Chemical Composition, Antibacterial, Enzyme-Inhibitory, and Anti-Inflammatory Activities of Essential Oil from Hedychium puerense Rhizome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Essential Oil
2.3. Analysis of Essential Oil
2.4. Antibacterial Activity
2.5. Enzyme Inhibitory Activities
2.6. Anti-Inflammatory Activity
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition
3.2. Antibacterial Activity
3.3. Enzyme Inhibitory Activity
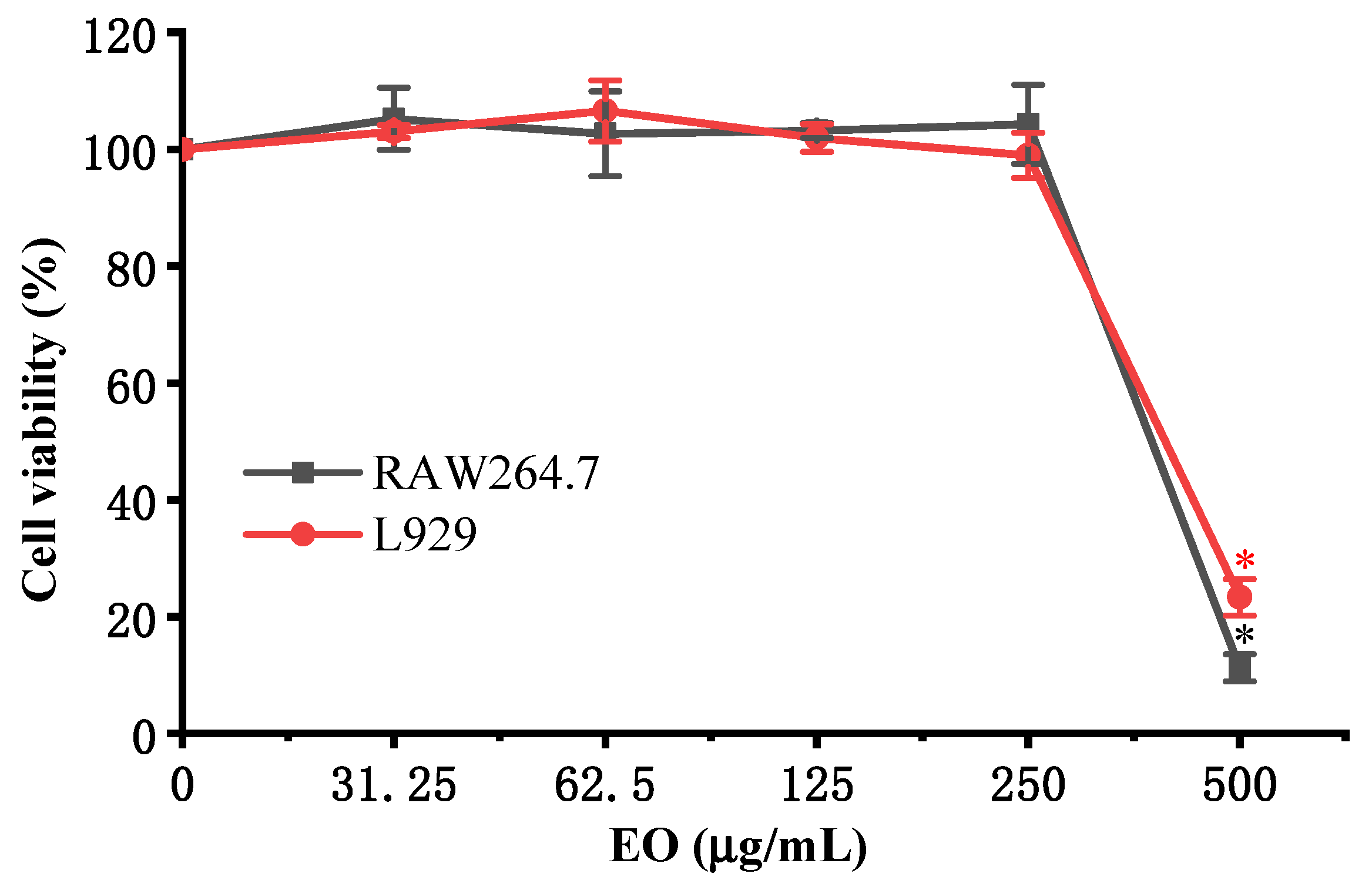
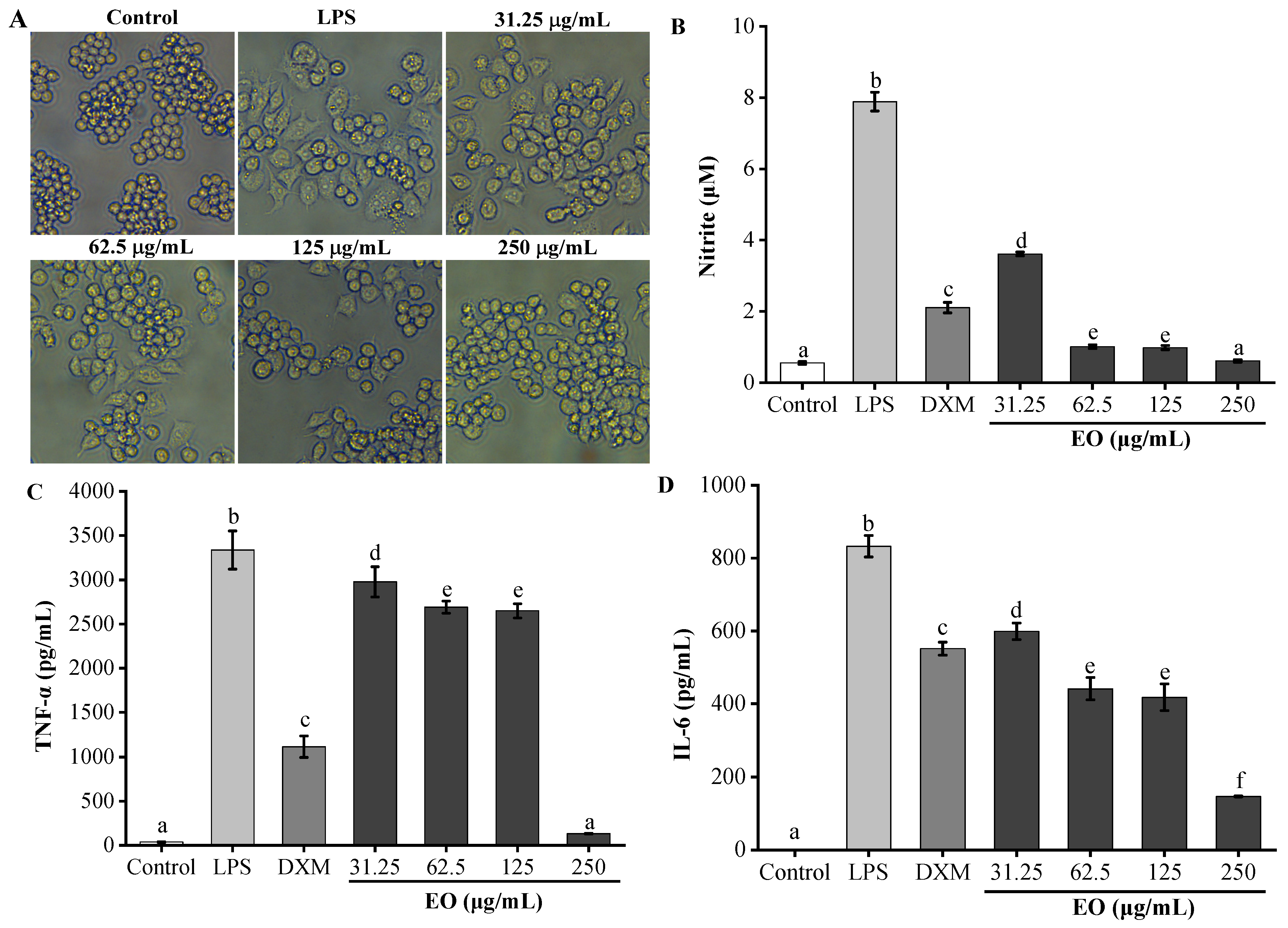
3.4. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evid. Based Complement. Alternat. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Buchbauer, G.; Bohusch, R. Biological activities of essential oils: An update. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 281–321. [Google Scholar]
- Harris, R. Phytotherapeutic uses of essential oils. In Handbook of Essential Oils: Science, Technology, and Applications; Baser, K.H.C., Buchbauer, G., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 381–431. [Google Scholar]
- Bayala, B.; Bassole, I.H.N.; Scifo, R.; Gnoula, C.; Morel, L.; Lobaccaro, J.M.; Simpore, J. Anticancer activity of essential oils and their chemical components—A review. Am. J. Cancer Res. 2014, 4, 591–607. [Google Scholar]
