Moringa oleifera Leaf Extract Enhanced Growth, Yield, and Silybin Content While Mitigating Salt-Induced Adverse Effects on the Growth of Silybum marianum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Moringa Leaves
2.2. Chemical Analysis of Moringa Leaves
2.3. Preparation of Moringa Leaf Extracts
2.4. Silybum Plant Material and Growth Conditions
2.5. Measurement of Vegetative Growth
2.6. Measurement of Yield
2.7. Determination of Photosynthetic Pigments
2.8. Determination of Mineral Composition
2.9. Determination of Silybin (A + B) Content by High-Performance Liquid Chromatography (HPLC)
2.9.1. Instrumentation
2.9.2. Preparation of Silybin (A + B) Standard Curve
2.9.3. Preparation of Silybum Fruit Methanolic Extract
2.9.4. HPLC Analysis Conditions
2.10. Analysis Chalcone Synthase 1, 2, and 3 Gene Expressions by Real-Time Reverse-Transcribed Polymerase Chain Reaction (Real Time RT-PCR)
2.10.1. Total RNA Preparation and cDNA Synthesis
2.10.2. Real-Time RT-PCR and Chalcone Synthase Gene Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. Analysis of Moringa Oleifera Leaf Composition
3.2. The Effects of Salinity and MLE Foliar Spray on the Growth and Yield of Silybum Marianum
3.3. The Effects of Salinity and MLE Foliar Spray on the Compositions of Chlorophyll a, Chlorophyll b, and Carotenoid in S. marianum Leaves
3.4. The Effects of Salinity and MLE Foliar Spray on the Mineral Contents in S. marianum Leaves
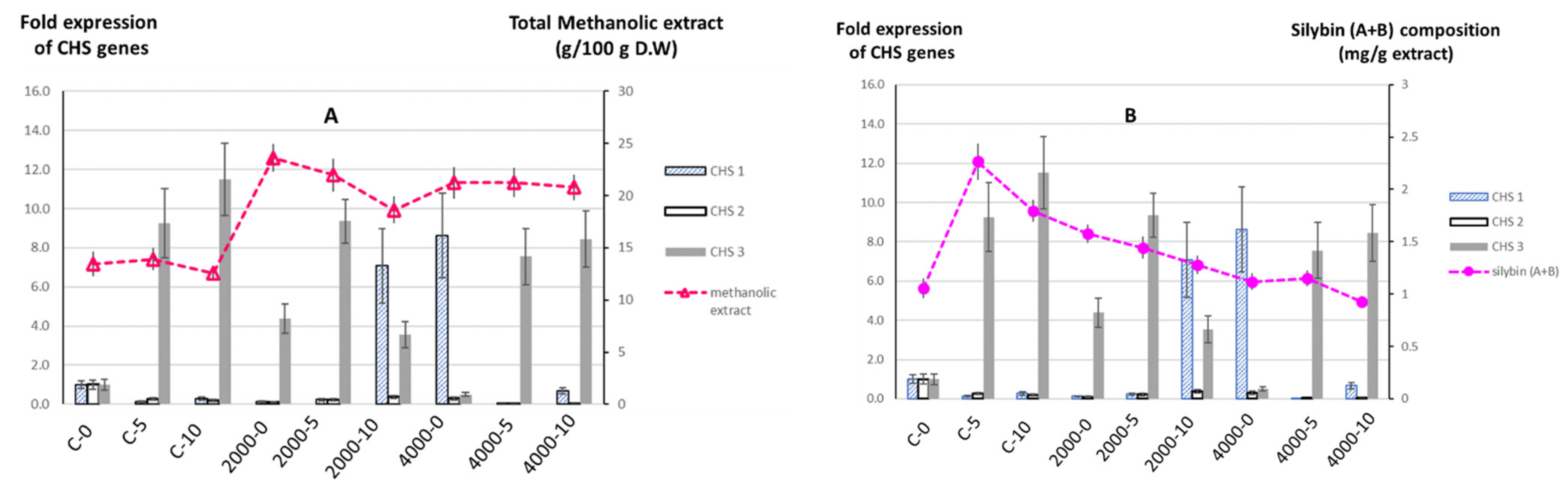
3.5. The Effects of Salinity and MLE Foliar Spray on the Total Amount of Methanolic Extract and Silybin (A + B) Composition of S. marianum Fruits
3.6. The Effects of Salinity and MLE Foliar Spray on the Expressions of Chalcone Synthase 1, 2, and 3 Genes in S. marianum Petals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez-Garcia, S.N.; Vazquez-Cruz, M.A.; Guevara-Gonzalez, R.G.; Torres-Pacheco, I.; Cruz-Hernandez, A.; Feregrino-Perez, A.A. Current Approaches for Enhanced Expression of Secondary Metabolites as Bioactive Compounds in Plants for Agronomic and Human Health Purposes—A Review. Pol. J. Food Nutr. Sci. 2013, 63, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Crocenzi, F.; Roma, M. Silymarin as a New Hepatoprotective Agent in Experimental Cholestasis: New Possibilities for an Ancient Medication. Curr. Med. Chem. 2006, 13, 1055–1074. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of Silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef]
- Ou, Q.; Weng, Y.; Wang, S.; Zhao, Y.; Zhang, F.; Zhou, J.; Wu, X. Silybin Alleviates Hepatic Steatosis and Fibrosis in NASH Mice by Inhibiting Oxidative Stress and Involvement with the Nf-ΚB Pathway. Dig. Dis. Sci. 2018, 63, 3398–3408. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Xu, D.; Wei, Q.; Zhang, B.; Aa, J.; Wang, G.; Xie, Y. Silybin Ameliorates Hepatic Lipid Accumulation and Modulates Global Metabolism in an NAFLD Mouse Model. Biomed. Pharmacother. 2020, 123, 109721. [Google Scholar] [CrossRef]
