The Effects of Reduced Mineral Fertilisation Combined with the Foliar Application of Biostimulants and Fertilisers on the Nutrition of Maiden Apple Trees and the Contents of Soil Nutrients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Biostimulants and Fertiliser Experiment
2.3. Chemical Analyses
2.4. Data Analysis
3. Results
3.1. Chemical Analysis of Soil Samples from Nursery with Maiden Apple Trees
3.2. Contents of Macro- and Micronutrients in Leaves of Maiden Apple Trees
4. Discussion
4.1. The Contents of Soluble Forms of Chemicals in Soil
4.2. The Contents of Macro- and Micronutrients in the Leaves of Maiden Apple Trees
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, S.; Neilsen, D.; Neilsen, G.H.; Fuchigami, L.H. Foliar N application reduces soil NO3–N leaching loss in apple orchards. Plant Soil 2005, 268, 357–366. [Google Scholar] [CrossRef]
- Khan, I.A.; Khatri, A.; Nizamani, G.S.; Siddiqui, M.A.; Raza, S.; Dahar, N. Effect of NPK fertilizers on the growth of sugarcane clone AEC 86347 developed at Nia. Tando Jam. Pak. J. Bot. 2005, 37, 355360. [Google Scholar] [CrossRef]
- Abdelaziz, M.; Pokluda, R.; Abdelwahab, M. Inflence of compost, microorganisms and NPK fertilizer upon growth, chemical composition and essential oil production of Rosmarinus officinalis L. Not. Bot. Hort. Agrobot. 2007, 35, 86–90. [Google Scholar] [CrossRef]
- Kang, M.S.; Aulakh, M.S.; Dhiman, J.S. Agricultural development in Punjab: Problems, possible solution and new initatives. Alumni Association, College of Agriculture, Punjab Agricultural University. Ludhiana 2008, 38, 26–34. [Google Scholar]
- Wójcik, P. Uptake of mineral nutrients from foliar fertilization. J. Fruit Ornam. Plant Res. 2004, 12, 201–218. [Google Scholar]
- Pfeiffer, B.; Eis, B.; Zimmer, J.; Fieger-Metag, N. Optimizing crop loading of apples and pears–results (foliar fertilizers, thinning). In Proceedings of the Ecofruit—13th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 18–20 February 2008; pp. 324–329. [Google Scholar]
- Naseri, L.; Arzani, K.; Babalar, M. Foliar boron, copper and manganese uptakes and cocentrations of apple leaves cv. Golden Delicious on M9 and B9 rootstocks. Acta Hortic. 2002, 594, 237–243. [Google Scholar]
- Amiri, M.E.; Fallahi, E.; Golchin, A. Influence of foliar and ground fertilization on yield, fruit quality, and soil, leaf, and fruit mineral nutrients in apple. J. Plant Nutr. 2008, 31, 515–525. [Google Scholar] [CrossRef]
- Karim, M.R.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Thalheimer, M.; Paoli, N. Effectiveness of various leaf-applied biostimulators on productivity and fruit quality of apple. Acta Hortic. 2002, 594, 335–339. [Google Scholar] [CrossRef]
