Properties of Humic Acids in Meadow Soils Irrigated with the Slope-and-Flooding System
Abstract
:1. Introduction
- -
- Agricultural land—61% (including arable land—73%, grassland—20%, orchards—2%, and other land—5%),
- -
- Forests and woodlands—31%,
- -
- Other land—8%.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Soil Analysis
2.2.2. Extraction of Humic Acids
2.2.3. Characteristics of Humic Acids
2.2.4. Statistical Analysis
3. Results and Discussion
3.1. Basic Parameters of Soils
3.2. Elemental Composition of Humic Acids
3.3. Spectrometric Parameters of Humic Acids in the UV–Vis Range
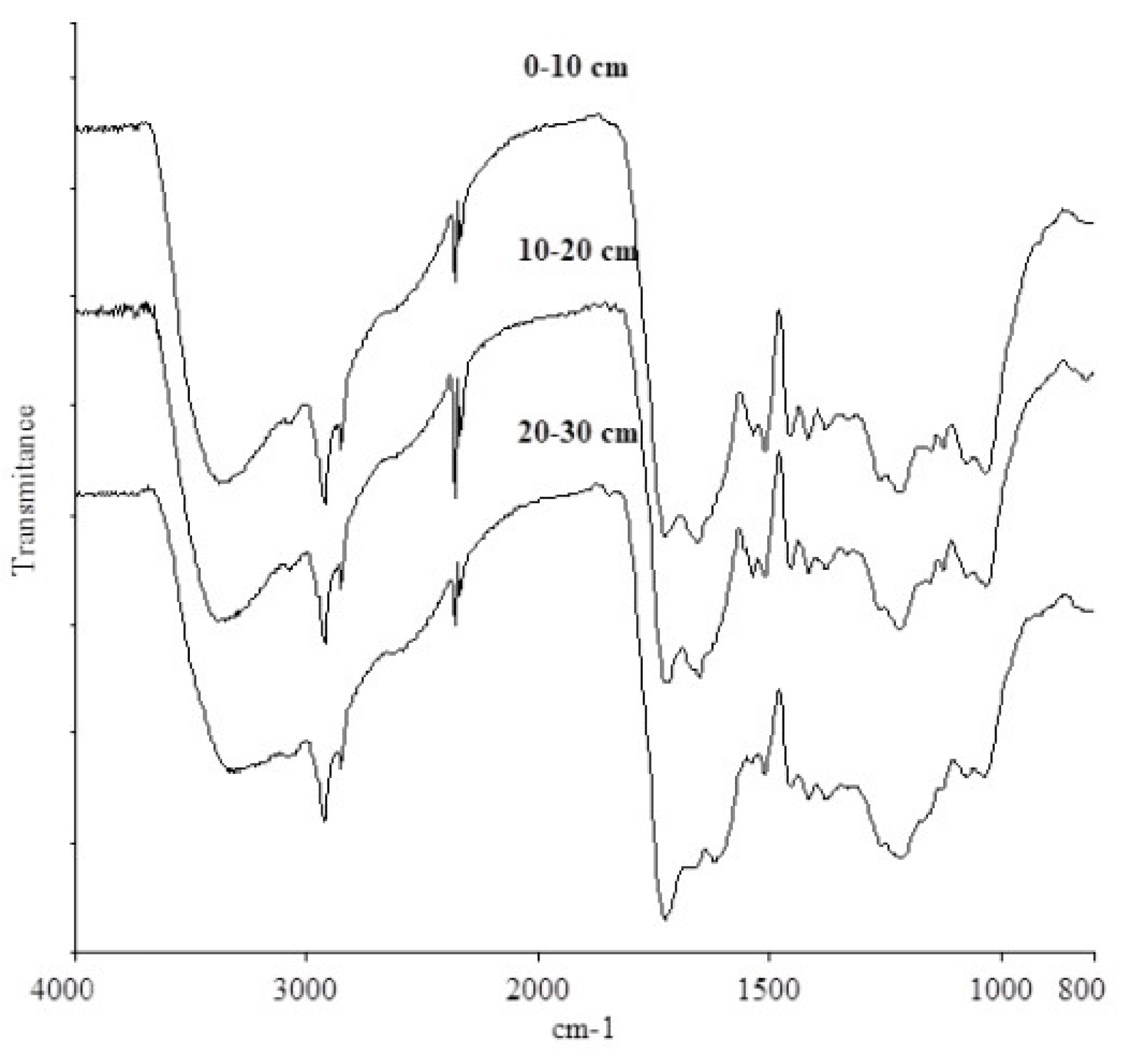
3.4. Analysis of the Fourier Transform Infrared (FTIR) Spectra of Humic Acids
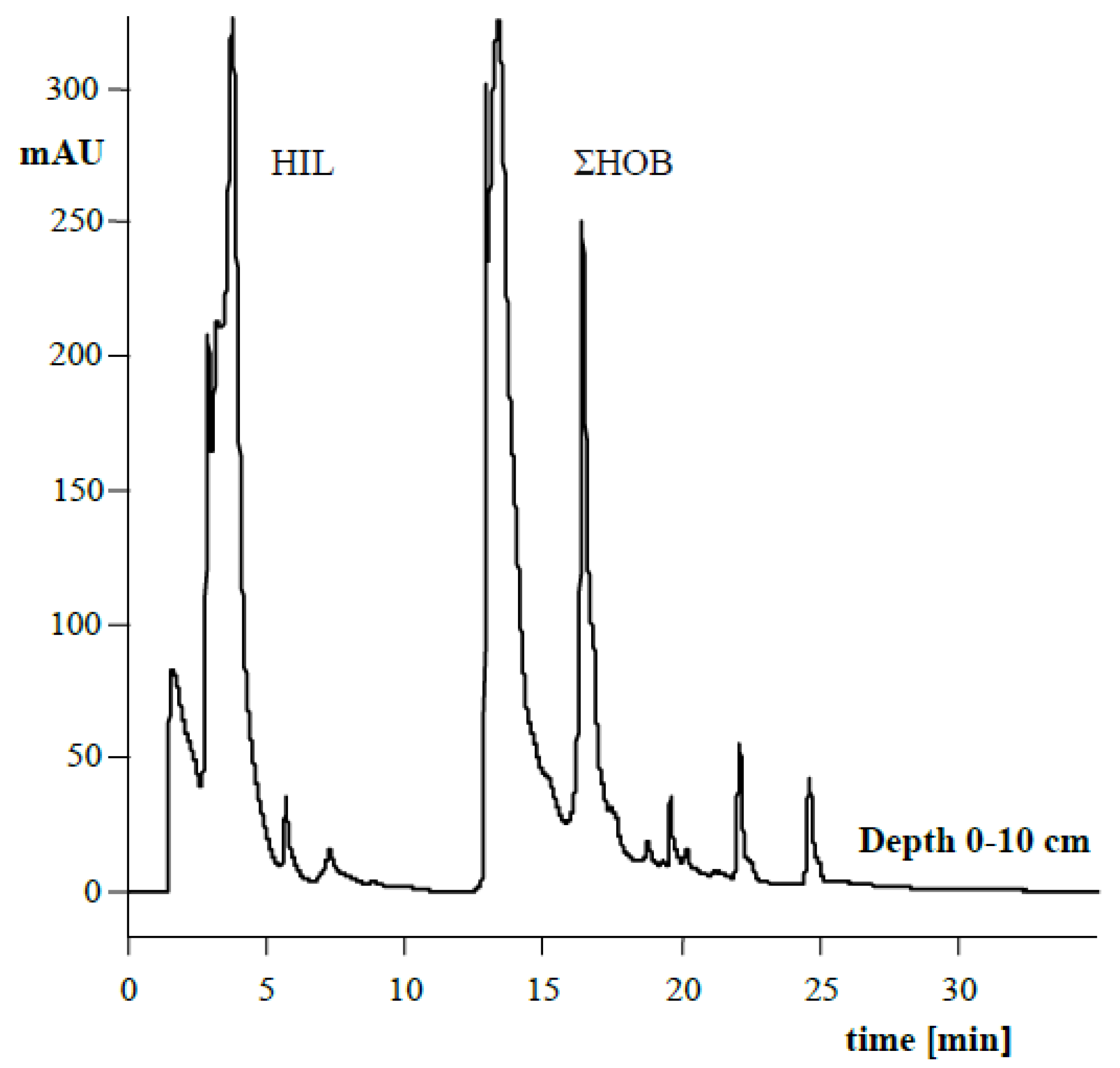
3.5. Hydrophilic and Hydrophobic Nature of Humic Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hayes, M.H.B.; Swift, R.S. Vindication of humic substances as a key component of organic matter in soil and water. Adv. Agron. 2020, 163, 1–37. [Google Scholar] [CrossRef]
- Lanyi, K. Assessment of the relations between the spectroscopic characteristics of soils and their ability to adsorb organic pollutants. Microchem. J. 2005, 79, 249–256. [Google Scholar] [CrossRef]
- McCarthy, P. The principles of humic substances. Soil Sci. 2001, 166, 738–751. [Google Scholar] [CrossRef]
- Yamashita, Y.; Jaffé, R.; Maie, N.; Tanoue, E. Assessing the dynamics of dissolved organic matter (DOM) in coastal environments by excitation emission matrix fluorescence and parallel factor analysis (EEM-PARAFAC). Limnol. Oceanogr. 2008, 53, 1900–1908. [Google Scholar] [CrossRef] [Green Version]
- Canellas, L.P.; Piccolo, A.; Dobbss, L.B.; Spaccini, R.; Olivares, F.L.; Zandonadi, D.B.; Façanha, A.R. Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 2010, 78, 457–466. [Google Scholar] [CrossRef]
- Lal, R. Soil Carbon Sequestration in Latin America. In Carbon Sequestration in Soils of Latin America; Lal, R., Cerri, C.C., Bernoux, M., Etcheves, J., Cerri, E., Eds.; Food Products Press: New York, NY, USA, 2006; pp. 49–64. [Google Scholar]
- Hayes, M.H.B.; Clapp, C.E. Humic substances: Considerations of compositions, aspects of structure, and environmental influences. Soil Sci. 2001, 166, 723–737. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.; Chen, Y.; Jamroz, E.; Miano, T. Preface: Humic substances in the environment. J. Soils Sediments 2018, 18, 2665–2667. [Google Scholar] [CrossRef] [Green Version]
- Visser, S.A. Application of van Krevelen’s graphical statistical method for the study of aquatic humic material. Environ. Sci. Technol. 1983, 17, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Baigorri, R.; González-Gaitano, G.; García-Mina, J.M. The complementary use of 1H NMR, 13C NMR, FTIR and size exclusion chromatography to investigate the principal structural changes associated with composting of organic materials with diverse origin. Org. Geochem. 2007, 38, 2012–2023. [Google Scholar] [CrossRef]
