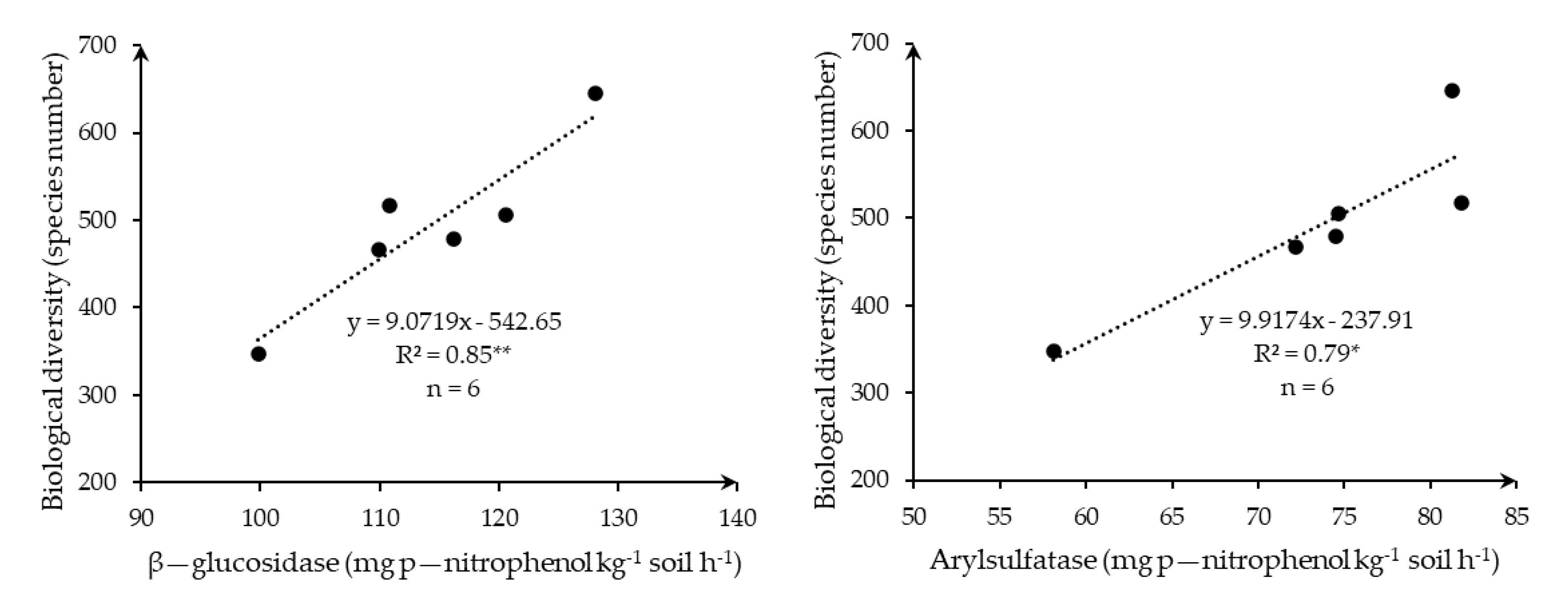
3.3. Enzyme Activity and Biodiversity under Varying Crop Yield Environments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kassam, A.; Friedrich, T.; Shaxson, F.; Pretty, J. The Spread of Conservation Agriculture: Justification, Sustainability and Uptake. Int. J. Agric. Sustain. 2009, 7, 292–320. [Google Scholar] [CrossRef]
- Shaxson, T.F. Re-Thinking the Conservation of Carbon, Water and Soil: A Different Perspective. Agron. Sustain. Dev. 2006, 26, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Leal, O.A.; Amado, T.J.C.; Fiorin, J.E.; Keller, C.; Reimche, G.B.; Rice, C.W.; Nicoloso, R.S.; Bortolotto, R.P.; Schwalbert, R. Linking Cover Crop Residue Quality and Tillage System to CO2-C Emission, Soil C and N Stocks and Crop Yield Based on a Long-Term Experiment. Agronomy 2020, 10, 1848. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Shah, K.K.; Tripathi, S.; Tiwari, I.; Shrestha, J.; Modi, B.; Paudel, N.; Das, B.D. Role of Soil Microbes in Sustainable Crop Production and Soil Health: A Review. AST 2021, 13, 109–118. [Google Scholar] [CrossRef]
- Kassam, A.; Friedrich, T.; Derpsch, R. Global Spread of Conservation Agriculture. Int. J. Environ. Stud. 2019, 76, 29–51. [Google Scholar] [CrossRef]
- Pires, C.A.B.; Amado, T.J.C.; Reimche, G.; Schwalbert, R.; Sarto, M.V.M.; Nicoloso, R.S.; Fiorin, J.E.; Rice, C.W. Diversified Crop Rotation with No-till Changes Microbial Distribution with Depth and Enhances Activity in a Subtropical Oxisol. Eur. J. Soil Sci. 2020, 71, 1173–1187. [Google Scholar] [CrossRef]
- Mendes, I.C.; Sousa, D.M.G.; Dantas, O.D.; Lopes, A.A.C.; Junior, F.B.R.; Oliveira, M.I.; Chaer, G.M. Soil Quality and Grain Yield: A Win–Win Combination in Clayey Tropical Oxisols. Geoderma 2021, 388, 114880. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and Assessing Soil Quality. In SSSA Special Publications; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1994; pp. 1–21. ISBN 978-0-89118-930-5. [Google Scholar]
- Garbisu, C.; Alkorta, I.; Epelde, L. Assessment of Soil Quality Using Microbial Properties and Attributes of Ecological Relevance. Appl. Soil Ecol. 2011, 49, 1–4. [Google Scholar] [CrossRef]
- Kremer, R.J. Biotechnology Impacts on Soil and Environmental Services. In Soil Health and Intensification of Agroecosytems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 353–375. ISBN 978-0-12-805317-1. [Google Scholar]
- Van Bruggen, A.H.C.; Semenov, A.M.; van Diepeningen, A.D.; de Vos, O.J.; Blok, W.J. Relation between Soil Health, Wave-like Fluctuations in Microbial Populations, and Soil-Borne Plant Disease Management. Eur. J. Plant Pathol. 2006, 115, 105–122. [Google Scholar] [CrossRef]
- Tripathi, S.; Srivastava, P.; Devi, R.S.; Bhadouria, R. Influence of synthetic fertilizers and pesticides on soil health and soil microbiology. In Agrochemicals Detection, Treatment and Remediation: Pesticides and Chemical Fertilizers; Prasad, M.N.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 25–54. ISBN 978-0-08-103017-2. [Google Scholar]
- Khan, N.; Bano, A.M.D.; Babar, A. Impacts of Plant Growth Promoters and Plant Growth Regulators on Rainfed Agriculture. PLoS ONE 2020, 15, e0231426. [Google Scholar] [CrossRef] [Green Version]
- Mendes, I.C.; Souza, D.M.G.; Reis Junior, F.B.; Lopes, A.A.C. Bioanálise de Solo: Como Acessar e Interpretar a Saúde Do Solo; Embrapa: Planaltina, Brazil, 2018. [Google Scholar]
- Van Bruggen, A.H.C.; Grünwald, N.J.; Bolda, M. Cultural Methods and Soil Nutrient Status in Low and High Input Agricultural Systems, as They Affect Rhizoctonia Species. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 407–421. ISBN 978-90-481-4597-3. [Google Scholar]
- Toor, M.D.; Adnan, M. Role of Soil Microbes in Agriculture, A Review. J. Biog. Sci. Res. 2020. [Google Scholar] [CrossRef]
