Harnessing Trichoderma in Agriculture for Productivity and Sustainability
Abstract
:1. Introduction
2. Roles of Trichoderma in Sustainable Crop Production
3. Roles of Trichoderma in Sustainable Plant Disease Management
3.1. Trichoderma as Biocontrol Agents against Plant Pathogenic Bacteria
3.2. Trichoderma as Biocontrol Agents against Phytopathogenic Fungi
3.3. Trichoderma as Biocontrol Agents against Pests and Plant-Parasitic Nematodes
4. Trichoderma Species as Abiotic Stress Relievers in Crops
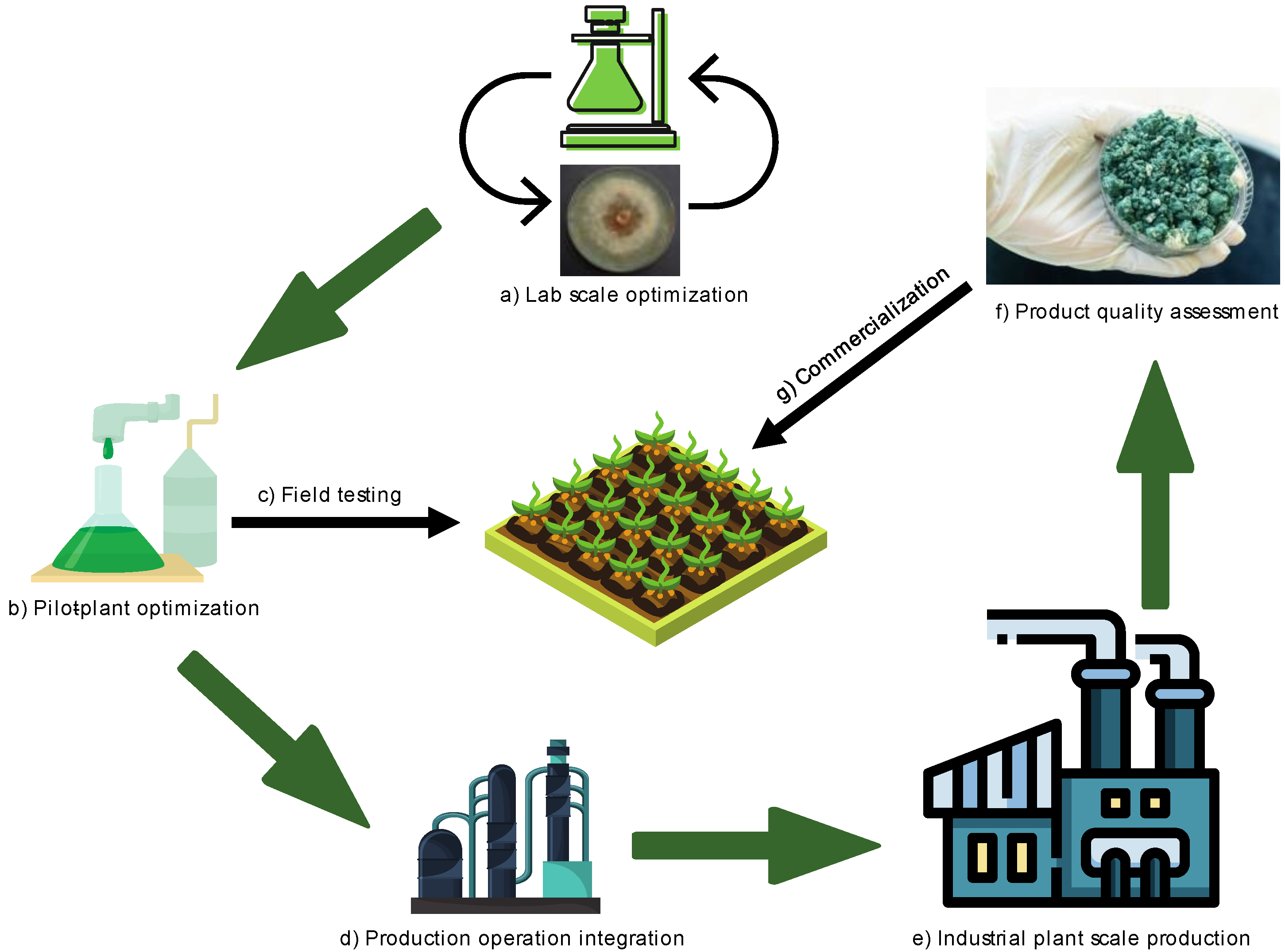
5. Challenges and Future Prospects for Up-Scaling the Use of Trichoderma for Sustainable Crop Production
Industrial Production of Trichoderma
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raza, A.; Razzaq, A.; Mehmood, S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafton, R.Q.; Daugbjerg, C.; Qureshi, M.E. Towards food security by 2050. Food Sec. 2015, 7, 179–183. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The 10 Elements of Agroecology. 2020. Available online: http://www.fao.org/3/i9037en/i9037en.pdf (accessed on 18 May 2020).