- Firouzi, A.; Mohammadi, S.A.; Khosrowchahli, M.; Movafeghi, A.; Hasanloo, T. Enhancement of Silymarin Production in Cell Culture of Silybum marianum (L) Gaertn by Elicitation and Precursor Feeding. J. Herbs Spices Med. Plants 2013, 19, 262–274. [Google Scholar] [CrossRef]
- Madrid, E.; Corchete, P.N. Silymarin Secretion and Its Elicitation by Methyl Jasmonate in Cell Cultures of Silybum marianum Is Mediated by Phospholipase D-Phosphatidic Acid. J. Exp. Bot. 2010, 61, 747–754. [Google Scholar] [CrossRef] [PubMed]
- El-Garhy, H.A.S.; Khattab, S.; Moustafa, M.M.A.; Abou Ali, R.; Abdel Azeiz, A.Z.; Elhalwagi, A.; el Sherif, F. Silybin Content and Overexpression of Chalcone Synthase Genes in Silybum marianum L. Plants under Abiotic Elicitation. Plant Physiol. Biochem. 2016, 108, 191–202. [Google Scholar] [CrossRef] [PubMed]
- El Sherif, F.; Khattab, S.; Ibrahim, A.K.; Ahmed, S.A. Improved Silymarin Content in Elicited Multiple Shoot Cultures of Silybum marianum L. Physiol. Mol. Biol. Plants 2013, 19, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Mangundayao, K.; Yasurin, P. Bioactivity of Moringa oleifera and Its Applications: A Review. J. Pure Appl. Microbiol. 2017, 11, 43–50. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An Overview of Plant-Based Natural Biostimulants for Sustainable Horticulture with a Particular Focus on Moringa Leaf Extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef]
- Alkuwayti, M.A.A.; El-Sherif, F.; Yap, Y.-K.; Khattab, S. Foliar Application of Moringa oleifera Leaves Extract Altered Stress-Responsive Gene Expression and Enhanced Bioactive Compounds Composition in Ocimum basilicum. S. Afr. J. Bot. 2020, 129, 291–298. [Google Scholar] [CrossRef]
- ElSherif, F.; Albotnoor, N.; Yap, Y.K.; Meligy, A.; Khattab, S. Enhanced Bioactive Compounds Composition in Lavandula officinalis In vitro Plantlets Using NaCl and Moringa oleifera, Aloe vera and Spirulina platensis Extracts. Ind. Crop. Prod. 2020, 157, 112890. [Google Scholar] [CrossRef]
- Batool, S.; Khan, S.; Basra, S.M.A. Foliar Application of Moringa Leaf Extract Improves the Growth of Moringa Seedlings in Winter. S. Afr. J. Bot. 2020, 129, 347–353. [Google Scholar] [CrossRef]
- Hoque, T.S.; Rana, M.S.; Zahan, S.A.; Jahan, I.; Abedin, M.A. Moringa Leaf Extract as a Bio-Stimulant on Growth, Yield and Nutritional Improvement in Cabbage. Asian J. Med Biol. Res. 2020, 6, 196–203. [Google Scholar] [CrossRef]
- Ngcobo, B.L.; Bertling, I. Influence of Foliar Moringa oleifera Leaf Extract (MLE) Application on Growth, Fruit Yield and Nutritional Quality of Cherry Tomato. Acta Hortic. 2021, 1306, 249–254. [Google Scholar] [CrossRef]