- Trivedi, P.C. Advantage in Plant Physiology; International Publishing House New Delhi: New Delhi, India, 2006; pp. 213–215. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Mancuso, S.; Azzarello, E.; Mugnai, S.; Briand, X. Marine bioactive substances (IPA extract) improve foliar ion uptake and water stress tolerance in potted Vitis vinifera plants. Adv. Hortic. Sci. 2006, 20, 156–161. [Google Scholar]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Zodape, S.T.; Kawarkhe, V.J.; Patolia, J.S.; Warade, A.D. Effect of liquid seaweed fertilizer on yield and quality of okra (Abelmoschus esculentus L.). J. Sci. Ind. Res. 2008, 67, 1115–1117. [Google Scholar]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Turan, M.; Köse, C. Seaweed extracts improve copper uptake of grapevine. Acta Agric. Scand. Plant Sci. 2004, 54, 213–220. [Google Scholar] [CrossRef]
- Shehata, S.M.; Abdel-Azem, H.S.; Abou El-Yazied, A.; El-Gizawy, A.M. Effect of foliar spraying with amino acids and seaweed extract on growth chemical constitutes, yield and its quality of celeriac plant. Eur. J. Sci. Res. 2011, 58, 257–265. [Google Scholar]
- Filipczak, J.; Żurawicz, E.; Sas Paszt, L. Wpływ wybranych biostymulatorów na wzrost i plonowanie roślin truskawki ‘Elkat’ (The influence of selected biostimulants on the growth and yielding of strawberry plants ‘Elkat’). Zesz. Nauk. ISiK Skiern. 2016, 24, 43–58. [Google Scholar]
- Furuya, S.; Umemiya, Y. The influence of chemical forms on foliar-applied nitrogen absorption for peach trees. Acta Hortic. 2002, 594, 97–103. [Google Scholar] [CrossRef]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Świerczyński, S.; Stachowiak, A. The influence of three fertilizers and preparation Gibrescol used as the foliage spraying on the growth and nutritional status of maiden apple trees in a nursery. Ann. UMCS Sec. E Agric. 2009, 64, 78–85. [Google Scholar]
- Świerczyński, S.; Borowiak, K.; Bosiacki, M.; Urbaniak, M.; Malinowska, A. Estimation of the growth of ‘Vanda’ maiden sweet cherry trees on three rootstocks and after application of foliar fertilization in a nursery. Acta Sci. Pol. Hortorum Cultus 2019, 18, 109–118. [Google Scholar] [CrossRef]
- Grzyb, Z.S.; Piotrowski, W.; Sas Paszt, L.; Pąśko, M. Badania wstępne nad wpływem różnych biopreparatów na zmiany odczynu i zawartość składników w glebie i liściach okulantów jabłoni i wiśni (Preliminary studies on the influence of various biopreparations on changes in the reaction and the content of components in the soil and leaves of apple and cherry maiden trees). J. Res. Appl. Agric. Eng. 2013, 58, 198–203. [Google Scholar]
- Breś, W.; Golcz, A.; Komosa, A.; Kozik, E.; Tyksiński, W. Żywienie Roślin Ogrodniczych. (Nutrition of Horticultural Plants); Wydawnictwo Uniwersytetu Przyrodniczego w Poznaniu: Poznan, Poland, 2009; p. 3. [Google Scholar]