- De Carvalho Mendes, I.; de Souza, L.M.; de Sousa, D.M.G.; de Castro Lopes, A.A.; dos Reis Junior, F.B.; Lacerda, M.P.C.; Malaquias, J.V. Critical Limits for Microbial Indicators in Tropical Oxisols at Post-Harvest: The FERTBIO Soil Sample Concept. Appl. Soil Ecol. 2019, 139, 85–93. [Google Scholar] [CrossRef]
- Alves de Castro Lopes, A.; Gomes de Sousa, D.M.; Chaer, G.M.; Bueno dos Reis Junior, F.; Goedert, W.J.; de Carvalho Mendes, I. Interpretation of Microbial Soil Indicators as a Function of Crop Yield and Organic Carbon. Soil Sci. Soc. Am. J. 2013, 77, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Mendes, I.C.; Tormena, C.A.; Cherubin, M.R.; Karlen, D.L. Soil health assessment and maintenance in Central and South-Central Brazil. In Burleigh Dodds Series in Agricultural Science; Reicosky, D., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2018; Volume 2, pp. 379–415. ISBN 978-1-78676-192-7. [Google Scholar]
- Lopes, A.A.C.; Sousa, D.M.G.; dos Reis, F.B.; Figueiredo, C.C.; Malaquias, J.V.; Souza, L.M.; Mendes, I.C. Temporal Variation and Critical Limits of Microbial Indicators in Oxisols in the Cerrado, Brazil. Geoderma Reg. 2018, 12, 72–82. [Google Scholar] [CrossRef]
- Pott, L.P.; Amado, T.J.C.; Schwalbert, R.A.; Gebert, F.H.; Reimche, G.B.; Pes, L.Z.; Ciampitti, I.A. Effect of Hairy Vetch Cover Crop on Maize Nitrogen Supply and Productivity at Varying Yield Environments in Southern Brazil. Sci. Total Environ. 2021, 759, 144313. [Google Scholar] [CrossRef]
- Pott, L.P.; Amado, T.J.C.; Leal, O.A.; Ciampitti, I.A. Mitigation of Soil Compaction for Boosting Crop Productivity at Varying Yield Environments in Southern Brazil. Eur. J. Soil Sci. 2019, 71, 1157–1172. [Google Scholar] [CrossRef]
- Corassa, G.M.; Santi, A.L.; Amado, T.J.C.; Reimche, G.B.; Gaviraghi, R.; Bisognin, M.B.; Pires, J.L.F. Performance of Soybean Varieties Differs According to Yield Class: A Case Study from Southern Brazil. Precis. Agric. 2019, 20, 520–540. [Google Scholar] [CrossRef]
- Schwalbert, R.A.; Amado, T.J.C.; Reimche, G.B.; Gebert, F. Fine-Tuning of Wheat (Triticum aestivum L.) Variable Nitrogen Rate by Combining Crop Sensing and Management Zones Approaches in Southern Brazil. Precis. Agric. 2019, 20, 56–77. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; Soil Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2. Microbiological and Biochemical Properties; Soil Science Society of America: Madison, WI, USA, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Embrapa. Manual de Métodos de Análise de Solo, 2nd ed.; Embrapa Solos: Rio de Janeiro, Brazil, 2011; Volume 1. [Google Scholar]
- Tedesco, M.J.; Gianello, C.; Bohnen, H.; Volkweiss, S.J. Análises de Solo, Plantas e Outros Materiais, 2nd ed.; Universidade Federal do Rio Grande do Sul: Porto Alegre, Brazil, 1995. [Google Scholar]
- Teixeira, P.C.; Donagemma, G.K.; Fontana, A.; Teixeira, W.G. Manual de Métodos de Análise de Solo, 3rd ed.; Embrapa: Brasília, Brazil, 2017. [Google Scholar]
- Imam, N.; Belda, I.; Duehl, A.J.; Doroghazi, J.R.; Almonacid, D.E.; Thomas, V.P.; Acedo, A. Soil Microbial Composition and Structure Allow Assessment of Biological Product Effectiveness and Crop Yield Prediction. BioRxiv 2021. [Google Scholar] [CrossRef]
- Vicini, L.; Souza, A.M.; Morales, F.E.C.; Souza, F.M. Técnicas Multivariadas: Teorias e Aplicações No Software Statistica; Editora da UFSM: Santa Maria, CA, USA, 2018. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J.; Anderson, R.E.; Tathan, R.L. Análise Multivariada de Dados, 5th ed.; Bookmam: Porto Alegre, Brazil, 2005. [Google Scholar]
- Ji, B.; Hu, H.; Zhao, Y.; Mu, X.; Liu, K.; Li, C. Effects of Deep Tillage and Straw Returning on Soil Microorganism and Enzyme Activities. Sci. World J. 2014, 2014, 451493. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.T.; Anderson, R.V.; Coleman, D.C.; Cole, C.V. Habitable Pore Space and Microbial Trophic Interactions. Oikos 1980, 35, 327. [Google Scholar] [CrossRef]
- Alvarez, G.; Chaussod, R.; Cluzeau, D. Biological Activities and Soil Fertility, Interest and Limitations of Analytical Methods Available, 1st ed.; Itab: London, UK, 2002. [Google Scholar]