- The Plant List. Version 1.1. Available online: http://www.theplantlist.org/1.1/browse/A/Zingiberaceae/Hedychium/ (accessed on 3 September 2021).
- Prakash, O.; Chandra, M.; Punetha, H.; Pant, A.K.; Rawat, D.S. Chapter 84-Spiked Ginger Lily (Hedychium spp.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V., Ed.; Academic Press: London, UK, 2016; pp. 737–750. [Google Scholar]
- Sakhanokho, H.F.; Rajasekaran, K. Hedychium Essential Oils: Composition and Uses. In Essential Oil Research; Malik, S., Ed.; Springer: Cham, Switzerland, 2019; pp. 49–60. [Google Scholar]
- Hartati, R.; Suganda, A.G.; Fidrianny, I. Botanical, phytochemical and pharmacological properties of Hedychium (Zingiberaceae)—A review. Procedia Chem. 2014, 13, 150–163. [Google Scholar] [CrossRef] [Green Version]
- Tushar; Basak, S.; Sarma, G.C.; Rangan, L. Ethnomedical uses of Zingiberaceous plants of Northeast India. J. Ethnopharmacol. 2010, 132, 286–296. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Kar, B.; Sahoo, A.; Panda, P.C.; Nayak, S.; Mahapatra, N. Volatile metabolite profiling of ten Hedychium species by gas chromatography mass spectrometry coupled to chemometrics. Ind. Crop. Prod. 2018, 126, 135–142. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghoshb, B.; Pandac, P.C.; Nayak, S.; Mahapatra, N. Chemical diversity, antioxidant and antimicrobial activities of the essential oils from Indian populations of Hedychium coronarium Koen. Ind. Crop. Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Lima, A.S.; Costa Junior, H.N.P.; Costa-Junior, L.M.; Monteiro, O.S.; Maia, J.G.S.; da Rocha, C.Q. Anthelmintic effect of essential rhizome oil from Hedychium coronarium Koenig (Zingiberaceae) introduced in Northeastern Brazil. Acta Trop. 2021, 218, 105912. [Google Scholar] [CrossRef]
- Tavares, W.R.; Barreto, M.C.; Seca, A.M.L. Uncharted source of medicinal products: The case of the Hedychium genus. Medicines 2020, 7, 23. [Google Scholar] [CrossRef]
- Qian, Y.Y. Two New Species of Hedychium from Yunnan, China. Acta Phytotaxon. Sin. 1996, 34, 443–446. [Google Scholar]
- Wu, D.L.; Larsen, K. Zingiberaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 2000; Volume 24, pp. 322–377. [Google Scholar]
- Plants of the World Online. Available online: http://plantsoftheworldonline.org/taxon/urn:lsid:ipni.org:names:991085-1 (accessed on 4 September 2021).