- Lipiński, W. Zasobność gleb Polski w mikroelementy. (The abundance of micronutrients in Polish soils). Studia Rap. IUNG-PIB 2013, 34, 121–131. [Google Scholar]
- Sas Paszt, L.; Żurawicz, E. The influence of nitrogen forms on root growth and pH changes in the rizosphere of strawberry plants. Acta Hort. 2004, 649, 217–221. [Google Scholar] [CrossRef]
- Ryabtseva, T.V.; Kapichnikova, N.G.; Mikhailovskaya, N.A. Influence of soil application of biological and mineral fertilizers on the growth, yield, and fruit biochemical components of ‘charavnitsa’ apple, and on some agrochemical soil characteristics. Acta Sci. Pol. Hortorum Cultus 2005, 4, 59–67. [Google Scholar]
- Van Diepeningen, A.D.; de Vos, O.J.; Korthals, G.W.; van Bruggen, A.H. Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl. Soil Ecol. 2006, 31, 120–135. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Yang, F.; Yaoyao, E.; Raza, W.; Huang, Q.; Shen, Q. Application of bioorganic fertilizer significantly increased apple yields and shaped bacterial community structure in orchard soil. Microb. Ecol. 2017, 73, 404–416. [Google Scholar] [CrossRef]
- Kleiber, T.; Markiewicz, B.; Kleiber, A. Wybrane właściwości chemiczne gleb zgrupowania “Szwajcaria Lwówecka” (Selected chemical properties of the soils of the “Lwówecka Switzerland” group). Apar. Badaw. Dydakt. 2010, 4, 69–74. [Google Scholar]
- Świerczyński, S.; Antonowicz, A.; Bykowska, J. The Effect of the Foliar Application of Biostimulants and Fertilisers on the Growth and Physiological Parameters of Maiden Apple Trees Cultivated with Limited Mineral Fertilisation. Agronomy 2021, 11, 1216. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Cristiano, G.; Pallozzi, E.; Conversa, G.; Tufarelli, V.; De Lucia, B. Effects of an Animal-Derived Biostimulant on the Growth and Physiological Parameters of Potted Snapdragon (Antirrhinum majus L.). Front. Plant Sci. 2018, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.L.; Madrid, R.; Gimeno, V.; Rodriguez-Ortega, W.M.; Nicolas, N.; Garcia-Sanchez, F. The effects of amino acids fertilization incorporated to the nutrient solution on mineral composition and growth in tomato seedlings. Span. J. Agric. Res. 2011, 9, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Nasir, M.; Khan, A.S.; Basra, S.A.; Malik, A.U. Foliar application of moringa leaf extract, potassium and zinc influence yield and fruit quality of Kinnow’mandarin. Sci. Hortic. 2016, 210, 227–235. [Google Scholar] [CrossRef]