- Soares, M.R.; Alleoni, L.R.F.; Vidal-Torrado, P.; Cooper, M. Mineralogy and Ion Exchange Properties of the Particle Size Fractions of Some Brazilian Soils in Tropical Humid Areas. Geoderma 2005, 125, 355–367. [Google Scholar] [CrossRef]
- Bayer, C.; Mielniczuk, J.; Amado, T.J.C.; Martin-Neto, L.; Fernandes, S.V. Organic Matter Storage in a Sandy Clay Loam Acrisol Affected by Tillage and Cropping Systems in Southern Brazil. Soil Tillage Res. 2000, 54, 101–109. [Google Scholar] [CrossRef]
- De Oliveira Ferreira, A.; Amado, T.J.C.; Rice, C.W.; Ruiz Diaz, D.A.; Briedis, C.; Inagaki, T.M.; Gonçalves, D.R.P. Driving Factors of Soil Carbon Accumulation in Oxisols in Long-Term No-till Systems of South Brazil. Sci. Total Environ. 2018, 622–623, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yu, G.; Zhang, X.; Ge, J.; He, N.; Wang, Q.; Wang, D. The Variations in Soil Microbial Communities, Enzyme Activities and Their Relationships with Soil Organic Matter Decomposition along the Northern Slope of Changbai Mountain. Appl. Soil Ecol. 2015, 86, 19–29. [Google Scholar] [CrossRef]
- Thor, K. Calcium—Nutrient and Messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Dalla Nora, D.; Amado, T.J.C.; Bortolotto, R.P.; Ferreira, A.O.; Reichardt, K.; Santi, A.L. Subsoil Chemical Amelioration and Crop Yields under Continuous Long-Term No-till in a Subtropical Oxisol. Afr. J. Agric. Res. 2014, 9, 3338–3349. [Google Scholar]
- Mankolo, R.; Reddy, C.; Senwo, Z.; Nyakatawa, E.; Sajjala, S. Soil Biochemical Changes Induced by Poultry Litter Application and Conservation Tillage under Cotton Production Systems. Agronomy 2012, 2, 187–198. [Google Scholar] [CrossRef] [Green Version]
- Tiecher, T.; Martins, A.P.; Peretto, E.J.S.; Fink, J.R.; Santos, L.S.; Denardin, L.G.O.; Tiecher, T.L. Evolução e Estado Da Fertilidade Do Solo No Norte Do Rio Grande Do Sul e Sudoeste de Santa Catarina; UFRGS: Porto Alegre, Brazil, 2016. [Google Scholar]
- Bayer, C.; Dieckow, J.; Amado, T.J.C.; Eltz, F.L.F.; Vieira, F.C.B. Cover Crop Effects Increasing Carbon Storage in a Subtropical No-Till Sandy Acrisol. Commun. Soil Sci. Plant Anal. 2009, 40, 1499–1511. [Google Scholar] [CrossRef]
- FAO. State of Knowledge of Soil Biodiversity-Status, Challenges and Potentialities; FAO: Rome, Italy, 2020; ISBN 978-92-5-133582-6. [Google Scholar]
- Bardgett, R.D.; Hobbs, P.J.; Frostegard, A. Changes in Soil Fungal:Bacterial Biomass Ratios Following Reductions in the Intensity of Management of an Upland Grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Lange, M.; Habekost, M.; Eisenhauer, N.; Roscher, C.; Bessler, H.; Engels, C.; Oelmann, Y.; Scheu, S.; Wilcke, W.; Schulze, E.-D.; et al. Biotic and Abiotic Properties Mediating Plant Diversity Effects on Soil Microbial Communities in an Experimental Grassland. PLoS ONE 2014, 9, e96182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Chen, H.Y.H.; Chen, X.; Huang, Z. Meta-Analysis Shows Positive Effects of Plant Diversity on Microbial Biomass and Respiration. Nat. Commun. 2019, 10, 1332. [Google Scholar] [CrossRef] [Green Version]
- De Vargas, R.L.; Schuch, L.O.B.; Barros, W.S.; Rigo, G.A.; Szareski, V.J.; Carvalho, I.R.; Pimentel, J.R.; Troyjack, C.; Jaques, L.B.A.; de Souza, V.Q.; et al. Macronutrients and Micronutrients Variability in Soybean Seeds. JAS 2018, 10, 209. [Google Scholar] [CrossRef] [Green Version]
- Dalla Nora, D.; Amado, T.J.C. Improvement in Chemical Attributes of Oxisol Subsoil and Crop Yields under No-Till. Agron. J. 2013, 105, 1393–1403. [Google Scholar] [CrossRef]
- Hansel, F.D.; Amado, T.J.C.; Bortolotto, R.P.; Trindade, B.S.; Hansel, D.S.S. Influence of Different Phosphorus Sources on Fertilization Efficiency. Rev. Bras. Tecnol. Apl. Ciênc. Agrár. 2014, 7, 103–111. [Google Scholar] [CrossRef]
- De Souza Nunes, R.; de Sousa, D.M.G.; Goedert, W.J.; de Oliveira, L.E.Z.; Pavinato, P.S.; Pinheiro, T.D. Distribution of Soil Phosphorus Fractions as a Function of Long-Term Soil Tillage and Phosphate Fertilization Management. Front. Earth Sci. 2020, 8, 350. [Google Scholar] [CrossRef]