- Saba, H.D.; Vibhash, M.; Manisha, K.S.; Prashant Farham, H.; Tauseff, A. Trichoderma—A promising plant growth stimulator and biocontrol agent. Mycosphere 2012, 3, 524–531. [Google Scholar] [CrossRef]
- Poveda, J. Beneficial effects of microbial volatile organic compounds (MVOCs) in plants. Appl. Soil Ecol. 2021, 168, 104118. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef]
- Poveda, J.; Zabalgogeazcoa, I.; Soengas, P.; Rodríguez, V.M.; Cartea, M.E.; Abilleira, R.; Velasco, P. Brassica oleracea var. acephala (kale) improvement by biological activity of root endophytic fungi. Sci. Rep. 2020, 10, 20224. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Singh, A.; Shukla, N.; Kabadwal, B.C.; Tewari, A.K.; Kumar, J. Review on plant-Trichoderma-pathogen interaction. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2382–2397. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P. Plant responses to Trichoderma spp. and their tolerance to abiotic stresses: A review. J. Pharmacog. Phytochem. 2018, 7, 758–766. [Google Scholar]
- Altieri, M.; Nicholls, C.; Montalba, R. Technological approaches to sustainable agriculture at a crossroads: An agroecological perspective. Sustainability 2017, 9, 349. [Google Scholar] [CrossRef] [Green Version]
- Altieri, M.; Nicholls, C. Agroecology and the reconstruction of a post-COVID-19 agriculture. J. Peasant Stud. 2020, 4, 881–898. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2016, 10, 19–21. [Google Scholar] [CrossRef]
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to plant health and productivity from enhancing plant microbial symbionts. Front. Plant Sci. 2021, 11, 610065. [Google Scholar] [CrossRef] [PubMed]
- Finkel, O.M.; Castrillo, G.; Herrera Paredes, S.; Salas González, I.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Mahato, D. Trichoderma’s contribution in environmentally friendly plant disease management. Biotica Res. Today 2021, 3, 591–594. [Google Scholar]
- Poveda, J.; Eugui, D.; Abril-Urias, P. Could Trichoderma Be a Plant Pathogen? Successful Root Colonization. In Trichoderma. Rhizosphere Biology; Sharma, A., Sharma, P., Eds.; Springer: Singapore, 2020; pp. 35–59. [Google Scholar] [CrossRef]
- Alonso-Ramírez, A.; Poveda, J.; Martín, I.; Hermosa, R.; Monte, E.; Nicolás, C. Salicylic acid Prevents Trichoderma harzianum from entering the vascular system of roots. Mol. Plant Pathol. 2014, 15, 823–831. [Google Scholar] [CrossRef]
- Poveda, J. Glucosinolates profile of Arabidopsis thaliana modified root colonization of Trichoderma species. Biol. Control 2021, 155, 104522. [Google Scholar] [CrossRef]
- Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem K., R. Sustainable Crop Production System. In Plant, Soil and Microbes; Hakeem, K., Akhtar, M., Abdullah, S., Eds.; Springer: Cham, Switzerland, 2016; pp. 103–116. [Google Scholar] [CrossRef]
- Al-Ani, L.K.T. Trichoderma: Beneficial Role in Sustainable Agriculture by Plant Disease Management. In Plant Microbiome: Stress Response. Microorganisms for Sustainability; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; pp. 105–126. [Google Scholar] [CrossRef]
- Molla, A.H.; Haque, M.M.; Haque, M.A.; Ilias, G.N.M. Trichoderma-enriched biofertilizer enhances production and nutritional quality of tomato (Lycopersicon esculentum Mill.) and minimizes NPK fertilizer Use. Agric. Res. 2012, 1, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Rudresh, D.L.; Shivaprakash, M.K.; Prasad, R.D. Tricalcium phosphate solubilizing abilities of Trichoderma spp. in relation to P uptake and growth and yield parameters of chickpea (Cicer arietinum L.). Can. J. Microbiol. 2005, 51, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Effect of Trichoderma spp. in plant growth promotion in chilli. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1574–1581. [Google Scholar] [CrossRef]