- Ye, Y.S.; Zhou, X.; Huang, J.P. Ex Situ Flora of China (Zingiberaceae); China Forestry Publishing House: Beijing, China, 2020; pp. 437–439. [Google Scholar]
- Chen, Q.; Zhao, X.G.; Lu, T.Y.; Yang, Y.; Hong, Y.; Tian, M.Y.; Zhou, Y. Chemical Composition, Antibacterial, and Anti-Inflammatory Activities of Essential Oils from Flower, Leaf, and Stem of Rhynchanthus beesianus. BioMed. Res. Int. 2021, 2021, 5562461. [Google Scholar] [CrossRef]
- Tian, M.Y.; Wu, X.H.; Lu, T.Y.; Zhao, X.G.; Wei, F.; Deng, G.D.; Zhou, Y. Phytochemical analysis, antioxidant, antibacterial, cytotoxic, and enzyme inhibitory activities of Hedychium flavum rhizome. Front. Pharmacol. 2020, 11, 572659. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.C.; Justino, A.B.; Prado, D.G.; Koch, G.A.; Martins, M.M.; de Souza Santos, P.; de Morais, S.A.L.; Goulart, L.R.; Cunha, L.C.S.; de Sousa, R.M.F.; et al. Chemical composition, antioxidant activity and inhibitory capacity of α-amylase, α-glucosidase, lipase and non-enzymatic glycation, in vitro, of the leaves of Cassia bakeriana Craib. Ind. Crop. Prod. 2019, 140, 111641. [Google Scholar] [CrossRef]
- Zardi-Bergaoui, A.; Jelizi, S.; Flamini, G.; Ascrizzi, R.; Jannet, B.H. Comparative study of the chemical composition and bioactivities of essential oils of fresh and dry seeds from Myoporum insulare R. Br. Ind. Crop. Prod. 2018, 111, 232–237. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherston, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Letizia, C.S.; Cocchiara, J.; Lalko, J.; Api, A.M. Fragrance material review on linalool. Food Chem. Toxicol. 2003, 41, 943–964. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool—A Review of a Biologically Active Compound of Commercial Importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Pereira, I.; Severino, P.; Santos, A.C.; Silva, A.M.; Souto, E.B. Linalool bioactive properties and potential applicability in drug delivery systems. Colloids Surf. B Biointerfaces 2018, 171, 566–578. [Google Scholar] [CrossRef]
- Monteiro, P.C.; Majolo, C.; Chaves, F.C.M.; Bizzo, H.R.; Almeida O’Sullivan, F.L.; Chagas, E.C. Antimicrobial activity of essential oils from Lippia sidoides, Ocimum gratissimum and Zingiber officinale against Aeromonas spp. J. Essent. Oil Res. 2021, 33, 152–161. [Google Scholar] [CrossRef]
- Guo, F.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Shewanella putrefaciens. Molecules 2021, 26, 245. [Google Scholar] [CrossRef]