- Hansel, F.D.; Amado, T.J.C.; Ruiz Diaz, D.A.; Rosso, L.H.M.; Nicoloso, F.T.; Schorr, M. Phosphorus Fertilizer Placement and Tillage Affect Soybean Root Growth and Drought Tolerance. Agron. J. 2017, 109, 2936–2944. [Google Scholar] [CrossRef] [Green Version]
- Bao, Z.; Matsushita, Y.; Morimoto, S.; Hoshino, Y.T.; Suzuki, C.; Nagaoka, K.; Takenaka, M.; Murakami, H.; Kuroyanagi, Y.; Urashima, Y.; et al. Decrease in Fungal Biodiversity along an Available Phosphorous Gradient in Arable Andosol Soils in Japan. Can. J. Microbiol. 2013, 59, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Liu, N.; Lu, W.; Wang, S.; Kan, H.; Zhang, Y.; Xu, L.; Chen, Y. The Interaction between Arbuscular Mycorrhizal Fungi and Soil Phosphorus Availability Influences Plant Community Productivity and Ecosystem Stability. J. Ecol. 2014, 102, 1072–1082. [Google Scholar] [CrossRef]
- Treseder, K.K. A Meta-analysis of Mycorrhizal Responses to Nitrogen, Phosphorus, and Atmospheric CO2 in Field Studies. New Phytol. 2004, 164, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu, C.H., Jr.; Muraoka, T.; Lavorante, A.F. Relationship between Acidity and Chemical Properties of Brazilian Soils. Sci. Agric. 2003, 60, 337–343. [Google Scholar] [CrossRef]
- Msimbira, L.A.; Smith, D.L. The Roles of Plant Growth Promoting Microbes in Enhancing Plant Tolerance to Acidity and Alkalinity Stresses. Front. Sustain. Food Syst. 2020, 4, 106. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a pH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Shen, C.; Shi, Y.; Fan, K.; He, J.-S.; Adams, J.M.; Ge, Y.; Chu, H. Soil pH Dominates Elevational Diversity Pattern for Bacteria in High Elevation Alkaline Soils on the Tibetan Plateau. FEMS Microbiol. Ecol. 2019, 95, fiz003. [Google Scholar] [CrossRef] [Green Version]
- Stark, S.; Männistö, M.K.; Eskelinen, A. Nutrient Availability and pH Jointly Constrain Microbial Extracellular Enzyme Activities in Nutrient-Poor Tundra Soils. Plant Soil 2014, 383, 373–385. [Google Scholar] [CrossRef]
- Donagemma, G.K.; de Freitas, P.L.; de Carvalho Balieiro, F.; Fontana, A.; Spera, S.T.; Lumbreras, J.F.; Viana, J.H.M.; de Araújo Filho, J.C.; dos Santos, F.C.; de Albuquerque, M.R.; et al. Characterization, Agricultural Potential, and Perspectives for the Management of Light Soils in Brazil. Pesq. Agropec. Bras. 2016, 51, 1003–1020. [Google Scholar] [CrossRef] [Green Version]
- Roques, S.; Kendall, S.; Smith, K.; Newell Price, P.; Berry, P. Review of the Non-NPKS Nutrient Requirements of UK Cereals and Oilseed Rape. HGCA Res. Rev. 2013, 78, 9–108. [Google Scholar]
- García-Mina, J.M.; Antolín, M.C.; Sanchez-Diaz, M. Metal-Humic Complexes and Plant Micronutrient Uptake: A Study Based on Different Plant Species Cultivated in Diverse Soil Types. Plant Soil 2004, 258, 57–68. [Google Scholar] [CrossRef]
- Alejandro, S.; Höller, S.; Meier, B.; Peiter, E. Manganese in Plants: From Acquisition to Subcellular Allocation. Front. Plant Sci. 2020, 11, 300. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, N.; McSpadden Gardener, B.B.; Lee, N.R.; Ramsier, C.; Dick, R.P. Soil Enzyme Activities Associated with Differential Outcomes of Contrasting Approaches to Soil Fertility Management in Corn and Soybean Fields. AEES 2020, 8, 517–525. [Google Scholar] [CrossRef]
- Bai, X.; Dippold, M.A.; An, S.; Wang, B.; Zhang, H.; Loeppmann, S. Extracellular Enzyme Activity and Stoichiometry: The Effect of Soil Microbial Element Limitation during Leaf Litter Decomposition. Ecol. Indic. 2021, 121, 107200. [Google Scholar] [CrossRef]
- Stout, J.D. The Role of Protozoa in Nutrient Cycling and Energy Flow. In Advances in Microbial Ecology; Alexander, M., Ed.; Springer: Boston, MA, USA, 1980; Volume 4. [Google Scholar]
- Clarholm, M. Interactions of Bacteria, Protozoa and Plants Leading to Mineralization of Soil Nitrogen. Soil Biol. Biochem. 1985, 17, 181–187. [Google Scholar] [CrossRef]