- Srivastava, S.N.; Singh, V.; Awasthi, S.K. Trichoderma induced improvement in growth, yield and quality of sugarcane. Sugar Tech 2006, 8, 166–169. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Tanwar, A.; Aggarwal, A.; Kaushish, S.; Chauhan, S. Interactive effect of AM fungi with Trichoderma viride and Pseudomonas fluorescens on growth and yield of broccoli. Plant Prot. Sci. 2013, 49, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Garnica-Vergara, A.; Barrera-Ortiz, S.; Muñoz-Parra, E.; Raya-González, J.; Méndez-Bravo, A.; Macías-Rodríguez, L.; Ruiz-Herrera, L.F.; López-Bucio, J. The volatile 6-pentyl-2H-pyran-2-one from Trichoderma atroviride regulates Arabidopsis thaliana root morphogenesis via auxin signaling and ETHYLENE INSENSITIVE 2 functioning. New Phytol. 2016, 209, 1496–1512. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Alfaro-Cuevas, R.; López-Bucio, J. Trichoderma spp. improve growth of Arabidopsis seedlings under salt stress through enhanced root development, osmolite production, and Na+ elimination through root exudates. Mol. Plant-Microbe Interact. 2014, 27, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Doni, F.; Isahak, A.; Zain, C.R.C.M.; Yusoff, W.M.W. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Express 2014, 4, 45. [Google Scholar] [CrossRef]
- Doni, F.; Zain, C.R.; Isahak, A.; Fathurrahman, F.; Sulaiman, N.; Uphoff, N.; Yusoff, W.M. Relationships observed between Trichoderma inoculation and characteristics of rice grown under System of Rice Intensification (SRI) vs. conventional methods of cultivation. Symbiosis 2016, 72, 45–59. [Google Scholar] [CrossRef]
- Doni, F.; Zain, C.R.; Isahak, A.; Fathurrahman, F.; Anhar, A.; Mohamad, W.N.; Wan Yusoff, W.M.; Uphoff, N. A simple, efficient, and farmer-friendly Trichoderma-based biofertilizer evaluated with the SRI Rice Management System. Org. Agric. 2018, 8, 207–223. [Google Scholar] [CrossRef]
- Akladious, S.A.; Abbas, S.M. Application of Trichoderma harzianum T22 as a biofertilizer supporting maize growth. Afr. J. Biotechnol. 2012, 11, 8672–8683. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E.; Mastouri, F. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species, opportunistic avirulent plant symbionts. Nature 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Shoresh, M.; Harman, G.E. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: A proteomic approach. Plant Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doni, F.; Fathurrahman, F.; Mispan, M.S.; Suhaimi, N.S.; Yusoff, W.M.; Uphoff, N. Transcriptomic profiling of rice seedlings inoculated with the symbiotic fungus Trichoderma asperellum SL2. J. Plant Growth Regul. 2019, 38, 1507–1515. [Google Scholar] [CrossRef]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- González-Pérez, E.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Bautista, E.; Douterlungne, D.; Jiménez-Bremont, J.F. The arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 2018, 8, 16427. [Google Scholar] [CrossRef] [Green Version]
- Poveda, J. Trichoderma parareesei favors the tolerance of rapeseed (Brassica napus L.) to salinity and drought due to a chorismate mutase. Agron. 2020, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef] [Green Version]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Gallou, A.; Cranenbrouck, S.; Declerck, S. Trichoderma harzianum elicits defence response genes in roots of potato plantlets challenged by Rhizoctonia solani. Eur. J. Plant Pathol. 2008, 124, 219–230. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, X.; Deng, J.; Yao, Z.; Lyu, M.; Zhang, R. Auxin response factor 1 acts as a positive regulator in the response of poplar to Trichoderma asperellum inoculation in overexpressing plants. Plants 2020, 9, 272. [Google Scholar] [CrossRef] [Green Version]