- Rouphael, Y.; Giordano, M.; Cardarelli, E.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant and seaweed-based extract increase yield but differentially modulate nutritional quality of greenhouse spinach through biostymulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Abdalla, M.M. The potential of Moringa oleifera extract as a biostimulant in enhancing the growth, biochemical and hormonal contents in rocket (Eruca vesicaria subsp. sativa) plants. Int. J. Plant Physiol. Biochem. 2013, 5, 42–49. [Google Scholar] [CrossRef]
- Soppelsa, S.; Kelderer, M.; Casera, C.; Bassi, M.; Robatscher, P.; Matteazzi, A.; Andreotti, C. Foliar applications of biostimulants promote growth, yield and fruit quality of strawberry plants grown under nutrient limitation. Agronomy 2019, 9, 483. [Google Scholar] [CrossRef] [Green Version]
- Chitu, V.; Chitu, E.; Marin, F.C.; Ionita, A.D.; Cirjaliu-Murgea, M.; Filipescu, L. Effects of foliar ecological products application on apple growth, yield and quality. Acta Hortic. 2010, 868, 409–416. [Google Scholar] [CrossRef]
- Crouch, I.J.; Beckett, R.P.; Van Staden, J. Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. J. Appl. Phycol. 1990, 2, 269–272. [Google Scholar] [CrossRef]
- Dobromilska, R.; Mikiciuk, M.; Gubarewicz, K. Evaluation of cherry tomato yielding and fruit mineral composition after using of Bio-algeen S-90 preparation. J. Elem. 2008, 13, 491–499. [Google Scholar]
- Kuwada, K.; Wamocho, L.S.; Utamura, M.; Matsushita, I.; Ishii, T. Effect of red and green algal extracts on hyphal growth of arbuscular fungi, and on mycorrhizal development and growth of papaya and passionfruit. Agron. J. 2006, 98, 1340–1344. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Castaings, L.; Marchive, C.; Meyer, C.; Krapp, A. Nitrogen signalling in Arabidopsis: How to obtain insights into a complex signalling network. J. Exp. Bot. 2011, 62, 1391–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Von Bennewitz, E.; Hlušek, J.; Lošák, T. Nutritional status, vegetative and generative behaviour of apple trees after the application of two biopreparations. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 56, 13–18. [Google Scholar]
- Maini, P. The experience of the first biostimulant, based on amino acids and peptides: A short retrospective review on the laboratory researches and the practical results. Fertil. Agrorum 2006, 1, 29–43. [Google Scholar]
- Westwood, M.N. Temperate–Zone Pomology. Physiology and Culture; Timber Press: Portland, OR, USA, 1993. [Google Scholar]
- Allen, M.F.; Swenson, W.; Querejeta, J.I.; Egerton-Warburton, L.M.; Treseder, K.K. Ecology of mycorrhizae: A conceptual framework for complex interactions among plants and fungi. Annu. Rev. Phytopathol. 2003, 41, 271–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wójcik, P.; Wójcik, M. Growth and nutrition of M.26 EMLA apple rootstock as influence by titanium fertilization. J. Plant Nutr. 2001, 24, 1575–1588. [Google Scholar] [CrossRef]