- Ozimek, E.; Hanaka, A. Mortierella Species as the Plant Growth-Promoting Fungi Present in the Agricultural Soils. Agriculture 2020, 11, 7. [Google Scholar] [CrossRef]
- Glick, B.R.; Todorovic, B.; Czarny, J.; Cheng, Z.; Duan, J.; McConkey, B. Promotion of Plant Growth by Bacterial ACC Deaminase. Crit. Rev. Plant Sci. 2007, 26, 227–242. [Google Scholar] [CrossRef]
- De Souza, R.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria as Inoculants in Agricultural Soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kang, S.-M.; Baek, I.-Y.; Lee, I.-J. Characterization of Plant Growth-Promoting Traits of Penicillium Species against the Effects of High Soil Salinity and Root Disease. J. Plant Interact. 2014, 9, 754–762. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced Systemic Resistance and Plant Responses to Fungal Biocontrol Agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [Green Version]
- Ethur, L.Z.; Blume, E.; Muniz, M.F.B.; Flores, M.G.V. Seleção de Antagonistas Fúngicos a Fusarium Solani e Fusarium Oxysporum Em Substrato Comercial Para Mudas. Cienc. Rural 2007, 37, 1801–1804. [Google Scholar] [CrossRef] [Green Version]
- Vanlauwe, B.; Hungria, M.; Kanampiu, F.; Giller, K.E. The Role of Legumes in the Sustainable Intensification of African Smallholder Agriculture: Lessons Learnt and Challenges for the Future. Agric. Ecosyst. Environ. 2019, 284, 106583. [Google Scholar] [CrossRef]
- Leandro, L.F.S.; Eggenberger, S.; Chen, C.; Williams, J.; Beattie, G.A.; Liebman, M. Cropping System Diversification Reduces Severity and Incidence of Soybean Sudden Death Syndrome Caused by Fusarium virguliforme. Plant Dis. 2018, 102, 1748–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.S.; Matos, K.S.; Tessmann, D.J.; Seixas, C.D.S.; Pfenning, L.H. Fusarium paranaense sp. nov., a Member of the Fusarium solani Species Complex Causes Root Rot on Soybean in Brazil. Fungal Biol. 2016, 120, 51–60. [Google Scholar] [CrossRef]
- Ranzi, C.; Camera, J.N.; Deuner, C.C. Influence of Continuous Cropping on Corn and Soybean Pathogens. Summa Phytopathol. 2017, 43, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Hartman, G.L.; Leandro, L.F.; Rupe, J.C. Sudden death syndrome. In Compendium of Soybean Diseases and Pests; Hartman, G.L., Rupe, J.C., Sikora, E.J., Domier, L.L., Davis, J.A., Steffey, K.L., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2015; pp. 88–90. [Google Scholar]
- Alam, S.S.; Sakamoto, K.; Inubushi, K. Biocontrol Efficiency of Fusarium Wilt Diseases by a Root-Colonizing Fungus Penicillium sp. Soil Sci. Plant Nutr. 2011, 57, 204–212. [Google Scholar] [CrossRef]
- Hariprasad, P.; Divakara, S.T.; Niranjana, S.R. Isolation and Characterization of Chitinolytic Rhizobacteria for the Management of Fusarium Wilt in Tomato. Crop Prot. 2011, 30, 1606–1612. [Google Scholar] [CrossRef]
- Saikia, R.; Singh, K.; Arora, D.K. Suppression of Fusarium Wilt and Charcoal Rot of Chickpea by Pseudomonas Aeruginosa RsB29. Indian J. Microbiol. 2006, 44, 181–184. [Google Scholar]
- Dimkpa, C.O.; Merten, D.; Svatoš, A.; Büchel, G.; Kothe, E. Siderophores Mediate Reduced and Increased Uptake of Cadmium by Streptomyces tendae F4 and Sunflower (Helianthus annuus), Respectively. J. Appl. Microbiol. 2009, 107, 1687–1696. [Google Scholar] [CrossRef]
- Grover, M.; Ali, S.Z.; Sandhya, V.; Rasul, A.; Venkateswarlu, B. Role of Microorganisms in Adaptation of Agriculture Crops to Abiotic Stresses. World J. Microbiol. Biotechnol. 2011, 27, 1231–1240. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Girardello, V.C.; Amado, T.J.C.; Santi, A.L.; Cherubin, M.R.; Kunz, J.; de Gregori Teixeira, T. Resistência à Penetração, Eficiência de Escarificadores Mecânicos e Produtividade Da Soja Em Latossolo Argiloso Manejado Sob Plantio Direto de Longa Duração. Rev. Bras. Ciênc. Solo 2014, 38, 1234–1244. [Google Scholar] [CrossRef]