- Kanchani, A.M.K.D.M.; Harris, K.D. Effect of Foliar Application of Moringa (Moringa oleifera) Leaf Extract with Recommended Fertilizer on Growth and Yield of Okra (Abelmoschus esculentus). Agriest 2019, 13, 38–54. [Google Scholar] [CrossRef]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone Synthase and Its Functions in Plant Resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepideh, S.; Shobbar, S.Z.; Ebrahimi, M.; Hasanloo, T.; Sadat-Noori, S.-A.; Tirnaz, S. Chalcone Synthase Genes from Milk Thistle (Silybum marianum): Isolation and Expression Analysis. J. Genet. 2015, 94, 611–617. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Becker, K. Nutrional Value and Antinutritional Components of Whole and Ethanol Extracted Moringa oleifera Leaves. Anim. Feed. Sci. Technol. 1996, 63, 211–228. [Google Scholar] [CrossRef]
- Horwitz, W.; Chichilo, P.; Reynolds, H. Official Methods of Analysis of the Association of Official Analytical Chemists; Association of Official Analytical Chemists: Washington, DC, USA, 1970. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Cambridge, MA, USA, 1942. [Google Scholar]
- Mazumdar, B.C.; Majumder, K. Methods of Physiochemical Analysis of Fruits; Daya Publisher: Delhi, India, 2003. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- StatSoft STATISTICA for Windows, Version 6; 2300; StatSoft Inc.: Tulsa, OK, USA, 2001.
- Ali, E.F.; Hassan, F.A.S.; Elgimabi, M. Improving the Growth, Yield and Volatile Oil Content of Pelargonium graveolens L. Herit by Foliar Application with Moringa Leaf Extract through Motivating Physiological and Biochemical Parameters. S. Afr. J. Bot. 2018, 119, 383–389. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhu, J.K. Salt and Drought Stress Signal Transduction in Plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslam, M.; Sultana, B.; Anwar, F.; Munir, H. Foliar Spray of Selected Plant Growth Regulators Affected the Biochemical and Antioxidant Attributes of Spinach in a Field Experiment. Turk. J. Agric. For. 2016, 40, 136–145. [Google Scholar] [CrossRef]
- Park, H.J.; Kim, W.Y.; Yun, D.J. A New Insight of Salt Stress Signaling in Plant. Mol. Cells 2016, 39, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, A.I.; El-Hamahmy, M.A.M.M.; Rafudeen, M.S.; Mohamed, A.H.; Omar, A.A. The Impact of Drought Stress on Antioxidant Responses and Accumulation of Flavonolignans in Milk Thistle (Silybum marianum (L.) Gaertn). Plants 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rady, M.M.; Mohamed, G.F. Modulation of Salt Stress Effects on the Growth, Physio-Chemical Attributes and Yields of Phaseolus vulgaris L. Plants by the Combined Application of Salicylic Acid and Moringa oleifera Leaf Extract. Sci. Hortic. 2015, 193, 105–113. [Google Scholar] [CrossRef]