- Verma, P.; Yadav, A.N.; Kumar, V.; Khan, A.; Saxena, A.K. Microbes in Termite Management: Potential Role and Strategies. In Termites and Sustainable Management: Volume 2—Economic Losses and Management; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 197–217. [Google Scholar] [CrossRef]
- Yan, L.; Khan, R.A. Biological control of bacterial wilt in tomato through the metabolites produced by the biocontrol fungus, Trichoderma harzianum. Egypt. J. Biol. Pest Control 2021, 31, 5. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of biological efficacy of Trichoderma asperellum against tomato bacterial wilt caused by Ralstonia solanacearum. Egypt. J. Biol. Pest 2018, 28, 63. [Google Scholar] [CrossRef] [Green Version]
- Fontenelle, A.; Guzzo, S.; Lucon, C.; Harakava, R. Growth promotion and induction of resistance in tomato plant against Xanthomonas euvesicatoria and Alternaria solani by Trichoderma spp. Crop Prot. 2011, 30, 1492–1500. [Google Scholar] [CrossRef]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant induction of systemic resistance to Pseudomonas syringae pv. lachrymans in cucumber by Trichoderma asperellum (T-203) and accumulation of phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, C.R. Mechanisms employed by Trichoderma species in the biological control of plant diseases: The history and evolution of current concepts. Plant Dis. 2003, 87, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Brand, A. Hyphal growth in human fungal pathogens and its role in virulence. Int. J. Microbiol. 2012, 2012, 517529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, D.A.; Wilson, C.R. The process of antagonism of Sclerotium cepivorum in white rot affected onion roots by Trichoderma koningii. Plant Pathol. 2001, 50, 249–257. [Google Scholar] [CrossRef]
- Howell, C.R. Understanding the mechanisms employed by Trichoderma virens to effect biological control of cotton diseases. Phytopathol. 2006, 96, 178–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howell, C.R. Cotton seedling preemergence damping-off incited by Rhizopus oryzae and Pythium spp. and its biological control with Trichoderma spp. Phytopathol. 2002, 92, 177–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Ashraf, S. Role of Trichoderma spp. as a Biocontrol Agent of Fungal Plant Pathogens. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 497–506. [Google Scholar] [CrossRef]
- de Lima, F.B.; Félix, C.; Osório, N.; Alves, A.; Vitorino, R.; Domingues, P.; da Silva Ribeiro, R.T.; Esteves, A.C. Trichoderma harzianum T1A constitutively secretes proteins involved in the biological control of Guignardia citricarpa. Biol. Control 2017, 106, 99–109. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Horwitz, B.A.; Kenerley, C.M. Secondary metabolism in Trichoderma—A genomic perspective. Microbiol. 2012, 158, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Howell, C.R.; Hanson, L.E.; Stipanovic, R.D.; Puckhaber, L.S. Induction of terpenoid synthesis in cotton roots and control of Rhizoctonia solani by seed treatment with Trichoderma virens. Phytopathology 2000, 90, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Rini, C.R.; Sulochana, K.K. Management of seedling rot of chilli (Capsicum annuum L.) using Trichoderma spp. and fluorescent pseudomonads (Pseudomonas fluorescens). J. Trop. Agric. 2006, 44, 79–82. [Google Scholar]
- Hwang, I.S.; Hwang, B.K. The pepper 9-lipoxygenase gene CaLOX1 functions in defense and cell death responses to microbial pathogens. Plant Physiol. 2010, 152, 948–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Téllez, V.I.; Cruz-Olmedo, A.K.; Plasencia, J.; Gavilanes-Ruíz, M.; Arce-Cervantes, O.; Hernández-León, S.; Saucedo-García, M. The protective effect of Trichoderma asperellum on tomato plants against Fusarium oxysporum and Botrytis cinerea diseases involves inhibition of reactive oxygen species production. Int. J. Mol. Sci. 2019, 20, 2007. [Google Scholar] [CrossRef] [Green Version]