- Cruz, D.R.; Leandro, L.F.S.; Mayfield, D.A.; Meng, Y.; Munkvold, G.P. Effects of Soil Conditions on Root Rot of Soybean Caused by Fusarium graminearum. Phytopathology 2020, 110, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Leandro, L.F.S.; Robertson, A.E.; Mueller, D.S.; Yang, X.-B. Climatic and Environmental Trends Observed During Epidemic and Non-Epidemic Years of Soybean Sudden Death Syndrome in Iowa. Plant Health Progress 2013, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Scherm, H.; Yang, X. Development of Sudden Death Syndrome of Soybean in Relation to Soil Temperature and Soil Water Matric Potential. Phytopathology 1996, 86, 642. [Google Scholar] [CrossRef]






| Field | Localization | Area | T | P | E | Soil Texture 1 | U.S. Soil Taxonomy 1 |
|---|---|---|---|---|---|---|---|
| (City-State) | (ha) | (°C) | (mm y–1) | (m) | |||
| S-1 | Carazinho-RS | 60.1 | 18.3 | 1856 | 565 | Clay loam | Typic Hapludox |
| S-2 | Não-Me-Toque-RS | 136.0 | 19.0 | 1771 | 500 | Clay loam | Typic Hapludox |
| S-3 | Rosário do Sul-RS | 25.0 | 19.5 | 1493 | 155 | Sandy loam | Paleudalf |
| CW-1 | Primavera do Leste-MT | 348.8 | 24.0 | 1471 | 650 | Sandy clay loam | Hapludox |
| CW-2 | Rio Verde-GO | 509.8 | 23.1 | 1294 | 875 | Clay loam | Hapludox |
| NE-1 | Luís Eduardo Magalhães-BA | 1376.1 | 23.6 | 881 | 830 | Sandy clay loam | Hapludox |
| NE-2 | Luís Eduardo Magalhães-BA | 690.9 | 25.0 | 1089 | 880 | Sandy clay loam | Hapludox |
| Field | YE | P | K+ | S | Al3+ | Ca2+ | Mg2+ | CEC | Ca2+/Mg2+ | BS |
|---|---|---|---|---|---|---|---|---|---|---|
| (mg dm−3) | (cmolc dm−3) | (%) | ||||||||
| S-1 | H | 35.5 ± 8.3 | 308.3 ± 35.8 | 28.2 ± 15.4 | 0.0 ± 0.0 | 6.9 ± 2.0 | 3.2 ± 0.8 | 13.5 ± 2.7 | 2.2 ± 0.1 | 79.9 ± 3.3 |
| M | 39.1 ± 8.5 | 327.3 ± 59.0 | 14.7 ± 2.8 | 0.0 ± 0.0 | 5.7 ± 1.2 | 2.3 ± 0.4 | 11.2 ± 1.9 | 2.5 ± 0.4 | 79.0 ± 0.5 | |
| L | 56.8 ± 13.2 | 205.3 ± 48.6 | 11.6 ± 0.4 | 0.0 ± 0.0 | 4.7 ± 0.9 | 1.9 ± 0.5 | 9.2 ± 1.6 | 2.4 ± 0.1 | 78.0 ± 1.5 | |
| S-2 | H | 41.3 ± 5.9 | 385.3 ± 66.4 | 6.7 ± 1.4 | 0.0 ± 0.0 | 5.8 ± 1.3 | 1.9 ± 0.3 | 11.5 ± 1.7 | 3.0 ± 1.0 | 75.9 ± 5.5 |
| M | 37.4 ± 4.1 | 293.3 ± 19.9 | 8.1 ± 0.7 | 0.0 ± 0.0 | 5.5 ± 0.5 | 1.5 ± 0.2 | 11.3 ± 0.3 | 3.6 ± 0.3 | 69.0 ± 5.5 | |
| L | 53.2 ± 37.8 | 283.7 ± 22.0 | 9.5 ± 0.4 | 0.0 ± 0.0 | 5.4 ± 1.2 | 1.8 ± 0.4 | 11.4 ± 0.7 | 3.0 ± 0.3 | 69.3 ± 5.0 | |
| S-3 | H | 61.3 ± 5.9 | 236.3 ± 3.8 | 7.9 ± 3.1 | 0.0 ± 0.0 | 9.9 ± 2.5 | 3.1 ± 0.6 | 16.2 ± 3.3 | 3.2 ± 0.0 | 83.7 ± 2.3 |
| M | 35.8 ± 0.6 | 125.7 ± 2.3 | 5.9 ± 0.8 | 0.0 ± 0.0 | 8.0 ± 1.3 | 2.9 ± 0.4 | 12.7 ± 1.7 | 2.8 ± 0.1 | 88.4 ± 1.2 | |
| L | 76.7 ± 3.9 | 246.3 ± 9.1 | 17.5 ± 4.5 | 0.0 ± 0.0 | 3.6 ± 0.2 | 1.4 ± 0.1 | 6.8 ± 0.3 | 2.5 ± 0.0 | 82.1 ± 0.5 | |
| CW-1 | H | 24.2 ± 7.5 | 160.3 ± 30.1 | 18.6 ± 2.9 | 0.0 ± 0.0 | 3.3 ± 0.1 | 1.4 ± 0.1 | 8.0 ± 0.4 | 2.3 ± 0.1 | 65.4 ± 5.2 |
| M | 22.1 ± 7.9 | 149.0 ± 12.8 | 16.2 ± 10.4 | 0.0 ± 0.0 | 4.0 ± 0.5 | 1.7 ± 0.3 | 8.4 ± 0.4 | 2.3 ± 0.3 | 72.0 ± 6.8 | |
| L | 18.4 ± 9.6 | 140.0 ± 12.8 | 13.1 ± 3.4 | 0.0 ± 0.0 | 3.9 ± 0.3 | 1.8 ± 0.3 | 8.3 ± 0.6 | 2.2 ± 0.4 | 73.2 ± 6.6 | |
| CW-2 | H | 21.1 ± 9.6 | 135.0 ± 48.6 | 32.4 ± 16.4 | 0.0 ± 0.0 | 5.2 ± 1.7 | 1.2 ± 0.3 | 9.7 ± 1.9 | 4.5 ± 0.4 | 68.6 ± 8.2 |
| M | 19.3 ± 5.7 | 142.0 ± 18.4 | 33.2 ± 16.1 | 0.0 ± 0.0 | 5.0 ± 1.2 | 1.2 ± 0.3 | 9.9 ± 1.6 | 4.1 ± 0.1 | 66.4 ± 6.0 | |
| L | 16.2 ± 8.2 | 178.3 ± 55.5 | 59.1 ± 18.5 | 0.0 ± 0.0 | 5.7 ± 1.1 | 1.4 ± 0.3 | 10.0 ± 0.9 | 4.0 ± 0.5 | 74.9 ± 5.3 | |
| NE-1 | H | 28.7 ± 6.4 | 88.3 ± 8.3 | 8.9 ± 0.7 | 0.0 ± 0.0 | 2.7 ± 0.8 | 1.2 ± 0.4 | 5.2 ± 1.0 | 2.3 ± 0.1 | 78.8 ± 6.7 |
| M | 40.4 ± 15.5 | 60.3 ± 5.1 | 8.1 ± 0.6 | 0.0 ± 0.0 | 3.0 ± 0.7 | 1.2 ± 0.2 | 5.5 ± 0.8 | 2.4 ± 0.2 | 79.2 ± 2.4 | |
| L | 45.4 ± 10.7 | 87.3 ± 23.7 | 7.9 ± 1.6 | 0.0 ± 0.0 | 2.7 ± 0.5 | 1.1 ± 0.3 | 5.1 ± 0.8 | 2.5 ± 0.2 | 77.6 ± 5.1 | |
| NE-2 | H | 48.4 ± 8.5 | 148.0 ± 34.0 | 36.6 ± 7.6 | 0.0 ± 0.0 | 3.2 ± 0.6 | 0.9 ± 0.3 | 5.6 ± 0.9 | 3.7 ± 0.2 | 79.2 ± 4.6 |