- Prakash, A.; Vadivel, V.; Rubini, D.; Nithyanand, P. Antibacterial and antibiofilm activities of linalool nanoemulsions against Salmonella Typhimurium. Food Biosci. 2019, 28, 57–65. [Google Scholar] [CrossRef]
- Silva, V.A.; Sousa, J.P.; Guerra, F.Q.S.; Pessôa, H.L.F.; Freitas, A.F.R.; Alves, L.B.N.; Lima, E.O. Antibacterial activity of Ocimum basilicum essential oil and linalool on bacterial isolates of clinical importance. IJPP 2015, 7, 1066–1071. [Google Scholar]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.S.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [Green Version]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The Antibacterial Mechanism of Terpinen-4-ol Against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Arunkumar, R.; Nair, S.A.; Rameshkumar, K.B.; Subramoniam, A. The essential oil constituents of Zornia diphylla (L.) Pers, and anti-inflammatory and antimicrobial activities of the oil. Rec. Nat. Prod. 2014, 8, 385–393. [Google Scholar]
- Fernandes, F.H.; Guterres, Z.D.; Violante, I.M.P.; Lopes, T.F.S.; Garcez, W.S.; Garcez, F.R. Evaluation of mutagenic and antimicrobial properties of brown propolis essential oil from the Brazilian Cerrado biome. Toxicol. Rep. 2015, 2, 1482–1488. [Google Scholar] [CrossRef] [Green Version]
- van de Laar, F.A.; Lucassen, P.L.; Akkermans, R.P.; van de Lisdonk, E.H.; Rutten, G.E.; van Weel, C. α-Glucosidase inhibitors for patients with type 2 diabetes: Results from a Cochrane systematic review and meta-analysis. Diabetes Care 2005, 28, 154–163. [Google Scholar] [CrossRef] [Green Version]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes: Diagnosis, Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.G.; Jo, S.H.; Ha, K.S.; Kim, S.C.; Kim, Y.C.; Apostolidis, E.; Kwon, Y.I. Effect of long-term supplementation of low molecular weight chitosan oligosaccharide (GO2KA1) on fasting blood glucose and HbA1c in db/db mice model and elucidation of mechanism of action. BMC Complement. Altern. Med. 2014, 14, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.G.; Chen, Q.; Lu, T.Y.; Wei, F.; Yang, Y.; Xie, D.; Wang, H.J.; Tian, M.Y. Chemical Composition, Antibacterial, Anti-Inflammatory, and Enzyme Inhibitory Activities of Essential Oil from Rhynchanthus beesianus Rhizome. Molecules 2020, 26, 167. [Google Scholar] [CrossRef] [PubMed]
- More, T.A.; Kulkarni, B.R.; Nalawade, M.L.; Arvindekar, A.U. Antidiabetic activity of linalool and limonene in streptozotocin-induced diabetic rat: A combinatorial therapy approach. Int. J. Pharm. Pharm. Sci. 2014, 6, 59–163. [Google Scholar]