- Trubetskaya, O.E.; Trubetskoj, O.A.; Voyard, G.; Richard, C. Determination of hydrophobicity and optical properties of soil humic acids isolated by different methods. J. Geochem. Explor. 2013, 132, 84–89. [Google Scholar] [CrossRef]
- Boguta, P.; D’Orazio, V.; Sokołowska, Z.; Senesi, N. Effects of selected chemical and physicochemical properties of humic acids from peat soils on their interaction mechanisms with copper ions at various pHs. J. Geochem. Explor. 2016, 168, 119–126. [Google Scholar] [CrossRef]
- Tan, K.H. Principles of Soil Chemistry; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Polak, J.; Bartoszek, M.; Żądło, M.; Kos, A.; Sułkowski, W.W. The spectroscopic studies of humic acid extracted from sediment collected at different seasons. Chemosphere 2011, 84, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Kumada, K. Chemistry of Soil Organic Matter; Elsevier: Amsterdam, The Netherlands, 1988. [Google Scholar]
- Gonet, S.S.; Debska, B. Properties of humic acids produced during decomposition of plant residues in soil. Rostl. Vyroba 1999, 45, 455–460. [Google Scholar]
- Vieyra, F.E.M.; Palazzi, V.I.; de Pinto, M.I.S.; Borsarelli, C.D. Combined UV–Vis absorbance and fluorescence properties of extracted humic substances-like for characterization of composting evolution of domestic solid wastes. Geoderma 2009, 151, 61–67. [Google Scholar] [CrossRef]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Schlenger, P.; García-Valverde, M. Monitoring changes in the structure and properties of humic substances following ozonation using UV-Vis, FTIR and H NMR techniques. Sci. Total Environ. 2016, 541, 626–637. [Google Scholar] [CrossRef]
- Banach-Szott, M.; Debska, B. Chromatographic Separation of Humic Acids of a Forest Soil. In Humic Substances-Linking Structure to Functions; Frimmel, F.H., Abbt-Braun, G., Eds.; Repository KITopen: Karlsruhe, Germany, 2006; pp. 225–228. [Google Scholar]
- Debska, B.; Banach-Szott, M.; Dziamski, A.; Gonet, S.S. Chromatographic characteristics (HPLC, HPSEC) of humic acids of soil fertilised with various organic fertilisers. Chem. Ecol. 2010, 26, 49–57. [Google Scholar] [CrossRef]
- Preuße, G.; Friedrich, S.; Salzer, R. Retention behavior of humic substances in reversed phase HPLC. Fresenius J. Anal. Chem. 2000, 368, 268–273. [Google Scholar] [CrossRef]
- Sierra, M.; Giovanela, M.; Parlanti, E.; Soriano-Sierra, E.J. 3D-fluorescence spectroscopic analysis of HPLC fractionated estuarine fulvic and humic acids. J. Brazilian Chem. Soc. 2006, 17, 113–124. [Google Scholar] [CrossRef]
- Woelki, G.; Friedrich, S.; Hanschmann, G.; Salzer, R. HPLC fractionation and structural dynamics of humic acids. Fresenius J. Anal. Chem. 1997, 357, 548–552. [Google Scholar] [CrossRef]