- Yasmeen, A.; Basra, S.M.A.; Farooq, M.; ur Rehman, H.; Hussain, N.; ur Rehman Athar, H. Exogenous Application of Moringa Leaf Extract Modulates the Antioxidant Enzyme System to Improve Wheat Performance under Saline Conditions. Plant Growth Regul. 2013, 69, 225–233. [Google Scholar] [CrossRef]
- Latif, H.H.; Mohamed, H.I. Exogenous Applications of Moringa Leaf Extract Effect on Retrotransposon, Ultrastructural and Biochemical Contents of Common Bean Plants under Environmental Stresses. S. Afr. J. Bot. 2016, 106, 221–231. [Google Scholar] [CrossRef]
- Howladar, S.M. A Novel Moringa oleifera Leaf Extract Can Mitigate the Stress Effects of Salinity and Cadmium in Bean (Phaseolus vulgaris L.) Plants. Ecotoxicol. Environ. Saf. 2014, 100, 69–75. [Google Scholar] [CrossRef] [PubMed]
- De Vasconcelos, A.C.F.; Garófalo Chaves, L.H. Biostimulants and Their Role in Improving Plant Growth under Abiotic Stresses. In Biostimulants in Plant Science; IntechOpen: London, UK, 2020. [Google Scholar]
- Khalofah, A.; Bokhari, N.A.; Migdadi, H.M.; Alwahibi, M.S. Antioxidant Responses and the Role of Moringa oleifera Leaf Extract for Mitigation of Cadmium Stressed Lepidium sativum L. S. Afr. J. Bot. 2020, 129, 341–346. [Google Scholar] [CrossRef]
- Abbas, S.; Zaglool, M.; El-Ghadban, E.; Abd El-Kareem, S.; Waly, A. Effect of Moringa Leaf Extract Spray on Sage (Salvia officinalis L.) Plant under Sandy Soil Conditions. Hortsci. J. Suez Canal Univ. 2016, 5, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Drouet, S.; Tungmunnithum, D.; Lainé, É.; Hano, C. Gene Expression Analysis and Metabolite Profiling of Silymarin Biosynthesis during Milk Thistle (Silybum marianum (L.) Gaertn.) Fruit Ripening. Int. J. Mol. Sci. 2020, 21, 4730. [Google Scholar] [CrossRef]
- Elwekeel, A.; Elfishawy, A.; Abouzid, S. Silymarin Content in Silybum marianum Fruits at Different Maturity Stages. J. Med. Plant Res. 2013, 7, 1665–1669. [Google Scholar] [CrossRef]
- Torres, M.; Corchete, P. Gene Expression and Flavonolignan Production in Fruits and Cell Cultures of Silybum marianum. J. Plant Physiol. 2016, 192, 111–117. [Google Scholar] [CrossRef] [PubMed]
| Chemical | Amount | Unit |
|---|---|---|
| Calcium | 440 | mg/100 g |
| Copper | 0.07 | mg/100 g |
| Iron | 0.85 | mg/100 g |
| Magnesium | 42 | mg/100 g |
| Phosphorus | 70 | mg/100 g |
| Potassium | 259 | mg/100 g |
| Zinc | 0.16 | mg/100 g |
| Protein | 6.70 | g/100 g |
| Fat | 1.70 | g/100 g |
| Carbohydrate | 12.5 | g/100 g |
| Arginine | 406.6 | mg/100 g |
| Histidine | 149.8 | mg/100 g |
| Isoleucine | 299.6 | mg/100 g |
| Leucine | 492.2 | mg/100 g |
| Lysine | 342.4 | mg/100 g |
| Methionine | 117.7 | mg/100 g |
| Phenylalanine | 310.3 | mg/100 g |