- Djonović, S.; Vargas, W.A.; Kolomiets, M.V.; Horndeski, M.; Wiest, A.; Kenerley, C.M. A Proteinaceous elicitor Sm1 from the beneficial fungus Trichoderma virens is required for induced systemic resistance in maize. Plant Physiol. 2007, 145, 875–889. [Google Scholar] [CrossRef] [Green Version]
- Nawrocka, J.; Małolepsza, U. Diversity in plant systemic resistance induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Utkhede, R.; Koch, C. Biological treatments to control bacterial canker of greenhouse tomatoes. BioControl 2004, 49, 305–313. [Google Scholar] [CrossRef]
- Brotman, Y.; Lisec, J.; Méret, M.; Chet, I.; Willmitzer, L.; Viterbo, A. Transcript and metabolite analysis of the Trichoderma-induced systemic resistance response to Pseudomonas syringae in Arabidopsis thaliana. Microbiology 2012, 158, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldahmani, J.H.; Abbasi, P.A.; Sahin, F.; Hoitink, H.A.J.; Miller, S.A. Reduction of bacterial leaf spot severity on radish, lettuce, and tomato plants grown in compost-amended potting mixes. Can. J. Plant Pathol. 2005, 27, 186–193. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Sharma, P.; Manokaran, R.; Lakshman, D.K.; Rokadia, P.; Jambhulkar, N. Assessing synergism of combined applications of Trichoderma harzianum and Pseudomonas fluorescens to control blast and bacterial leaf blight of rice. Eur. J. Plant Pathol. 2018, 152, 747–757. [Google Scholar] [CrossRef]
- Escande, A.R.; Laich, F.S.; Pedraza, M.V. Field testing of honeybee-dispersed Trichoderma spp. to manage sunflower head rot (Sclerotinia sclerotiorum). Plant Pathol. 2002, 51, 346–351. [Google Scholar] [CrossRef]
- Perelló, A.; Mónaco, C.; Simón, M.R.; Sisterna, M.; Bello, G.D. Biocontrol efficacy of Trichoderma isolates for tan spot of wheat in Argentina. Crop Prot. 2003, 22, 1099–1106. [Google Scholar] [CrossRef]
- Sivakumar, D.; Wijeratnam, R.S.; Wijesundera, R.L.; Marikar, F.M.; Abeyesekere, M. Antagonistic effect of Trichoderma harzianum on postharvest pathogens of rambutan (Nephelium lappaceum). Phytoparasitica 2000, 28, 240–247. [Google Scholar] [CrossRef]
- Poveda, J. Biological control of Fusarium oxysporum f. sp. ciceri and Ascochyta rabiei infecting protected geographical indication Fuentesaúco-Chickpea by Trichoderma species. Eur. J. Plant Pathol. 2021, 160, 825–840. [Google Scholar] [CrossRef]
- Poveda, J.; Hermosa, R.; Monte, E.; Nicolás, C. The Trichoderma harzianum Kelch protein ThKEL1 plays a key role in root colonization and the induction of systemic defense in Brassicaceae plants. Front. Plant Sci. 2019, 10, 1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poveda, J. Trichoderma as biocontrol agent against pests: New uses for a mycoparasite. Biol. Control 2021, 159, 104634. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Tahna Maafi, Z. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2011; pp. 21–43. [Google Scholar] [CrossRef]
- Moosavi, M.R.; Askary, T.H. Nematophagous fungi-commercialization. In Biocontrol Agents of Phytonematodes; CABI Publishing: Wallingford, UK, 2015; pp. 187–202. [Google Scholar]
- Harman, G.E.; Uphoff, N. Symbiotic root-endophytic soil microbes improve crop productivity and provide environmental benefits. Scientifica 2019, 2019, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, P.P.; Rao, M.S.; Nagesh, M. Management of citrus nematode, Tylenchulus semipenetrans, by integration of Trichoderma harzianum with oil cakes. Nematol. Mediterr. 1996, 24, 265–267. [Google Scholar]