- Sahin Basak, S.; Candan, F. Effect of Laurus nobilis L. Essential Oil and its Main Components on α-glucosidase and Reactive Oxygen Species Scavenging Activity. Iran. J. Pharm. Res. 2013, 12, 367–379. [Google Scholar]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Masum, M.N.; Yamauchi, K.; Mitsunaga, T. Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci. 2019, 7, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef]
- Matsuura, R.; Ukeda, H.; Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem. 2006, 54, 2309–2313. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.K.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef] [PubMed]
- Grutzendler, J.; Morris, J.C. Cholinesterase inhibitors for Alzheimer’s disease. Drugs 2001, 61, 41–52. [Google Scholar] [CrossRef]
- Howes, M.J.; Perry, N.S.L.; Houghton, P.J. Plants with traditional uses and activities, relevant to the management of Alzheimer’s disease and other cognitive disorders. Phytother. Res. 2003, 17, 1–18. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Houghton, P.J.; Theobald, A.; Jenner, P.; Perry, E.K. In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 2000, 52, 895–902. [Google Scholar] [CrossRef]
- Miyazawa, M.; Watanabe, H.; Kameoka, H. Inhibition of acetylcholinesterase activity by monoterpenoids with a p-menthane skeleton. J. Agric. Food Chem. 1997, 45, 677–679. [Google Scholar] [CrossRef]
- Kim, S.W.; Kang, J.; Park, I.K. Fumigant toxicity of Apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J. Asia Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Kang, J.; Koh, S.H.; Ahn, Y.J.; Kang, K.S.; Park, I.K. Fumigant toxicity and acetylcholinesterase inhibitory activity of 4 Asteraceae plant essential oils and their constituents against Japanese termite (Reticulitermes speratus Kolbe). Pestic. Biochem. Physiol. 2014, 113, 55–61. [Google Scholar] [CrossRef]
- Owona, B.A.; Njayou, N.F.; Laufer, S.; Moundipa, P.F.; Schluesener, H.J. A fraction of stem bark extract of Entada africana suppresses lipopolysaccharide-induced inflammation in RAW 264.7 cells. J. Ethnopharmacol. 2013, 149, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, E47–E54. [Google Scholar] [CrossRef]