| Threonine | 117.7 | mg/100 g |
| Tryptophan | 107 | mg/100 g |
| Valine | 374.5 | mg/100 g |
| Vitamin A | 6.78 | mg/100 g |
| Vitamin B1 | 0.06 | mg/100 g |
| Vitamin B2 | 0.05 | mg/100 g |
| Vitamin B3 | 0.8 | mg/100 g |
| Vitamin C | 220 | mg/100 g |
| Gibberellin | 5.4 | mg/100 g |
| Zeatin | 3.0 | mg/100 g |
| Indole acetic acid (IAA) | 0.52 | mg/100 g |
| Total polyphenol | 28.05 | mg/g |
| Total flavonoid | 80 | mg/g |
| Total tocopherol | 5 | μg/g |
| Total rutin | 0.27810 | mg/g |
| Total gallic acid | 0.12786 | mg/g |
| Salinity Level (ppm) | Cations (meq/L) | Anions (meq/L) | SAR | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Na+ | K+ | CO32− | HCO3− | SO42− | Cl− | ||
| 864 | 5.72 | 2.02 | 7.27 | 0.38 | 0.28 | 2.68 | 4.03 | 8.4 | 3.43 |
| Gene | Primer’s Sequence | Amplicon Length (bp) | GenBank Accession Number |
|---|---|---|---|
| Chalcone synthase1 (CHS1) | CHS1F 5-TCTTGATTCCCTCGTTGGTC-3 CHS1R 5-TCTCAAACAACGGCCTCTCT-3 | 101 | JN182805.1 |
| Chalcone synthase2 (CHS2) | CHS2F 5-AGGACATTGCGGAAAACAAC-3 CHS2R 5-AACGGCCTCTCTGTCTTCAA-3 | 184 | JN182806.1 |
| Chalcone synthase3 (CHS3) | CHS3F 5-ACCCACCTCATCTTTTGCAC-3 CHS3R 5--CATCATGAGGCGTTTGATTG-3 | 105 | JN182807.1 |
| NADH | ndhchs_L 5-TTCCGCATTTTGGAAATACC-3 ndhchs_R 5-CCCGTCTTGATTGAAAGGAA-3 | 134 | KC589999.1 |
| Treatment | Plant Height (cm) | No. of Leaves (n) | No. of Branches (n) | Root Dry Weight (g) | Aerial Part Dry Weight (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinity (ppm) | MLE (g/L) | Season (2018) | Season (2019) | Season (2018) | Season (2019) | Season (2018) | Season (2019) | Season (2018) | Season (2019) | Season (2018) | Season (2019) | ||
| (a) Main effect salinity | |||||||||||||
| Control | 62.3 a * | 66.9 a | 60.9 a | 64.2 a | 19.4 a | 20.7 a | 6.2 a | 4.8 a | 66.1 a | 79.6 a | |||
| 2000 | 43.5 b | 48.8 b | 39.6 b | 39.5 b | 12.4 b | 12.9 b | 2.3 b | 2.9 b | 33.6 b | 36.4 b | |||
| 4000 | 24.7 c | 25.9 c | 24.6 c | 22.1 c | 7.8 c | 7.7 c | 1.2 c | 0.9 c | 13.4 c | 14.2 c | |||
| (b) Main effect of MLE | |||||||||||||
| Control | 39.1 b | 47.9 a | 38.1 a | 30.5 b | 11.7 a | 11.6 b | 3.8 a | 3.7 a | 34.3 a | 46.9 ab | |||
| 5 | 42.7 ab | 48.6 a | 42.1 a | 42.1 b | 14.0 a | 16.9 a | 3.1 a | 2.3 b | 36.2 a | 40.7 b | |||
| 10 | 48.8 a | 51.1 a | 44.6 a | 51.0 a | 13.8 a | 14.7 ab | 2.8 a | 3.1 ab | 42.8 a | 51.9 a | |||
| (c) Interacting effects of salinity and MLE | |||||||||||||
| Control | 41.7 bc | 62.0 abc | 45.3 b | 54.5 b | 13 bc | 15.5 b | 6.2 a | 5.1 ab | 41.4 b | 76.3 b | |||
| Control | 5 | 70.3 a | 73.0 a | 66.7 a | 60.0 b | 24.7 a | 24.5 a | 6.6 a | 3.4 bc | 68.9 b | 65.2 b | ||
| 10 | 75.0 a | 65.8 ab | 70.7 a | 79.0a | 20.7 a | 22.0 a | 5.9 a | 5.8 a | 87.9 a | 97.3 a | |||