- Debska, B.; Drąg, M.; Banach-Szott, M. Molecular size distribution and hydrophilic and hydrophobic properties of humic acids isolated from forest soil. Soil Water Res. 2007, 2, 45–53. [Google Scholar] [CrossRef] [Green Version]
- Debska, B.; Gonet, I. Share of hydrophilic and hydrophobic fractions in humic acids formed as a result of post-harvest residue decomposition. Pol. J. Soil Sci. 2007, 40, 57–65. [Google Scholar]
- Cocozza, C.; Miano, T. Structural Resolution of Metal-Humic Acids Interactions through Deconvolution FT-IR Spectroscopy. In Proceedings of the 11th IHSS Meeting, Boston, MA, USA, 21–26 July 2002; Davis, G., Ghabbour, E.A., Eds.; IHSS: Boston, MA, USA, 2002; pp. 264–266. [Google Scholar]
- Pajączkowska, J.; Sułkowska, A.; Sułkowski, W.W.; Jędrzejczyk, M. Spectroscopic study of the humification process during sewage sludge treatment. J. Mol. Struct. 2003, 651, 141–149. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lutzow, M.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Lieb, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soil—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- Minami, K.; Goudriaan, J.E.A.; Lantinga, E.A.; Kimura, T. Significance of Grasslands in Emission and Absorption of Greenhouse Gases. In Proceedings of the XVIIth International Grassland Congress, Palmerston North, Hamilton, Lincoln, New Zealand, 13–16 February 1993; pp. 1231–1238. [Google Scholar]
- Conant, R.T.; Paustian, K.; Elliott, E.T. Grassland management and conversion into grassland: Effects on soil carbon. Ecol. Appl. 2001, 11, 343–355. [Google Scholar] [CrossRef]
- Mannetje, L.T. Advances in grassland science. NJAS Wagen. J. Life Sci. 2003, 50, 195–221. [Google Scholar] [CrossRef] [Green Version]
- Lal, R. Sequestering carbon in soils of agro-ecosystems. Food Policy 2011, 36, 533–539. [Google Scholar] [CrossRef]
- Kämpf, I.; Hölzel, N.; Störrle, M.; Broll, G.; Kiehl, K. Potential of temperate agricultural soils for carbon sequestration: A meta-analysis of land-use effects. Sci. Total Environ. 2016, 566, 428–435. [Google Scholar] [CrossRef]
- Environmental Protection. Study of the Central Statistical Office 2019. Available online: https://stat.gov.pl/obszary-tematyczne/srodowisko-energia/srodowisko/ochrona-srodowiska-2019,1,20.html (accessed on 29 November 2019). (In Polish)
- Kondratowicz-Maciejewska, K.; Kobierski, M.; Murawska, A. Effect of Brunic Arenosols use on selected physicochemical properties in organic matter. Soil Sci. Ann. 2012, 63, 19–24. [Google Scholar] [CrossRef] [Green Version]
- EIP-AGRI, Focus Group. Profitability of Permanent Grassland 2019. Available online: https://ec.europa.eu/eip/agriculture/sites/default/files/fg9_permanent_grassland_profitability_starting_paper_2014_en.pdf (accessed on 28 May 2014).