- Ramalho, T.R.; Filgueiras, L.R.; Pacheco de Oliveira, M.T.; Oliveira, M.T.; Lima, A.L.; Bezerra-Santos, C.R.; Jancar, S.; Piuvezam, M.R. Gamma-Terpinene modulation of LPS-Stimulated macrophages is dependent on the PGE2/IL-10 axis. Planta Med. 2016, 82, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Valente, J.; Zuzarte, M.; Liberal, J.; Gonçalves, M.J.; Lopes, M.C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Margotia gummifera essential oil as a source of anti-inflammatory drugs. Ind. Crop. Prod. 2013, 47, 86–91. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Anti-inflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Bonjardim, L.R.; Cunha, E.S.; Guimarães, A.G.; Santana, M.F.; Oliveira, M.G.B.; Serafini, M.R.; Araújo, A.A.S.; Antoniolli, A.R.; Cavalcanti, S.C.H.; Santos, M.R.V.; et al. Evaluation of the anti-inflammatory and antinociceptive properties of p-cymene in mice. Z. Naturforsch. C J. Biosci. 2012, 67, 15–21. [Google Scholar] [CrossRef] [PubMed]
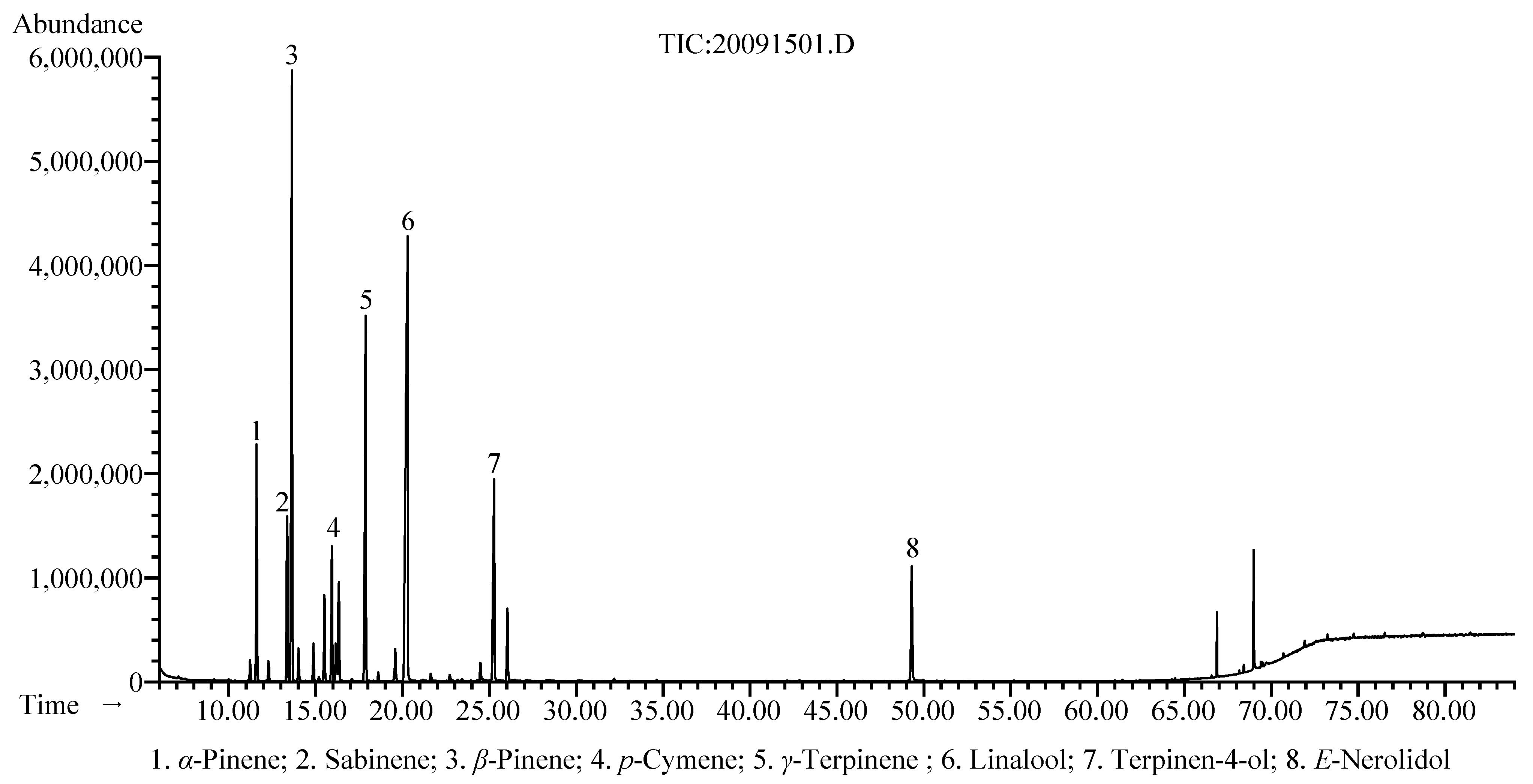
| Compounds a | RI b | RI c | RT (min) | % Area | Identification d |
|---|---|---|---|---|---|
| Octane | 800 | 800 | 7.074 | tr e | MS, RI |
| Tricyclene | 926 | 925 | 11.132 | tr e | MS, RI |
| α-Thujene | 928 | 929 | 11.224 | 0.5 | MS, RI |
| α-Pinene | 937 | 937 | 11.599 | 5.8 | MS, RI |
| Camphene | 953 | 952 | 12.29 | 0.5 | MS, RI |
| Sabinene | 977 | 974 | 13.357 | 4.9 | MS, RI |
| β-Pinene | 983 | 979 | 13.641 | 18.6 | MS, RI |
| β-Myrcene | 992 | 991 | 14.013 | 0.8 | MS, RI |
| α-Phellandrene | 1009 | 1005 | 14.87 | 1.0 | MS, RI |
| 3-Carene | 1014 | 1011 | 15.186 | 0.1 | MS, RI |
| α-Terpinene | 1020 | 1017 | 15.504 | 2.2 | MS, RI |
| p-Cymene | 1028 | 1025 | 15.931 | 3.6 | MS, RI |
| Limonene | 1032 | 1030 | 16.163 | 1.3 | MS, RI |
| 1,8-Cineole | 1035 | 1032 | 16.335 | 2.6 | MS, RI |
| α-Ocimene | 1048 | 1047 | 17.076 | 0.1 | MS, RI |
| γ-Terpinene | 1062 | 1060 | 17.881 | 12.1 | MS, RI |
| 4-Thujanol | 1070 | 1070 | 18.326 | 0.0 | MS, RI |
| Linolool oxide | 1075 | 1074 | 18.596 | 0.2 | MS, RI |
| α-Terpinolene | 1092 | 1088 | 19.585 | 1.1 | MS, RI |
| Linalool | 1104 | 1099 | 20.294 | 26.5 | MS, RI |
| 1,3,8-p-Menthatriene | 1116 | 1119 | 21.047 | tr e | MS, RI |
| Fenchol | 1118 | 1120 | 21.19 | tr e | MS, RI |
| p-Menth-2-en-1-ol | 1125 | 1126 | 21.619 | 0.2 | MS, RI |
| trans-p-2-Menthen-1-ol | 1143 | 1140 | 22.713 | 0.2 | MS, RI |
| Camphor | 1150 | 1145 | 23.173 | tr e | MS, RI |
| Camphene hydrate | 1154 | 1148 | 23.43 | 0.1 | MS, RI |
| Pinocarvone | 1168 | 1164 | 24.299 | 0.0 | MS, RI |
| endo-Borneol | 1171 | 1167 | 24.485 | 0.5 | MS, RI |