- Wójcik, P. Vigor and nutrition of apple trees in nursery as influenced by titanium sprays. J. Plant Nutrit. 2002, 25, 1129–1138. [Google Scholar] [CrossRef]
- Serrano, M.; Martinem-Romero, D.; Castillo, S.; Guillen, F.; Valero, D. Effect of preharvest sprays containing calcium, magnesium and titanium on the quality of peaches and nectarines at harvest and during postharvest storage. J. Sci. Food Agric. 2004, 84, 1270–1276. [Google Scholar] [CrossRef]
- Malusà, E.; Sas Paszt, L.; Popińska, W.; Żurawicz, E. The effect of a substrate containing arbuscular mycorrhizal fungi and rhizosphere microorganisms (Trichoderma, Bacillus, Pseudomonas and Streptomyces) and foliar fertilization on growth response and rhizosphere pH of three strawberry cultivars. Int. J. Fruit Sci. 2007, 6, 25–41. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmianski, J. Influence of titanium treatment on antioxidants and antioxidant activity of strawberries. Acta Sci. Pol. Technol. Aliment. 2007, 6, 83–94. [Google Scholar]



| Dose of Mineral Fertilisation | pH | EC (mS·cm−1) | Na | Cl | S-SO4 |
|---|---|---|---|---|---|
| (mg·dm−3) | |||||
| Full | 6.40 | 0.27 | 14 | 4.4 | 6 |
| Half | 7.19 | 0.065 | 17 | 8 | 5 |
| Dose of Mineral Fertilisation | N-NH4 | N-NO3 | P | K | Ca | Mg |
|---|---|---|---|---|---|---|
| (mg∙dm−3) | ||||||
| Full | 28 | trace amounts | 107 | 145 | 520 | 96 |
| Half | trace amounts | trace amounts | 82 | 69 | 675 | 65 |
| Dose of Mineral Fertilisation | Fe | Mn | Zn | Cu |
|---|---|---|---|---|
| (mg∙dm−3) | ||||
| Full | 115.9 | 73.7 | 21.2 | 3.9 |
| Half | 121.9 | 70.4 | 18.3 | 2.6 |
| Element | Cultivar | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Amino Plant | Biamino Plant | Bispeed | Fylloton | Basfoliar 6-12-6 | Basfoliar 12-4-6+S | Mean for Cultivar | ||
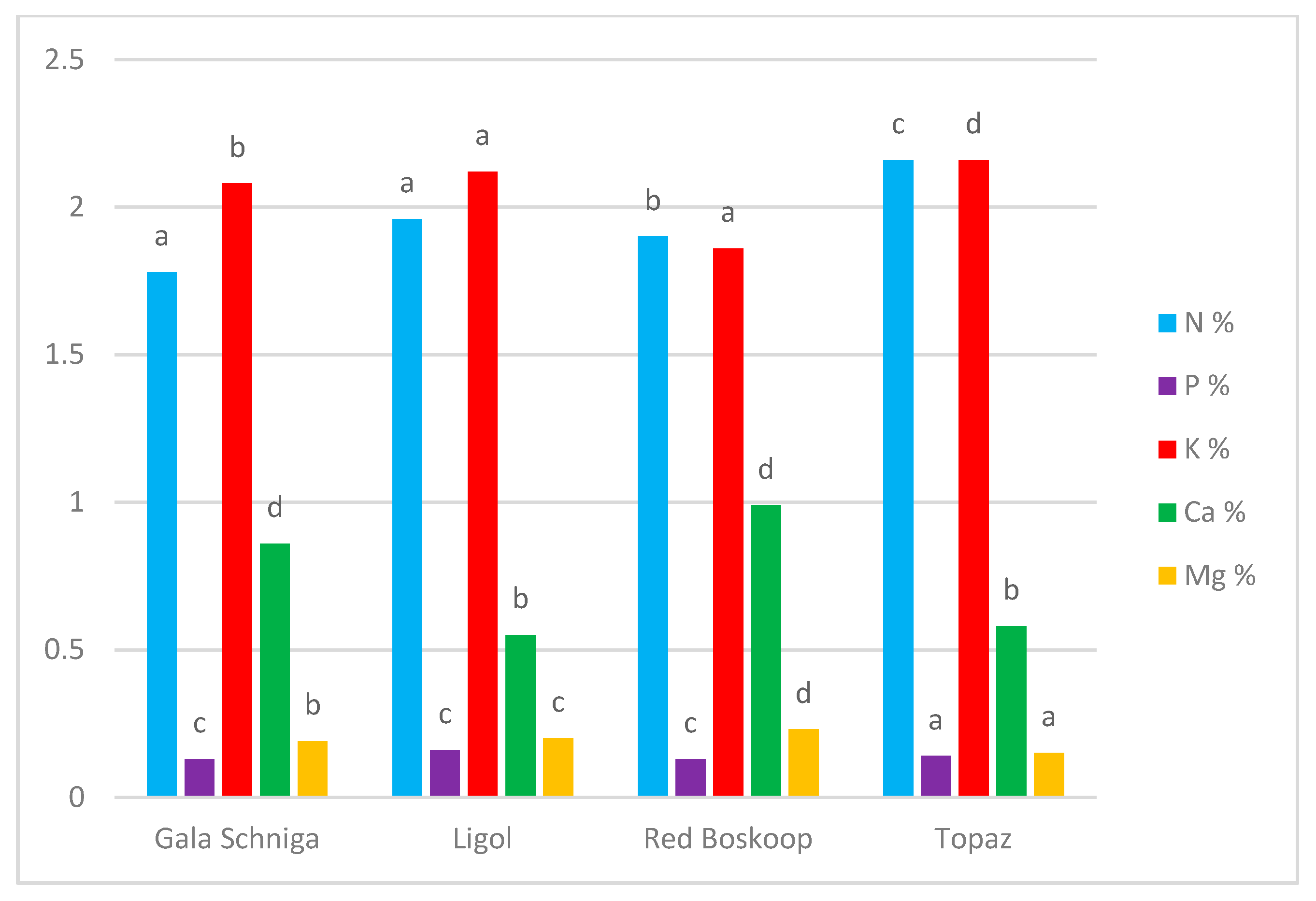
| N (%) | Gala Schniga | 1.96 e–g | 1.72 bc | 1.79 cd | 1.77 b–d | 1.96 e–g | 1.72 bc | 1.56 a | 1.78 a |
| Ligol | 2.07 g–i | 1.79 cd | 1.82 cd | 1.82 cd | 1.89 d–f | 2.17 jk | 2.17 jk | 1.96 c | |
| R. Boskoop | 2.15 ij | 1.86 de | 1.70 bc | 1.87 de | 1.66 ab | 1.98 e–h | 2.10 hi | 1.90 b | |
| Topaz | 2.28 kl | 2.00 f-h | 2.31 l | 2.00 f–h | 2.00 f–h | 2.24 j–l | 2.35 l | 2.16 d | |
| Average for treatment | 2.11 d | 1.84 a | 1.90 b | 1.86 ab | 1.87 ab | 2.02 c | 2.04 c | ||
| P (%) | Gala Schniga | 0.13 a–c | 0.12 a–c | 0.11 ab | 0.13 a–c | 0.13 a–c | 0.14 bc | 0.13 a–c | 0.13 a |
| Ligol | 0.15 c | 0.13 a–c | 0.13 a–c | 0.12 a–c | 0.13 a–c | 0.24 d | 0.22 d | 0.16 c | |
| R. Boskoop | 0.13 a–c | 0.14 bc | 0.13 a–c | 0.13 a–c | 0.10 a | 0.13 a–c | 0.12 a–c | 0.13 a | |