- Ghosh, S.K.; Pal, S. Entomopathogenic potential of Trichoderma longibrachiatum and its comparative evaluation with malathion against the insect pest Leucinodes orbonalis. Environ. Monit. Assess. 2016, 188, 37. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, Á.; Mayo, S.; González-López, Ó.; Reinoso, B.; Gutierrez, S.; Casquero, P.A. Inhibitory activity of Beauveria bassiana and Trichoderma spp. on the insect pests Xylotrechus arvicola (Coleoptera: Cerambycidae) and Acanthoscelides obtectus (Coleoptera: Chrisomelidae: Bruchinae). Environ. Monit. Assess. 2017, 189, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, X.F.; Guo, J.; dos-Santos, M.L.; Vivanco, J.M. Trichoderma gamsii affected herbivore feeding behaviour on Arabidopsis thaliana by modifying the leaf metabolome and phytohormones. Microb. Biotechnol. 2018, 11, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; del-Val, E.; Larsen, J. The root endophytic fungus Trichoderma atroviride induces foliar herbivory resistance in maize plants. Appl. Soil Ecol. 2018, 124, 45–53. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Lelio, I.D.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma atroviride P1 colonization of tomato plants enhances both direct and indirect defense barriers against insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef] [Green Version]
- Gad, H.A.; Al-Anany, M.S.; Atta, A.A.; Abdelgaleil, S.A. Efficacy of low-dose combinations of diatomaceous earth, spinosad and Trichoderma harzianum for the control of Callosobruchus maculatus and Callosobruchus chinensis on stored cowpea seeds. J. Stored Prod. Res. 2021, 91, 101778. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Fathi, A.; Tari, D.B. Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 2016, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shukla, N.; Awasthi, R.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N.; Karaoglu, S.A.; Guzel, S.; Bozdeveci, A. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defence in maize seedlings. Acta Physiol. Plant 2016, 38, 132. [Google Scholar] [CrossRef]
- Heidarvand, L.; Maali Amiri, R. What happens in plant molecular responses to cold stress? Acta Physiol. Plant 2010, 32, 419–431. [Google Scholar] [CrossRef]
- Rawat, L.; Singh, Y.; Shukla, N.; Kumar, J. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed biopriming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil 2011, 347, 387–400. [Google Scholar] [CrossRef]
- Betzen, B.M.; Smart, C.M.; Maricle, K.L.; Maricle, B.R. Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species. Trans. Kans. Acad. Sci. 2019, 122, 49. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Zhang, Y.-Q. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agric. 2015, 14, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, N. Effects of salinity and solidicity on root growth. Ann. Rev. Phytopathol. 1975, 13, 295–312. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd_Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Zhang, F.; Huo, Y.; Cobb, A.B.; Luo, G.; Zhou, J.; Yang, G.; Wilson, G.W.; Zhang, Y. Trichoderma biofertilizer links to altered soil chemistry, altered microbial communities, and improved grassland biomass. Front. Microbiol. 2018, 9, 848. [Google Scholar] [CrossRef]
- Dugassa, A.; Alemu, T.; Woldehawariat, Y. In-vitro compatibility assay of indigenous Trichoderma and Pseudomonas species and their antagonistic activities against black root rot disease (Fusarium solani) of faba bean (Vicia faba L.). BMC Microbiol. 2021, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Santos, U.R.; Costa, M.C.; de Freitas, G.J.; de Oliveira, F.S.; Santos, B.R.; Silva, J.F.; Santos, D.A.; Dias, A.A.; de Carvalho, L.D.; Augusto, D.G.; et al. Exposition to biological control agent Trichoderma stromaticum increases the development of cancer in mice injected with murine melanoma. Front. Cell. Infect. Microbiol. 2020, 10, 252. [Google Scholar] [CrossRef]