| Terpinen-4-ol | 1183 | 1177 | 25.272 | 7.7 | MS, RI |
| α-Terpineol | 1195 | 1190 | 26.041 | 2.2 | MS, RI |
| Bornyl acetate | 1290 | 1285 | 32.181 | 0.1 | MS, RI |
| cis-β-Farnesene | 1459 | 1444 | 42.841 | tr e | MS, RI |
| E-Nerolidol | 1568 | 1564 | 49.313 | 4.1 | MS, RI |
| Coronarin E | 2161 | 2136 | 66.874 | 0.8 | MS, RI |
| Monoterpene hydrocarbons | 52.7 | ||||
| Oxygenated monoterpenes | 40.5 | ||||
| Sesquiterpene hydrocarbons | tr e | ||||
| Oxygenated sesquiterpenes | 4.1 | ||||
| Diterpenes | 0.8 | ||||
| Total identified | 98.2 | ||||
| Bacterial Strains a | EO | Streptomycin | ||||
|---|---|---|---|---|---|---|
| DIZ b (mm) | MIC c (mg/mL) | MBC c (mg/mL) | DIZ b (mm) | MIC c (μg/mL) | MBC c (μg/mL) | |
| Gram positive | ||||||
| E. faecalis | 10.30 ± 0.32 | 3.13 | 3.13 | 7.01 ± 0.29 | 12.50 | 25.00 |
| B. subtilis | 9.80 ± 0.88 | 3.13 | 3.13 | 18.39 ± 0.71 | 1.56 | 3.13 |
| S. aureus | 7.44 ± 0.34 | 6.25 | 12.50 | 22.03 ± 0.61 | 0.39 | 0.78 |
| Gram negative | ||||||
| P. vulgaris | 10.16 ± 0.43 | 3.13 | 6.25 | 14.83 ± 0.51 | 0.78 | 1.56 |
| P. aeruginosa | 7.76 ± 0.39 | 6.25 | 12.50 | 10.17 ± 0.43 | 3.13 | 12.50 |
| E. coli | 7.73 ± 0.14 | 6.25 | 12.50 | 16.25 ± 0.89 | 0.78 | 3.13 |
| Samples | Enzyme Inhibitory Activity (IC50, mg/mL) 1 | |||
|---|---|---|---|---|
| α-Glucosidase | Tyrosinase | Acetylcholinesterase | Butyrylcholinesterase | |
| EO | 5.42 ± 0.32 a | 3.23 ± 0.21 a | 0.94 ± 0.02 a | 1.32 ± 0.06 a |
| Acarbose | 0.23 ± 0.03 b | – | – | – |
| Arbutin | – | 0.26 ± 0.01 b | – | – |
| Galanthamine * | – | – | 0.45 ± 0.03 b | 6.94 ± 0.29 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Liu, X.; Wang, H.; Zhang, M.; Tian, M. Chemical Composition, Antibacterial, Enzyme-Inhibitory, and Anti-Inflammatory Activities of Essential Oil from Hedychium puerense Rhizome. Agronomy 2021, 11, 2506. https://doi.org/10.3390/agronomy11122506
Hong Y, Liu X, Wang H, Zhang M, Tian M. Chemical Composition, Antibacterial, Enzyme-Inhibitory, and Anti-Inflammatory Activities of Essential Oil from Hedychium puerense Rhizome. Agronomy. 2021; 11(12):2506. https://doi.org/10.3390/agronomy11122506
Chicago/Turabian StyleHong, Yi, Xiongli Liu, Huijuan Wang, Min Zhang, and Minyi Tian. 2021. "Chemical Composition, Antibacterial, Enzyme-Inhibitory, and Anti-Inflammatory Activities of Essential Oil from Hedychium puerense Rhizome" Agronomy 11, no. 12: 2506. https://doi.org/10.3390/agronomy11122506