| Topaz | 0.15 c | 0.13 a–c | 0.12 a–c | 0.12 a–c | 0.14 bc | 0.14 bc | 0.15 c | 0.14 b | |
| Average for treatment | 0.14 b | 0.13 ab | 0.12 a | 0.13 a | 0.13 a | 0.16 c | 0.16 c | ||
| K (%) | Gala Schniga | 2.13 i–k | 2.00 e–h | 1.88 c–e | 2.13 i–k | 2.22 k–m | 2.29 mn | 1.92 c–f | 2.08 b |
| Ligol | 2.04 f–i | 2.14 i–l | 2.11 h–k | 2.14 i–l | 2.11 h–k | 2.21 j–m | 2.14 i–l | 2.12 c | |
| R. Boskoop | 2.09 h–j | 1.85 cd | 1.80 bc | 1.68 ab | 1.60 a | 2.26 l–n | 1.96 d–g | 1.86 a | |
| Topaz | 2.12 h–k | 2.27 mn | 2.13 i–k | 2.36 n | 2.05 g–i | 2.23 k–m | 2.14 i–l | 2. d | |
| Average for treatment | 2.09 d | 2.06 cd | 1.98 a | 2.07 cd | 1.99 ab | 2.24 e | 2.04 bc | ||
| Ca (%) | Gala Schniga | 0.67 d–g | 0.74 h–j | 0.68 e–h | 0.97 lm | 0.94 kl | 1.10 op | 0.90 k | 0.86 c |
| Ligol | 0.71 g–i | 0.64 d–f | 0.62 de | 0.62 de | 0.64 d–f | 0.23 a | 0.38 c | 0.55 a | |
| R. Boskoop | 1.04 no | 1.02 mn | 0.76 ij | 0.98 l–n | 0.79 j | 1.18 r | 1.14 pr | 0.99 d | |
| Topaz | 0.31 b | 0.72 g–i | 0.71 g–i | 0.61 d | 0.63 d–f | 0.37 bc | 0.69 f–h | 0.58 b | |
| Average for treatment | 0.68 a | 0.78 d | 0.69 a | 0.80 d | 0.75 c | 0.72 b | 0.78 d | ||
| Mg (%) | Gala Schniga | 0.19 c–f | 0.22 f–h | 0.21 e–g | 0.16 bc | 0.19 c–f | 0.20 d–f | 0.17 b–d | 0.19 b |
| Ligol | 0.22 f–h | 0.15 ab | 0.15 ab | 0.16 bc | 0.18 b–e | 0.28 jk | 0.26 ij | 0.20 c | |
| R. Boskoop. | 0.21 e–g | 0.30 k | 0.20 d–f | 0.25 h–j | 0.24 g–i | 0.21 e–g | 0.20 d–f | 0.23 d | |
| Topaz | 0.16 bc | 0.15 ab | 0.16 bc | 0.15 ab | 0.12 a | 0.19 c–f | 0.15 ab | 0.15 a | |
| Average for treatment | 0.20 b | 0.21 b | 0.18 a | 0.18 a | 0.18 a | 0.22 c | 0.20 b | ||
| Na (%) | Gala Schniga | 0.003 a–c | 0.003 a–c | 0.002 ab | 0.005 b–e | 0.007d–f | 0.004 a–d | 0.004 a–d | 0.004 b |
| Ligol | 0.004 a–d | 0.005 b–e | 0.001 a | 0.007d–f | 0.004 a–d | 0.004 a–d | 0.004 a–d | 0.004 b | |
| R. Boskoop | 0.004 a–d | 0.005 b–e | 0.006 c–f | 0.004 a–d | 0.00 f | 0.006 c–f | 0.006 c–f | 0.006 c | |
| Topaz | 0.004 a–d | 0.003 a–c | 0.001 a | 0.001 a | 0.008 ef | 0.002 ab | 0.002 ab | 0.003 a | |
| Average for treatment | 0.004 b | 0.004 b | 0.003 a | 0.004 b | 0.007 c | 0.004 b | 0.004 b | ||
| Micro-Nutrient | Cultivar | Treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Amino Plant | Biamino Plant | Bispeed | Fylloton | Basfoliar 6-12-6 | Basoliar 12-4-6+S | Average for Cultivar | ||
| Fe ppm | Gala Schniga | 144.4 h | 165.6 kl | 173.7 m | 190.7 o | 176.1 mn | 125.8 f | 119.4 e | 156.5 c |
| Ligol | 101.1 d | 168.5 l | 195.9 p | 164.0 k | 178.4 n | 131.0 g | 85.8 c | 146.4 b | |
| R. Boskoop | 199.2 r | 241.2 u | 225.9 t | 268.6 x | 219.4 s | 263.4 w | 152.7 i | 224.3 d | |
| Topaz | 82.0 b | 129.1 g | 160.0 j | 131.8 g | 147.1 h | 71.8 a | 79.4 b | 114.5 a | |