- Sabiniarz, A. Historic outline of the Czersk Meadows. Grassl. Sci. Pol. 2006, 9, 184–194. (In Polish) [Google Scholar]
- Papke, R. The formation of plant communities of the Czerskie Meadows depending on water conditions. Zesz. Probl. Post. Nauk Roln. 1958, 13, 97–117. (In Polish) [Google Scholar]
- Grzyb, S. Characteristics of soils and vegetation in Czerskie Meadows. Mater. Semin. 1969, 8, 14–26. (In Polish) [Google Scholar]
- Lorenc, K. Changes in the structure of plant communities in Czerskie Meadows in terms of phytosociology. IMUZ Falenty. Mat. Konf. 1969, 8, 79–85. (In Polish) [Google Scholar]
- Sabiniarz, A.; Kozłowski, S. Czerskie Meadows in the landscape aspect. Grassl. Sci. Pol. 2009, 12, 141–154. (In Polish) [Google Scholar]
- Sabiniarz, A.; Kozłowski, S. Łąki Czerskie w aspekcie paszowym (Czerskie Meadows in terms of forage). Łąkarstwo w Polsce. Grassl. Sci. Pol. 2009, 12, 155–163. (In Polish) [Google Scholar]
- Kochsiek, A.E.; Knops, J.M.H.; Walters, D.T.; Arkebauer, T.J. Impacts of management on decomposition balance in irrigated and rainfed no till agricultural systems. Agr. Forest Meteorol. 2009, 149, 1983–1993. [Google Scholar] [CrossRef]
- Trost, B.; Prochnow, A.; Drastig, K.; Meyer-Aurich, A.; Ellmer, F.; Baumecker, M. Irrigation, soil organic carbon and N2O emissions. A review. Agron. Sustain. Dev. 2013, 33, 733–749. [Google Scholar] [CrossRef] [Green Version]
- Getaneh, F.; Deressa, A.; Negassa, W. Influence of Small Scale Irrigation on Selected Soil Chemical Properties. In Proceedings of the Tropentag, Witzenhausen, Germany, 9–11 October 2007. [Google Scholar]
- Nunes, J.M.; López-Piñeiro, A.; Albarrán, A.; Muñoz, A.; Coelho, J. Changes in selected soil properties caused by 30 years of continuous irrigation under Mediterranean conditions. Geoderma 2007, 139, 321–328. [Google Scholar] [CrossRef]
- Costantini, E.A.C.; Lorenzetti, R. Soil degradation processes in the Italian agricultural and forest ecosystems. Ital. J. Agron. 2013, 8, e28. [Google Scholar] [CrossRef] [Green Version]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Howell, T.A. Irrigation’s Role in Enhancing Water Use Efficiency. In National Irrigation Symposium, Proceedings of the 4th Decennial Symposium, Phoenix, AZ, USA, 14–16 November 2000; Evans, R.G., Benham, B.L., Trooien, T.P., Eds.; American Society of Agricultural Engineers: Phoenix, AZ, USA, 2000; pp. 66–80. [Google Scholar]
- Fallahzade, J.; Hajabbasi, M.A. The effects of irrigation and cultivation on the quality of desert soil in central Iran. Land Degrad. Dev. 2012, 23, 53–61. [Google Scholar] [CrossRef]
- Follett, R.F.; Jantalia, C.P.; Halvorson, A.D. Soil carbon dynamics for irrigated corn under two tillage systems. Soil Sci. Soc. Am. J. 2013, 77, 951–963. [Google Scholar] [CrossRef]
- Entry, J.A.; Sojka, R.E.; Shewmaker, G.E. Management of irrigated agriculture to increase organic carbon storage in soils. Soil Sci. Soc. Am. J. 2002, 66, 1957–1964. [Google Scholar] [CrossRef] [Green Version]
- Trost, B.; Ellmer, F.; Baumecker, M.; Meyer-Aurich, A.; Prochnow, A.; Drastig, K. Effects of irrigation and nitrogen fertilizer on yield, carbon inputs from above ground harvest residues and soil organic carbon contents of a sandy soil in Germany. Soil Use Manag. 2014, 30, 209–218. [Google Scholar] [CrossRef]
- Kenngott, K.G.; Riess, K.; Muñoz, K.; Schaumann, G.E.; Buhk, C.; Diehl, D. Flood Pulse Irrigation of Meadows Shapes Soil Chemical and Microbial Parameters More Than Mineral Fertilization. Soil Syst. 2021, 5, 24. [Google Scholar] [CrossRef]
- Buhk, C.; Schirmel, J.; Rebekka, G.; Frör, O. Traditional Water Meadows: A Sustainable Management Type for the Future? In Irrigation in Agroecosystems; IntechOpen: London, UK, 2018. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition Reactions; Wiley: Chichester, UK, 1994. [Google Scholar]
- Senesi, N.; Miano, T.M. Humic Substances in the Global Enviro nment and Implications on Human Health; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Schlesinger, W.H. Biogeochemistry: An Analysis of Global Change; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- COM 231 Final. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions; Thematic Strategy for Soil Protection: Croatia, Turkey, 2006; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52006DC0231&from=EN (accessed on 26 September 2006).