| M | 62.5 ± 16.7 | 157.3 ± 38.8 | 65.6 ± 37.8 | 0.0 ± 0.0 | 2.8 ± 0.2 | 0.6 ± 0.2 | 4.9 ± 0.4 | 4.7 ± 0.3 | 77.9 ± 2.1 | |
| L | 54.3 ± 25.0 | 147.3 ± 54.6 | 39.6 ± 15.7 | 0.0 ± 0.0 | 2.4 ± 0.6 | 0.5 ± 0.2 | 4.3 ± 1.0 | 4.7 ± 0.3 | 74.0 ± 5.1 | |
| Field | YE | pH | H + Al3+ | Sand | Silt | Clay | Zn2+ | Cu2+ | B | Mn2+ |
|---|---|---|---|---|---|---|---|---|---|---|
| (cmolc dm−3) | (%) | (mg dm−3) | ||||||||
| S-1 | H | 6.1 ± 0.1 | 2.7 ± 0.2 | 43.2 ± 5.2 | 18.5 ± 4.1 | 38.3 ± 1.2 | 4.3 ± 0.8 | 3.4 ± 0.5 | 0.8 ± 0.1 | 7.0 ± 1.0 |
| M | 6.3 ± 0.3 | 2.3 ± 0.4 | 41.7 ± 1.0 | 23.3 ± 1.2 | 35.0 ± 2.0 | 4.5 ± 0.7 | 3.1 ± 0.2 | 0.6 ± 0.1 | 3.3 ± 2.1 | |
| L | 6.5 ± 0.1 | 2.0 ± 0.3 | 49.7 ± 15.1 | 22.3 ± 19.0 | 28.0 ± 5.0 | 5.5 ± 1.4 | 2.4 ± 0.1 | 0.7 ± 0.0 | 2.3 ± 0.6 | |
| S-2 | H | 6.2 ± 0.2 | 2.7 ± 0.6 | 36.7 ± 4.4 | 25.7 ± 4.7 | 37.7 ± 0.6 | 3.7 ± 0.7 | 2.5 ± 0.4 | 0.5 ± 0.1 | 7.0 ± 3.5 |
| M | 6.0 ± 0.2 | 3.5 ± 0.7 | 41.5 ± 4.5 | 19.2 ± 1.8 | 39.3 ± 3.5 | 4.1 ± 1.3 | 3.0 ± 0.9 | 0.4 ± 0.0 | 9.7 ± 2.9 | |
| L | 5.9 ± 0.3 | 3.5 ± 0.5 | 41.6 ± 11.6 | 18.7 ± 3.7 | 39.7 ± 10.2 | 3.3 ± 0.6 | 2.5 ± 1.0 | 0.4 ± 0.1 | 7.3 ± 2.1 | |
| S-3 | H | 6.0 ± 0.0 | 2.6 ± 0.1 | 61.9 ± 1.1 | 18.1 ± 2.8 | 20.0 ± 1.7 | 1.4 ± 0.3 | 0.3 ± 0.0 | 0.4 ± 0.1 | 3.7 ± 1.2 |
| M | 6.6 ± 0.1 | 1.4 ± 0.1 | 48.4 ± 1.9 | 33.3 ± 1.1 | 18.3 ± 1.2 | 1.1 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.1 | 1.7 ± 0.6 | |
| L | 6.4 ± 0.0 | 1.2 ± 0.0 | 78.1 ± 0.4 | 7.9 ± 1.4 | 14.0 ± 1.0 | 4.2 ± 0.3 | 0.8 ± 0.1 | 0.4 ± 0.0 | 5.3 ± 0.6 | |
| CW-1 | H | 6.2 ± 0.4 | 2.8 ± 0.6 | 51.9 ± 2.4 | 18.7 ± 3.0 | 29.3 ± 3.1 | 3.5 ± 0.7 | 0.8 ± 0.1 | 0.6 ± 0.2 | 1.3 ± 0.6 |
| M | 6.5 ± 0.3 | 2.3 ± 0.6 | 53.1 ± 3.6 | 20.9 ± 1.2 | 26.0 ± 3.0 | 3.8 ± 0.8 | 0.9 ± 0.2 | 0.7 ± 0.2 | 1.0 ± 0.0 | |
| L | 6.6 ± 0.3 | 2.2 ± 0.7 | 50.8 ± 1.6 | 21.2 ± 1.3 | 28.0 ± 2.6 | 3.2 ± 0.6 | 0.8 ± 0.2 | 0.5 ± 0.1 | 1.3 ± 0.6 | |
| CW-2 | H | 6.2 ± 0.3 | 3.0 ± 0.3 | 27.0 ± 2.4 | 45.3 ± 6.2 | 27.7 ± 3.8 | 7.5 ± 0.9 | 1.3 ± 0.2 | 0.7 ± 0.1 | 1.3 ± 0.6 |
| M | 6.0 ± 0.2 | 3.3 ± 0.3 | 23.8 ± 7.2 | 48.5 ± 10.1 | 27.7 ± 3.8 | 10.1 ± 6.6 | 1.5 ± 0.6 | 0.6 ± 0.1 | 2.0 ± 0.0 | |
| L | 6.2 ± 0.3 | 2.5 ± 0.3 | 16.7 ± 2.6 | 50.3 ± 3.1 | 33.0 ± 3.6 | 2.7 ± 0.8 | 0.9 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.0 | |
| NE-1 | H | 7.0 ± 0.5 | 1.1 ± 0.2 | 63.9 ± 0.5 | 10.1 ± 2.1 | 26.0 ± 1.7 | 4.0 ± 0.9 | 1.2 ± 0.7 | 0.5 ± 0.1 | 1.0 ± 0.0 |
| M | 6.9 ± 0.1 | 1.1 ± 0.1 | 65.9 ± 3.2 | 9.1 ± 5.1 | 25.0 ± 2.0 | 6.2 ± 3.0 | 1.6 ± 0.6 | 0.4 ± 0.1 | 1.0 ± 0.0 | |
| L | 7.0 ± 0.2 | 1.1 ± 0.1 | 58.3 ± 4.6 | 12.4 ± 3.6 | 29.3 ± 4.0 | 2.7 ± 1.0 | 0.7 ± 0.3 | 0.4 ± 0.0 | 1.0 ± 0.0 | |
| NE-2 | H | 6.5 ± 0.3 | 1.1 ± 0.3 | 69.5 ± 3.0 | 8.5 ± 3.0 | 22.0 ± 4.4 | 8.4 ± 0.2 | 2.3 ± 0.5 | 0.4 ± 0.0 | 2.7 ± 2.9 |
| M | 6.7 ± 0.2 | 1.1 ± 0.2 | 71.2 ± 4.0 | 5.4 ± 1.7 | 23.3 ± 4.0 | 8.0 ± 0.8 | 2.1 ± 0.4 | 0.4 ± 0.1 | 1.0 ± 0.0 | |
| L | 6.6 ± 0.1 | 1.1 ± 0.0 | 70.5 ± 0.4 | 5.8 ± 0.8 | 23.7 ± 1.2 | 8.1 ± 3.5 | 2.2 ± 0.4 | 0.4 ± 0.1 | 1.0 ± 0.0 | |
| Field | YE | Crop Yield | NDVI | β-Glucosidase | Arylsulfatase | SOM ¹ | TOC ² | TN ² | C/N |
|---|---|---|---|---|---|---|---|---|---|
| (kg ha−1) | (mg p-Nitrophenol kg−1 soil h−1) | (%) | (Mg ha–1) | ||||||
| S-1 | H | 5532 ± 1210 | 0.90 ± 0.02 | 209.8 ± 25.5 | 287.3 ± 8.1 | 3.2 ± 0.12 | 27.6 ± 1.97 | 2.36 ± 0.15 | 11.7 ± 0.23 |
| M | 4514 ± 287 | 0.73 ± 0.05 | 180.0 ± 23.7 | 259.5 ± 2.4 | 2.8 ± 0.38 | 23.8 ± 1.83 | 2.06 ± 0.17 | 11.6 ± 017 | |
| L | 4582 ± 288 | 0.43 ± 0.10 | 205.2 ± 7.8 | 235.9 ± 2.7 | 2.7 ± 0.06 | 19.8 ± 1.15 | 1.71 ± 0.10 | 11.5 ± 0.26 | |
| S-2 | H | 6120 ± 106 | 0.88 ± 0.03 | 210.5 ± 18.3 | 253.5 ± 17.1 | 3.3 ± 0.00 | 26.5 ± 1.60 | 2.25 ± 0.16 | 11.8 ± 0.15 |
| M | 5686 ± 733 | 0.74 ± 0.04 | 198.3 ± 27.3 | 240.4 ± 47.8 | 3.2 ± 0.32 | 26.7 ± 1.67 | 2.27 ± 0.29 | 11.8 ± 0.73 | |
| L | 5144 ± 268 | 0.31 ± 0.06 | 215.4 ± 10.8 | 249.8 ± 53.5 | 3.1 ± 0.25 | 25.4 ± 1.65 | 2.08 ± 0.18 | 12.2 ± 0.44 | |
| S-3 | H | 4530 ± 570 | 0.77 ± 0.08 | 213.0 ± 12.0 | 255.7 ± 54.8 | 2.7 ± 0.00 | 23.5 ± 0.42 | 2.14 ± 0.08 | 11.0 ± 0.37 |