| Average for treatment | 131.7 b | 176.1 d | 188.9 f | 188.8 f | 180.3 e | 148.0 c | 109.3 a | ||
| Mn ppm | Gala Schniga | 26.5 bc | 48.6 m–o | 43.6 i–l | 59.8 s | 52.5 p | 42.2 h–j | 29.0 cd | 43.2 c |
| Ligol | 46.5 l–n | 42.4 h–k | 41.5 g–i | 31.7 d | 35.0 e | 36.1 ef | 44.8 j–l | 39.7 b | |
| R. Boskoop | 39.3 gh | 45.5k–m | 43.9 i–l | 50.8 op | 41.2 g–i | 51.2 op | 25.9 bc | 42.5 c | |
| Topaz | 25.0 ab | 48.8 no | 55.7 r | 49.2 no | 38.5 fg | 25.8 ab | 22.7 a | 38.0 a | |
| Average for treatment | 34.3 b | 46.3 e | 46.2 e | 47.9 f | 41.8 d | 38.8 c | 30.6 a | ||
| Zn ppm | Gala Schniga | 21.1 k | 13.3 a | 14.9 b | 16.1 e | 19.7 j | 24.4 p | 18.8 h | 18.3 a |
| Ligol | 15.3 c | 14.8 b | 17.5 g | 16.5 f | 15.9 d | 26.5 t | 22.4 l | 18.4 b | |
| R. Boskoop | 30.7 u | 24.4 p | 26.6 t | 26.1 s | 19.1 i | 39.3 w | 25.4 r | 27.4 d | |
| Topaz | 22.6 m | 24.2 o | 24.1 o | 19.6 j | 22.9 n | 19.1 i | 21.0 k | 21.9 c | |
| Average for treatment | 22.4 f | 19.2 a | 20.8 d | 19.6 c | 19.4 b | 27.3 g | 21.9 e | ||
| Cu ppm | Gala Schiga | 6.6 j | 5.0 b | 5.0 b | 6.1 gh | 6.3 i | 6.8 k | 5.5 e | 5.9 a |
| Ligol | 8.1 m | 5.3 cd | 5.0 b | 4.3 a | 5.2 c | 9.5 r | 8.8 n | 6.6 c | |
| R. Boskoop | 6.1 gh | 5.7 f | 5.4 de | 6.0 g | 4.4 a | 8.7 n | 7.1 l | 6.2 b | |
| Topaz | 10.0 s | 6.9 k | 6.2 hi | 6.5 j | 9.3 p | 9.1 o | 10.0 s | 8.3 d | |
| Average for treatment | 7.7 d | 5.7 b | 5.4 a | 5.7 b | 6.3 c | 8.5 f | 7.9 e | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świerczyński, S.; Antonowicz, A. The Effects of Reduced Mineral Fertilisation Combined with the Foliar Application of Biostimulants and Fertilisers on the Nutrition of Maiden Apple Trees and the Contents of Soil Nutrients. Agronomy 2021, 11, 2438. https://doi.org/10.3390/agronomy11122438
Świerczyński S, Antonowicz A. The Effects of Reduced Mineral Fertilisation Combined with the Foliar Application of Biostimulants and Fertilisers on the Nutrition of Maiden Apple Trees and the Contents of Soil Nutrients. Agronomy. 2021; 11(12):2438. https://doi.org/10.3390/agronomy11122438
Chicago/Turabian StyleŚwierczyński, Sławomir, and Agnieszka Antonowicz. 2021. "The Effects of Reduced Mineral Fertilisation Combined with the Foliar Application of Biostimulants and Fertilisers on the Nutrition of Maiden Apple Trees and the Contents of Soil Nutrients" Agronomy 11, no. 12: 2438. https://doi.org/10.3390/agronomy11122438
APA StyleŚwierczyński, S., & Antonowicz, A. (2021). The Effects of Reduced Mineral Fertilisation Combined with the Foliar Application of Biostimulants and Fertilisers on the Nutrition of Maiden Apple Trees and the Contents of Soil Nutrients. Agronomy, 11(12), 2438. https://doi.org/10.3390/agronomy11122438