- Debska, B.; Dlugosz, J.; Piotrowska-Dlugosz, A.; Banach-Szott, M. The impact of bio-fertilizer on the soil organic matter status and carbon sequestration—results from a fiel-scale study. J. Soils Sediments 2016, 16, 2335–2343. [Google Scholar] [CrossRef] [Green Version]
- van Reeuwijk, L.P. Procedures for Soil Analysis; International Soil Reference and Information Centre: Wageningen, The Netherlands, 2002. [Google Scholar]
- Kobierski, M. Texture of different types of soils formed from glacial till in the aspect of PTG 2008 classification system. Soil Sci. Ann. 2010, 61, 67–76. (In Polish) [Google Scholar]
- Kobierski, M.; Kondratowicz-Maciejewska, K.; Kociniewska, K. Soil quality assessment of Phaeozems and Luvisols from the Kujawy region (central Poland). Soil Sci. Ann. 2015, 66, 111–118. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical grouping to optimise an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Jadczyszyn, T.; Kowalczyk, J.; Lipiński, W. Fertilization recommendations for field crops and permanent grasslands. In Teaching Materials; IUNG-PIB: Puławy, Poland, 2010; Volume 95, p. 24. (In Polish) [Google Scholar]
- Pizzeghello, D.; Francioso, O.; Concheri, G.; Muscolo, A.; Nardi, S. Land use affects the soil C sequestration in alpine environment. NE Italy For. 2017, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Drąg, M.; Debska, B.; Dziamski, A. Properties of humic substances of forest and meadow soil in the area of the Wierzchlas Reserve. Humic Subst. Ecosys. 2007, 7, 141–151. [Google Scholar]
- Jonczak, J. Soil organic matter properties in stagnic luvisols under different land use types. Acta Agroph. 2013, 20, 565–576. [Google Scholar]
- Sun, S.; Che, T.; Gentine, P.; Chen, Q.; Wang, L.; Yan, Z.; Chen, B.; Song, Z. Shallow groundwater inhibits soil respiration and favors carbon uptake in a wet alpine meadow ecosystem. Agric. For. Meteorol. 2021, 297, 108254. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Review: Factors affecting rhizosphere priming effects. J. Plant Nutr. Soil Sci. 2002, 165, 382–396. [Google Scholar] [CrossRef]
- van Camp, G.; Gentile, A.; Bujarrabal, B.; Jones, R.; Montanarella, L.; Olazabal, C.; Selvaradjou, S. Reports of the Technical Working Groups Establish under the Thematic Strategy for Soil Protection; Vol. IV: Contamination and Land Management. EUR 21319 EN; Office for Official Publications of the European Communities: Luxembourg, 2004; p. 28870. [Google Scholar]
- Khalid, M.; Soleman, N.; Jones, D.L. Grassland plants affect dissolved organic carbon and nitrogen dynamics in soil. Soil Biol. Biochem. 2007, 39, 378–381. [Google Scholar] [CrossRef]
- Becher, M.; Banach-Szott, M.; Godlewska, A. Organic matter properties of spent button mushroom substrate in the context of soil organic matter reproduction. Agronomy 2021, 11, 204. [Google Scholar] [CrossRef]
- Filip, Z.; Tesarova, M. Microbial degradation and transformation of humic acids from permanent meadow and forest soils. Int. Biodeterior. Biodegr. 2004, 54, 225–231. [Google Scholar] [CrossRef]
- Orlov, D.S. Humus Acids of Soils; Balkema: Rotterdam, The Netherlands, 1986. [Google Scholar]
- Debska, B.; Drąg, M.; Tobiasova, E. Effect of post-harvest residue of maize, rapeseed, and sunflower on humic acids properties in various soils. Pol. J. Environ. Stud. 2012, 21, 603–613. [Google Scholar]
- Dergacheva, M.I.; Nekrasova, O.A.; Okoneshnikova, M.V.; Vasileva, D.I.; Gavrilov, D.A.; Ochur, K.O.; Ondar, E.E. Ratio of elements in humic acids as a source of information on the environment of soil formation. Contemp. Probl. Ecol. 2012, 5, 497–504. [Google Scholar] [CrossRef]