| M | 3620 ± 151 | 0.55 ± 0.05 | 183.0 ± 4.3 | 222.5 ± 19.8 | 2.3 ± 0.06 | 20.8 ± 0.98 | 1.73 ± 0.04 | 12.0 ± 0.27 | |
| L | 3600 ± 60 | 0.38 ± 0.02 | 119.4 ± 5.9 | 78.6 ± 8.6 | 1.7 ± 0.00 | 15.1 ± 1.88 | 1.52 ± 0.18 | 9.9 ± 0.13 | |
| CW-1 | H | 3629 ± 158 | 0.69 ± 0.12 | 162.1 ± 16.6 | 91.8 ± 6.2 | 3.3 ± 0.06 | 27.7 ± 1.65 | 1.90 ± 0.15 | 14.6 ± 0.28 |
| M | 3627 ± 289 | 0.59 ± 0.12 | 136.7 ± 27.5 | 70.6 ± 6.7 | 3.1 ± 0.26 | 24.6 ± 1.96 | 1.75 ± 0.15 | 14.1 ± 0.11 | |
| L | 3932 ± 159 | 0.17 ± 0.08 | 130.0 ± 20.3 | 68.1 ± 7.8 | 3.1 ± 0.44 | 27.2 ± 1.37 | 1.87 ± 0.13 | 14.6 ± 0.40 | |
| CW-2 | H | 4711 ± 197 | 0.79 ± 0.13 | 207.4 ± 6.2 | 160.2 ± 12.2 | 3.9 ± 0.40 | 36.3 ± 5.13 | 2.66 ± 0.48 | 13.7 ± 0.63 |
| M | 4496 ± 360 | 0.55 ± 0.19 | 233.0 ± 29.5 | 199.3 ± 47.6 | 3.8 ± 0.12 | 35.3 ± 4.82 | 2.63 ± 0.39 | 13.4 ± 0.20 | |
| L | 4032 ± 393 | 0.28 ± 0.08 | 256.0 ± 12.9 | 232.7 ± 10.6 | 4.2 ± 0.47 | 36.9 ± 4.05 | 2.74 ± 0.37 | 13.5 ± 0.33 | |
| NE-1 | H | 5443 ± 193 | 0.87 ± 0.15 | 157.1 ± 1.2 | 49.4 ± 13.1 | 1.3 ± 0.10 | 12.1 ± 0.95 | 1.01 ± 0.14 | 12.1 ± 0.80 |
| M | 4956 ± 283 | 0.80 ± 0.18 | 140.4 ± 36.6 | 48.3 ± 12.9 | 1.7 ± 0.21 | 13.6 ± 2.20 | 1.10 ± 0.30 | 12.6 ± 1.56 | |
| L | 5042 ± 416 | 0.41 ± 0.04 | 112.8 ± 13.5 | 32.6 ± 6.8 | 1.5 ± 0.23 | 13.1 ± 3.83 | 0.85 ± 0.22 | 15.3 ± 0.73 | |
| NE-2 * | H | 11,333 ± 885 | 0.80 ± 0.03 | 133.3 ± 24.4 | 28.2 ± 3.3 | 1.5 ± 0.15 | 11.7 ± 2.45 | 0.89 ± 0.20 | 13.3 ± 0.79 |
| M | 12,442 ± 282 | 0.74 ± 0.03 | 121.8 ± 18.5 | 38.2 ± 12.3 | 1.4 ± 0.26 | 11.0 ± 3.27 | 0.80 ± 0.22 | 13.6 ± 0.50 | |
| L | 12,827 ± 558 | 0.53 ± 0.06 | 90.8 ± 11.4 | 32.3 ± 4.9 | 1.4 ± 0.29 | 12.9 ± 2.50 | 0.94 ± 0.23 | 13.9 ± 0.78 | |
| Fields in Agro-Ecoregion | SOM 1 | TN 2 | Sand | Silt | Clay | CEC | Ca2+ | |
|---|---|---|---|---|---|---|---|---|
| (%) | (Mg ha–1) | (%) | (cmolc dm–3) | |||||
| β-glucosidase (mg p-nitrophenol kg−1 soil h−1) | South | 0.78 ** | 0.72 ** | −0.61 ** | 0.39 * | 0.48 * | 0.49 * | 0.35 ns |
| Central-West | 0.83 ** | 0.80 ** | −0.91 ** | 0.85 ** | 0.43 ns | 0.58 * | 0.56 * | |
| Northeast | 0.67 ** | 0.36 ns | −0.07 ns | 0.24 ns | −0.13 ns | 0.31 ns | 0.20 ns | |
| Average | 0.77 ** | 0.81 ** | −0.76 ** | 0.70 ** | 0.41 ** | 0.67 ** | 0.59 ** | |
| Arylsulfatase (mg p-nitrophenol kg−1 soil h−1) | South | 0.79 ** | 0.70 ** | −0.72 ** | 0.35 ns | 0.67 ** | 0.55 ** | 0.38 ns |
| Central-West | 0.80 ** | 0.74 ** | −0.89 ** | 0.82 ** | 0.47 * | 0.51 * | 0.53 * | |
| Northeast | −0.13 ns | 0.19 ns | −0.08 ns | −0.06 ns | 0.18 ns | −0.14 ns | −0.18 ns | |
| Average | 0.65 ** | 0.73 ** | −0.64 ** | 0.49 ** | 0.53 ** | 0.82 ** | 0.72 ** | |
| SOM | South | - | 0.89 ** | −0.78 ** | 0.24 ns | 0.84 ** | 0.37 ns | 0.13 ns |
| Central-West | - | 0.92 ** | −0.83 ** | 0.81 ** | 0.26 ns | 0.72 ** | 0.75 ** | |
| Northeast | - | 0.61 ** | −0.29 ns | 0.30 ns | 0.13 ns | 0.54 * | 0.38 ns | |
| Average | - | 0.95 ** | −0.86 ** | 0.78 ** | 0.49 ** | 0.61 ** | 0.46 ** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passinato, J.H.; Amado, T.J.C.; Kassam, A.; Acosta, J.A.A.; Amaral, L.d.P. Soil Health Check-Up of Conservation Agriculture Farming Systems in Brazil. Agronomy 2021, 11, 2410. https://doi.org/10.3390/agronomy11122410
Passinato JH, Amado TJC, Kassam A, Acosta JAA, Amaral LdP. Soil Health Check-Up of Conservation Agriculture Farming Systems in Brazil. Agronomy. 2021; 11(12):2410. https://doi.org/10.3390/agronomy11122410
Chicago/Turabian StylePassinato, Jardel H., Telmo J. C. Amado, Amir Kassam, José A. A. Acosta, and Lúcio de P. Amaral. 2021. "Soil Health Check-Up of Conservation Agriculture Farming Systems in Brazil" Agronomy 11, no. 12: 2410. https://doi.org/10.3390/agronomy11122410
APA StylePassinato, J. H., Amado, T. J. C., Kassam, A., Acosta, J. A. A., & Amaral, L. d. P. (2021). Soil Health Check-Up of Conservation Agriculture Farming Systems in Brazil. Agronomy, 11(12), 2410. https://doi.org/10.3390/agronomy11122410