| Control | 52.3 b | 55.0 bcd | 44.3 b | 40.3 c | 14.3 b | 16.7 bc | 3.1 b | 4.3 abc | 40.8 c | 40.9 c | |||
| 2000 | 5 | 35.0 cd | 42.3 de | 36.8 b | 38.3 c | 11.8 b | 11.3 bcd | 2.1 bc | 2.3 cd | 32.2 cd | 31.5 cde | ||
| 10 | 46.0 b | 49.0 cd | 38.7 b | 39.8 c | 11.3 bcd | 12.8 bcd | 1.9 bc | 2.3 cd | 28.1 cd | 36.6 cd | |||
| Control | 23.3 e | 27.7 ef | 24.3 c | 20.7 d | 7.7 de | 7.5 d | 2.0 bc | 1.3 d | 8.7 e | 13.6 e | |||
| 4000 | 5 | 25.3 de | 22.3 f | 24.5 c | 21.3 d | 6.3 e | 6.7 d | 0.8 c | 0.7 d | 20.6 de | 17.2 de | ||
| 10 | 25.1 de | 27.9 ef | 24.8 c | 24.3 d | 9.3 cde | 9.0 cd | 0.7 c | 0.7 d | 12.3 e | 11.8 e | |||
| Treatment | Capitula Number (n) | Fruit Dry Weight (g) | |||
|---|---|---|---|---|---|
| Salinity (ppm) | MLE (g/L) | Season (2018) | Season (2019) | Season (2018) | Season (2019) |
| (a) Main effect of salinity | |||||
| Control | 18.3 a * | 14.3 a | 38.8 a | 41.8 a | |
| 2000 | 17.6 ab | 14.2 a | 23.5 b | 24.4 b | |
| 4000 | 9.0 b | 12.9 a | 9.3 c | 4.5 c | |
| (b) Main effect of MLE | |||||
| Control | 14.3 a | 11.7 b | 22.6 a | 33.9 a | |
| 5 | 14.8 a | 12.1 b | 25.7 a | 20.9 b | |
| 10 | 17.9 a | 16.9 a | 23.2 a | 25.8 b | |
| (c) Interacting effects of salinity and MLE | |||||
| control | 16.3 abc | 13.7 ab | 28.4 c | 52.9 a | |
| control | 5 | 19.0 ab | 11.0 b | 49.4 a | 31.1 bc |
| 10 | 21.5 a | 17.7 a | 38.9 b | 41.4 b | |
| control | 14.3 abc | 11.7 ab | 28.0 c | 28.7 c | |
| 2000 | 5 | 16.0 abc | 13 ab | 22.1 c | 21.3 cd |
| 10 | 21.0 a | 16.7 ab | 20.9 c | 23.6 cd | |
| control | 12.3 bc | 10.3 b | 11.7 d | 13.8 de | |
| 4000 | 5 | 9.0 c | 12.5 ab | 6.5 d | 6.8 e |
| 10 | 11.3 bc | 10.0 b | 9.8 d | 7.8 e | |
| Treatment | Chl a (mg/100 g F.W.) | Chl b (mg/100 g F.W.) | Carotenoids (mg/100 g F.W.) | ||||
|---|---|---|---|---|---|---|---|
| Salinity (ppm) | MLE (g/L) | Season (2018) | Season (2019) | Season (2018) | Season (2019) | Season (2018) | Season (2019) |
| (a) Main effect of salinity | |||||||
| Control | 89.69 a * | 92.46 a | 24.41 a | 26.20 a | 85.46 a | 89.05 a | |
| 2000 | 86.94 a | 93.10 a | 23.35 a | 25.28 a | 86.42a | 91.47 a | |
| 4000 | 87.53 a | 86.72 a | 23.52 a | 23.37 a | 87.38 a | 87.23 a | |
| (b) Main effect of MLE | |||||||
| Control | 94.44 a | 92.97 a | 25.47 a | 25.03 a | 91.56 a | 91.15 a | |
| 5 | 86.70 a | 86.66 a | 23.38 a | 23.81 a | 86.29 a | 86.94 a | |
| 10 | 83.02 a | 92.65 a | 22.43 a | 26.00 a | 81.40 a | 89.67 a | |
| (c) Interacting effects of salinity and MLE | |||||||
| Control | 109.96 a | 93.93 a | 29.57 a | 26.30 a | 85.02 ab | 89.62 a | |
| Control | 5 | 89.26 ab | 93.14 a | 24.18 ab | 27.08 a | 84.90 ab | 91.99 a |
| 10 | 69.86 b | 90.32 a | 19.27 b | 25.21 a | 67.17 b | 85.53 a | |
| Control | 87.76 ab | 104.12 a | 23.52 ab | 27.98 a | 90.31 a | 101.67 a | |