- Amir, S.; Jouraiphy, A.; Meddich, A.; El Gharous, M.; Winterton, P.; Hafidi, M. Structural study of humic acids during composting of activated sludge-green waste: Elemental analysis, FTIR and 13C NMR. J. Hazard. Mater. 2010, 177, 524–529. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cegarra, J.; Garcia, D.; Roig, A. Chemical and structural evolution of humic acids during organic waste composting. Biodegradation 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Gonet, S.S.; Debska, B.; Zaujec, A.; Banach-Szott, M.; Szombathova, N. Effect of the Tree Species and Soil-and-Climate Conditions on the Properties of Humus in Forest Soils. In Role of Organic Matter in the Environment; Gonet, S.S., Markiewicz, M., Eds.; PTSH: Wroclaw, Poland, 2007; pp. 61–98. [Google Scholar]
- Senesi, N.; D’Orazio, V.; Ricca, G. Humic acids in the first generation of Eurosoils. Geoderma 2003, 116, 325–344. [Google Scholar] [CrossRef]
- Kobierski, M.; Kondratowicz-Maciejewska, K.; Banach-Szott, M.; Wojewódzki, P.; Castejón, J.M.P. Humic substances and aggregate stability in rhizospheric and non-rhizospheric soil. J. Soils Sediments 2018, 18, 2777–2789. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, P.; Almendros, G.; González-Vila, F.; Sanz, J.; González-Pérez, J. Revisiting molecular characteristics responsive for the aromaticity of soil humic acids. J. Soils Sediment. 2015, 15, 781–791. [Google Scholar] [CrossRef] [Green Version]
- Filcheva, E.; Hristova, M.; Nikolova, P.; Popova, T.; Chakalov, K.; Savov, V. Quantitative and qualitative characterisation of humic products with spectral parameters. J. Soils Sediments 2018, 18, 2863–2867. [Google Scholar] [CrossRef]
- Enev, V.; Pospisilova, L.; Klucakova, M.; Liptaj, T.; Doskocil, L. Spectral characterization of selected humic substances. Soil Water Res. 2014, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.; An, T.; Wei, D.; Chi, F.; Zhou, B. Effects of long-term fertilization on soil humic acid composition and structure in Black Soil. PLoS ONE 2017, 12, e0186918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, M.H.B.; Swift, R.S. An appreciation of the contribution of Frank Stevenson to the advancement of studies of soil organic matter and humic substances. J. Soils Sediments 2018, 18, 1212–1231. [Google Scholar] [CrossRef]
- Banach-Szott, M.; Kobierski, M.; Kondratowicz-Maciejewska, K. Humic substances in Fluvisols of the Lower Vistula floodplain, North Poland. Environ. Sci. Pollut. Res. 2018, 25, 23999–24002. [Google Scholar] [CrossRef] [PubMed]
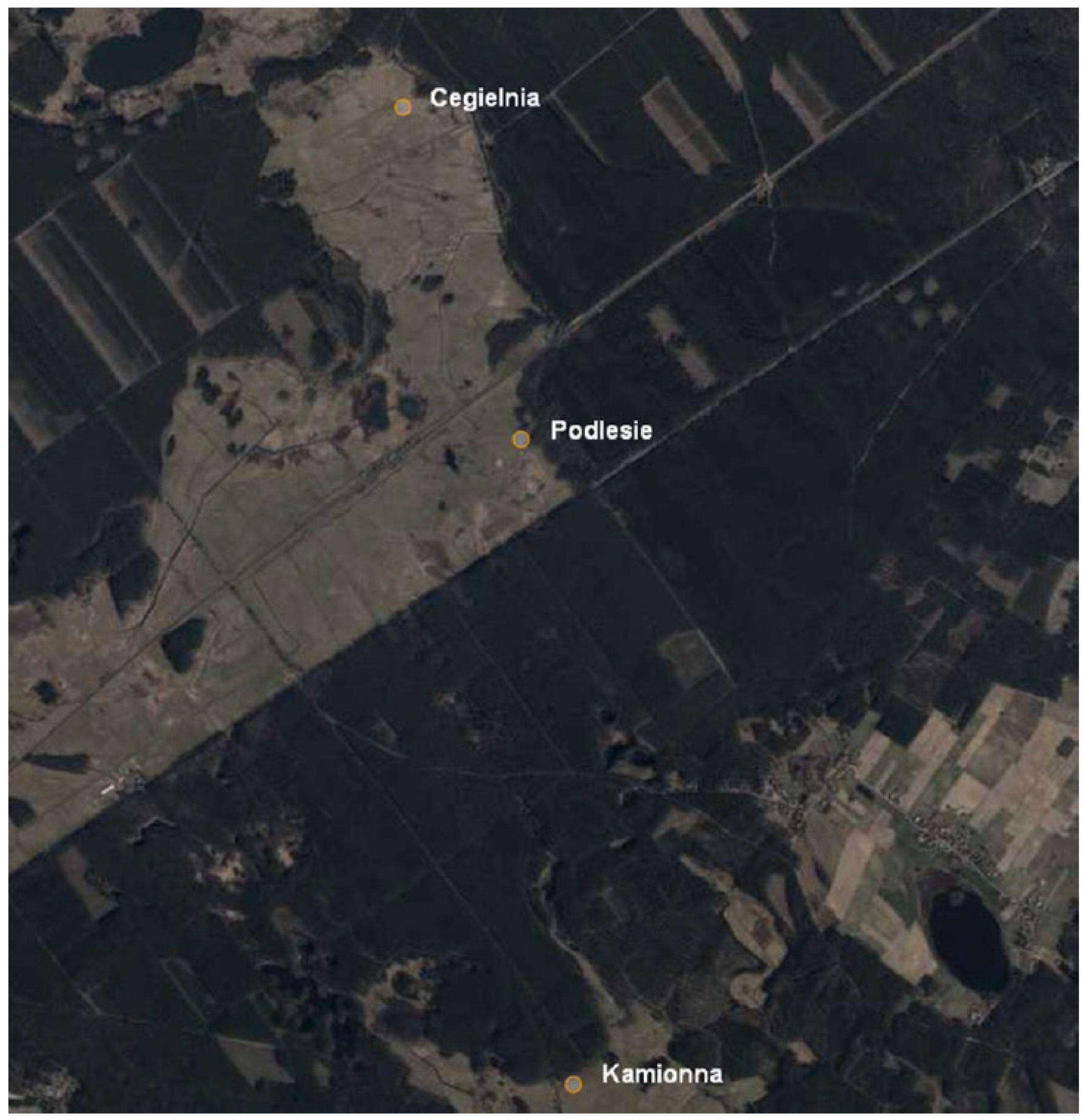

| Factor | pH | Sand (%) | Silt (%) | Clay (%) | |
|---|---|---|---|---|---|
| Kamionna | |||||
| Distance (m) | 5 | 5.5–6.4 | 90.8 | 8.8 | 0.4 |
| 15 | 5.6–6.3 | 91.0 | 8.6 | 0.4 | |
| 25 | 6.2–6.9 | 90.0 | 9.6 | 0.4 | |
| LSD | - | 0.816 | 0.827 | ns | |
| Depth (cm) | 0–10 | 5.5–6.2 | 92.5 | 7.4 | 0.1 |
| 10–20 | 5.6–6.3 | 91.8 | 8.1 | 0.1 | |
| 20–30 | 6.3–6.9 | 87.3 | 11.6 | 1.1 | |
| LSD | - | 0.307 | 0.318 | 0.141 | |
| Cegielnia | |||||
| Distance (m) | 5 | 6.7–7.0 | 88.0 | 11.7 | 0.3 |
| 15 | 6.0–6.8 | 90.4 | 9.4 | 0.2 | |
| 25 | 6.9–7.0 | 91.0 | 8.9 | 0.1 | |
| LSD | - | 0.144 | 0.118 | 0.051 | |
| Depth (cm) | 0–10 | 6.0–6.7 | 90.7 | 9.2 | 0.1 |
| 10–20 | 6.3–7.0 | 88.8 | 11.1 | 0.1 | |
| 20–30 | 6.8–7.0 | 90.0 | 9.6 | 0.4 | |
| LSD | - | 0.161 | 0.161 | 0.101 | |
| Podlesie | |||||
| Distance (m) | 5 | 5.3–6.5 | 91.4 | 8.2 | 0.4 |
| 15 | 5.8–6.4 | 91.8 | 7.9 | 0.3 | |
| 25 | 6.1–6.5 | 91.2 | 8.7 | 0.1 | |
| LSD | - | 0.235 | 0.177 | 0.112 | |
| Depth (cm) | 0–10 | 5.3–6.1 | 92.8 | 7.0 | 0.2 |
| 10–20 | 6.0–6.3 | 91.7 | 8.2 | 0.1 | |
| 20–30 | 6.4–6.5 | 89.9 | 9.7 | 0.4 | |
| LSD | - | 0.249 | 0.129 | 0.163 | |
| Parameter | 0–10 cm n = 27 | 10–20 cm n = 27 | Significant Level (p) * | 0–10 cm n = 27 | 20–30 cm n = 27 | Significant Level (p) * |
|---|---|---|---|---|---|---|
| TOC (g kg−1) | 14.7 | 49.5 | 0.0001 * | 14.7 | 34.9 | 0.0001 * |
| Nt (g kg−1) | 1.09 | 4.30 | 0.0001 * | 1.09 | 2.51 | 0.0001 * |
| TOC/Nt | 15.2 | 11.6 | 0.0002 * | 15.2 | 14.7 | 0.689 |
| Sand (%) | 92.0 | 90.8 | 0.009 * | 92.0 | 90.0 | 0.004 * |
| Silt (%) | 7.92 | 9.13 | 0.011 * | 7.92 | 10.3 | 0.0001 * |
| Clay (%) | 0.18 | 0.13 | 0.145 | 0.18 | 0.73 | 0.0001 * |
| pH | 6.01 | 6.28 | 0.268 | 6.01 | 6.64 | 0.006 * |
| Bulk density (g cm−3) | 1.29 | 1.43 | 0.0002 * | 1.29 | 1.52 | 0.0002 * |
| H/C | 1.34 | 1.30 | 0.005 * | 1.34 | 1.23 | 0.0001 * |
| ω | 0.97 | 0.136 | 0.014 * | 0.97 | 0.164 | 0.0001 * |
| A4/6 | 5.00 | 4.71 | 0.015 * | 5.00 | 4.52 | 0.0002 * |
| ΔLogk | 0.678 | 0.638 | 0.024 * | 0.678 | 0.581 | 0.0001 * |
| HIL (%) | 38.73 | 39.85 | 0.011 * | 38.73 | 40.53 | 0.0005 * |
| HIL/ΣHOB | 0.633 | 0.663 | 0.011 * | 0.633 | 0.684 | 0.0004 * |
| Factor | TOC (g kg−1) | Nt (g kg−1) | TOC/Nt | TOC (g kg−1) | Nt (g kg−1) | TOC/Nt | TOC (g kg−1) | Nt (g kg−1) | TOC/Nt | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kamionna | Cegielnia | Podlesie | ||||||||
| Distance (m) | 5 | 22.7 | 1.83 | 14.55 | 39.2 | 2.35 | 17.35 | 35.0 | 2.78 | 13.19 |
| 15 | 29.4 | 2.02 | 16.87 | 27.3 | 2.46 | 12.76 | 34.2 | 2.97 | 11.71 | |
| 25 | 40.2 | 3.04 | 14.20 | 32.1 | 3.16 | 10.93 | 37.4 | 3.07 | 12.85 | |
| LSD | 1.58 | 0.431 | Ns | 0.56 | 0.136 | 1.293 | 2.01 | 0.168 | 1.262 | |
| Depth (cm) | 0–10 | 14.2 | 0.99 | 17.00 | 12.3 | 0.77 | 16.06 | 17.8 | 1.51 | 12.44 |
| 10–20 | 47.5 | 4.17 | 11.43 | 44.6 | 3.81 | 11.81 | 56.5 | 4.91 | 11.52 | |
| 20–30 | 30.6 | 1.73 | 17.19 | 41.8 | 3.39 | 13.17 | 32.4 | 2.40 | 13.78 | |
| LSD | 1.05 | 0.045 | 2.138 | 0.32 | 0.163 | 2.871 | 1.59 | 0.132 | 1.356 | |
| Factor | H/C | N/C | O/C | O/H | ω | |
|---|---|---|---|---|---|---|
| Kamionna | ||||||
| Distance (m) | 5 | 1.29 | 0.068 | 0.622 | 0.484 | 0.160 |
| 15 | 1.27 | 0.067 | 0.635 | 0.501 | 0.198 | |
| 25 | 1.28 | 0.067 | 0.614 | 0.481 | 0.162 | |
| LSD | 0.005 | ns | 0.010 | 0.010 | ns | |
| Depth (cm) | 0–10 | 1.34 | 0.077 | 0.620 | 0.462 | 0.131 |
| 10–20 | 1.28 | 0.071 | 0.626 | 0.488 | 0.181 | |
| 20–30 | 1.21 | 0.054 | 0.625 | 0.515 | 0.208 | |
| LSD | 0.025 | 0.006 | ns | 0.026 | 0.062 | |
| Cegielnia | ||||||
| Distance (m) | 5 | 1.10 | 0.061 | 0.529 | 0.478 | 0.132 |
| 15 | 1.24 | 0.071 | 0.582 | 0.464 | 0.121 | |
| 25 | 1.35 | 0.081 | 0.620 | 0.450 | 0.108 | |
| LSD | 0.053 | 0.015 | 0.014 | ns | ns | |
| Depth (cm) | 0–10 | 1.29 | 0.072 | 0.580 | 0.449 | 0.082 |
| 10–20 | 1.24 | 0.071 | 0.570 | 0.462 | 0.115 | |
| 20–30 | 1.16 | 0.070 | 0.582 | 0.480 | 0.165 | |
| LSD | 0.051 | ns | ns | 0.015 | 0.033 | |
| Podlesie | ||||||
| Distance (m) | 5 | 1.27 | 0.068 | 0.609 | 0.473 | 0.145 |
| 15 | 1.26 | 0.069 | 0.591 | 0.467 | 0.124 | |
| 25 | 1.25 | 0.063 | 0.542 | 0.435 | 0.041 | |
| LSD | ns | ns | 0.022 | 0.020 | 0.063 | |
| Depth (cm) | 0–10 | 1.32 | 0.073 | 0.586 | 0.444 | 0.080 |
| 10–20 | 1.28 | 0.070 | 0.595 | 0.459 | 0.111 | |
| 20–30 | 1.18 | 0.058 | 0.562 | 0.472 | 0.120 | |
| LSD | 0.060 | 0.006 | 0.021 | 0.012 | 0.024 | |
| Factor | A2/4 | A2/6 | A4/6 | ΔlogK | |
|---|---|---|---|---|---|
| Kamionna | |||||
| Distance (m) | 5 | 6.45 | 33.81 | 5.23 | 0.675 |
| 15 | 5.25 | 24.49 | 4.66 | 0.628 | |