| 2000 | 5 | 79.27 b | 77.52 a | 21.12 ab | 19.82 a | 81.41 ab | 78.12 a |
| 10 | 93.79 ab | 97.65 a | 25.41 ab | 28.03 a | 92.47 a | 94.63 a | |
| Control | 85.59 ab | 80.86 a | 23.32 ab | 20.81 a | 85.37 ab | 85.53 a | |
| 4000 | 5 | 91.58 ab | 89.32 a | 24.83 ab | 24.54 a | 92.54 ab | 90.62 a |
| 10 | 85.42 ab | 89.99 a | 22.39 ab | 24.78 a | 84.58 ab | 88.85 a | |
| Treatment | % Na+ | % K+ | |||
|---|---|---|---|---|---|
| Salinity (ppm) | MLE (g/L) | Season (2018) | Season (2019) | Season (2018) | Season (2019) |
| (a) Main effect of salinity | |||||
| Control | 0.212 c * | 0.187 c | 1.139 a | 0.975 a | |
| 2000 | 0.329 b | 0.389 b | 0.696 b | 0.608 b | |
| 4000 | 0.538 a | 0.532 a | 0.420 c | 0.440 c | |
| (b) Main effect of MLE | |||||
| Control | 0.334 b | 0.372 a | 0.880 a | 0.663 a | |
| 5 | 0.394 a | 0.360 a | 0.673 b | 0.673 a | |
| 10 | 0.351 b | 0.376 a | 0.703 b | 0.686 a | |
| (c) Interacting effects of salinity and MLE | |||||
| Control | 0.216 c | 0.179 d | 1.488 a | 1.103 a | |
| Control | 5 | 0.244 c | 0.223 d | 0.818 c | 0.828 b |
| 10 | 0.176 c | 0.158 d | 1.113 b | 0.995 a | |
| Control | 0.222 c | 0.404 c | 0.759 cd | 0.483 cd | |
| 2000 | 5 | 0.411 b | 0.366 c | 0.739 cd | 0.759 b |
| 10 | 0.355 b | 0.399 c | 0.591 de | 0.581 c | |
| Control | 0.561 a | 0.571 a | 0.394 f | 0.404 d | |
| 4000 | 5 | 0.528 a | 0.493 b | 0.463 ef | 0.433 d |
| 10 | 0.520 a | 0.532 ab | 0.404 ef | 0.483 cd | |
| Treatment | Total Methanolic Extract (g/100 g D.W.) | Silybin (A + B) Composition (mg/g Extract) | |
|---|---|---|---|
| Salinity (ppm) | MLE (g/L) | ||
| Control | Control | 13.421 d * | 1.056 a |
| 5 | 13.910 d | 2.262 cd | |
| 10 | 12.560 cd | 1.796 bc | |
| 2000 | Control | 23.614 a | 1.578 d |
| 5 | 21.960 b | 1.444 c | |
| 10 | 18.620 bc | 1.279 b | |
| 4000 | Control | 21.240 b | 1.119 b |
| 5 | 21.261 b | 1.148 b | |
| 20.790 b | 0.926 a | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, Y.-K.; El-Sherif, F.; Habib, E.S.; Khattab, S. Moringa oleifera Leaf Extract Enhanced Growth, Yield, and Silybin Content While Mitigating Salt-Induced Adverse Effects on the Growth of Silybum marianum. Agronomy 2021, 11, 2500. https://doi.org/10.3390/agronomy11122500
Yap Y-K, El-Sherif F, Habib ES, Khattab S. Moringa oleifera Leaf Extract Enhanced Growth, Yield, and Silybin Content While Mitigating Salt-Induced Adverse Effects on the Growth of Silybum marianum. Agronomy. 2021; 11(12):2500. https://doi.org/10.3390/agronomy11122500
Chicago/Turabian StyleYap, Yun-Kiam, Fadia El-Sherif, Eman S. Habib, and Salah Khattab. 2021. "Moringa oleifera Leaf Extract Enhanced Growth, Yield, and Silybin Content While Mitigating Salt-Induced Adverse Effects on the Growth of Silybum marianum" Agronomy 11, no. 12: 2500. https://doi.org/10.3390/agronomy11122500