| 25 | 5.28 | 23.51 | 4.46 | 0.632 | |
| LSD | 0.417 | 3.60 | 0.63 | 0.025 | |
| Depth (cm) | 0–10 | 6.05 | 29.59 | 4.86 | 0.688 |
| 10–20 | 5.55 | 26.50 | 4.73 | 0.654 | |
| 20–30 | 5.38 | 25.72 | 4.75 | 0.593 | |
| LSD | 0.409 | Ns | ns | 0.051 | |
| Cegielnia | |||||
| Distance (m) | 5 | 5.21 | 24.72 | 4.74 | 0.697 |
| 15 | 4.94 | 23.37 | 4.72 | 0.614 | |
| 25 | 5.33 | 24.19 | 4.53 | 0.535 | |
| LSD | 0.288 | Ns | ns | 0.042 | |
| Depth (cm) | 0–10 | 5.54 | 27.06 | 4.88 | 0.645 |
| 10–20 | 5.14 | 24.11 | 4.69 | 0.614 | |
| 20–30 | 4.79 | 21.11 | 4.42 | 0.588 | |
| LSD | 0.230 | 0.994 | 0.420 | 0.028 | |
| Podlesie | |||||
| Distance (m) | 5 | 5.05 | 24.47 | 4.90 | 0.671 |
| 15 | 5.19 | 24.90 | 4.71 | 0.629 | |
| 25 | 5.18 | 25.12 | 4.77 | 0.610 | |
| LSD | 0.128 | Ns | ns | ns | |
| Depth (cm) | 0–10 | 5.87 | 30.94 | 5.28 | 0.700 |
| 10–20 | 5.12 | 24.16 | 4.71 | 0.647 | |
| 20–30 | 4.41 | 19.39 | 4.39 | 0.564 | |
| LSD | 0.195 | 2.44 | 0.32 | 0.031 | |
| Wavenumber (cm−1) | Assignment a |
|---|---|
| 3400–3100 | O-H stretching of alcohols, phenols and acids, N-H stretching |
| 3100–3000 | C-H groups of aromatic and alicyclic compounds |
| 2960–2920; 2850 | asymmetric and symmetric C-H stretching of CH3 and CH2 group |
| 1730–1710 | C=O stretching of carboxyl, aldehyde, ketone group |
| 1660–1620 | C=O of stretching of amide groups; N-H deformation |
| 1610–1600 | C-C stretching of aromatic rings |
| 1550–1530 | N-H deformation, C=N stretching (amide II bands) |
| 1520–1500 | C-C stretching of aromatic rings |
| 1460–1440 | C-H asymmetric of CH3 and CH2 |
| 1420–1400 | C-O stretching and OH deformation of phenols |
| 1380–1320 | C-N aromatic amine, COO-, C-H stretching |
| 1280–1200 | C-O stretching of aryl ethers, esters and phenols |
| 1160–1030 | C-O stretching alcohols, ethers and polysaccharides |
| Factor | HIL | ΣHOB | HIL/ΣHOB | HIL | ΣHOB | HIL/ΣHOB | HIL | ΣHOB | HIL/ΣHOB | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kamionna | Cegielnia | Podlesie | ||||||||
| Distance (m) | 5 | 38.88 | 61.12 | 0.637 | 39.62 | 60.38 | 0.657 | 39.85 | 60.15 | 0.663 |
| 15 | 40.04 | 59.96 | 0.668 | 38.18 | 61.82 | 0.620 | 40.16 | 59.84 | 0.671 | |
| 25 | 39.81 | 60.19 | 0.662 | 40.42 | 59.58 | 0.680 | 40.48 | 59.52 | 0.682 | |
| LSD | ns | ns | ns | ns | ns | 0.057 | ns | Ns | ns | |
| Depth (cm) | 0–10 | 38.57 | 61.43 | 0.628 | 37.71 | 62.29 | 0.606 | 39.90 | 60.10 | 0.664 |
| 10–20 | 39.83 | 60.17 | 0.662 | 39.92 | 60.08 | 0.664 | 39.82 | 60.18 | 0.690 | |
| 20–30 | 40.34 | 59.66 | 0.676 | 40.59 | 59.41 | 0.686 | 40.76 | 59.24 | 0.663 | |
| LSD | 1.374 | 1.374 | 0.037 | 2.264 | 2.264 | 0.061 | ns | Ns | ns | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banach-Szott, M.; Dziamski, A.; Markiewicz, M. Properties of Humic Acids in Meadow Soils Irrigated with the Slope-and-Flooding System. Agronomy 2021, 11, 2553. https://doi.org/10.3390/agronomy11122553
Banach-Szott M, Dziamski A, Markiewicz M. Properties of Humic Acids in Meadow Soils Irrigated with the Slope-and-Flooding System. Agronomy. 2021; 11(12):2553. https://doi.org/10.3390/agronomy11122553
Chicago/Turabian StyleBanach-Szott, Magdalena, Andrzej Dziamski, and Maciej Markiewicz. 2021. "Properties of Humic Acids in Meadow Soils Irrigated with the Slope-and-Flooding System" Agronomy 11, no. 12: 2553. https://doi.org/10.3390/agronomy11122553
APA StyleBanach-Szott, M., Dziamski, A., & Markiewicz, M. (2021). Properties of Humic Acids in Meadow Soils Irrigated with the Slope-and-Flooding System. Agronomy, 11(12), 2553. https://doi.org/10.3390/agronomy11122553