- Konstantinovas, C.; de Oliveira Mendes, T.A.; Vannier-Santos, M.A.; Lima-Santos, J. Modulation of human immune response by fungal biocontrol agents. Front. Microbiol. 2017, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Bhardwaj, R.; Singh, I.K. Biocontrol Agents: Potential of Biopesticides for Integrated Pest Management. In Biofertilizers for Sustainable Agriculture and Environment; Giri, B., Prasad, R., Wu, Q.S., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 55. [Google Scholar] [CrossRef]
- Rostaminia, M.; Habibi, D.; Shahbazi, S.; Sani, B.; Pazoki, A. Biological effects of agricultural bio-materials on some blood and tissue factors in Balb/c mice. Iran. J. Vet. Sci. Technol. 2020, 12, 63–69. [Google Scholar]
- Kumar, S.M.; Thakur, M.A.; Rani, A. Trichoderma: Mass production, formulation, quality control, delivery and its scope in commercialization in India for the management of plant diseases. Afr. J. Agric. Res. 2014, 9, 3838–3852. [Google Scholar] [CrossRef]
- Elad, Y. TRICHODEX: Commercialization of Trichoderma harzianum T39—A casestudy. In Agro Report, Biopesticides: Trends and Opportunities; Jarvis, P., Ed.; PJB: Richmond, UK, 2001; pp. 45–50. [Google Scholar]
- Topolovec-Pintarić, S. Trichoderma: Invisible partner for visible impact on agriculture. In Trichoderma: The Most Widely Used Fungicide; Shah, M.M., Sharif, U., Buhari, T.R., Eds.; IntechOpen: London, UK, 2019; pp. 15–35. [Google Scholar] [CrossRef] [Green Version]
- Nakkeeran, S.; Renukadevi, P.; Aiyanathan, K.E. Exploring the potential of Trichoderma for the management of seed and soil-borne diseases of crops. In Integrated Pest Management of Tropical Vegetable Crops; Muniappan, R., Heinrichs, E., Eds.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Prasad, R.D.; Rangeshwaran, R. Shelf life and bioefficacy of Trichoderma harzianum formulated in various carrier materials. Plant Dis. Res. 2000, 15, 38–42. [Google Scholar]
- Kolombet, L.V.; Zhigletsova, S.K.; Kosareva, N.I.; Bystrova, E.V.; Derbyshev, V.V.; Krasnova, S.P.; Schisler, D. Development of an extended shelf-life, liquid formulation of the biofungicide Trichoderma asperellum. World J. Microbiol. Biotechnol. 2007, 24, 123–131. [Google Scholar] [CrossRef]
- Locatelli, G.O.; dos Santos, G.F.; Botelho, P.S.; Finkler, C.L.; Bueno, L.A. Development of Trichoderma sp. formulations in encapsulated granules (CG) and evaluation of conidia shelf-life. Biol. Control 2018, 117, 21–29. [Google Scholar] [CrossRef]
- Peil, S.; Beckers, S.J.; Fischer, J.; Wurm, F.R. Biodegradable, lignin-based encapsulation enables delivery of Trichoderma reesei with programmed enzymatic release against grapevine trunk diseases. Mater. Today Bio 2020, 7, 100061. [Google Scholar] [CrossRef] [PubMed]
- Mitter, E.K.; Tosi, M.; Obregón, D.; Dunfield, K.E.; Germida, J.J. Rethinking crop nutrition in times of modern microbiology: Innovative biofertilizer technologies. Front. Sustain. Food Syst. 2021, 5, 29. [Google Scholar] [CrossRef]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Steiner, W.; Labudova, I.; Hermann, A.; Hayn, M. Production of Trichoderma cellulase in laboratory and pilot scale. Bioresour. Technol. 1991, 36, 51–65. [Google Scholar] [CrossRef]
- Al-Taweil, H.I.; Osman, M.B.; Aidil, A.H.; Yussof, W.M. Optimizing of Trichoderma viride cultivation in submerged state fermentation. Am. J. Appl. Sci. 2009, 6, 1284. [Google Scholar] [CrossRef]
- Agosin, E.; Auilera, J.M. Industrial Production of Active Propagules of Trichoderma for Agricultural Uses. In Trichoderma and Gliocladium, Volume 2: Enzymes, Biological Control, and Commercial Application; Harman, G.E., Kubicek, C.P., Eds.; Taylor & Francis: London, UK, 1998; pp. 205–227. [Google Scholar]
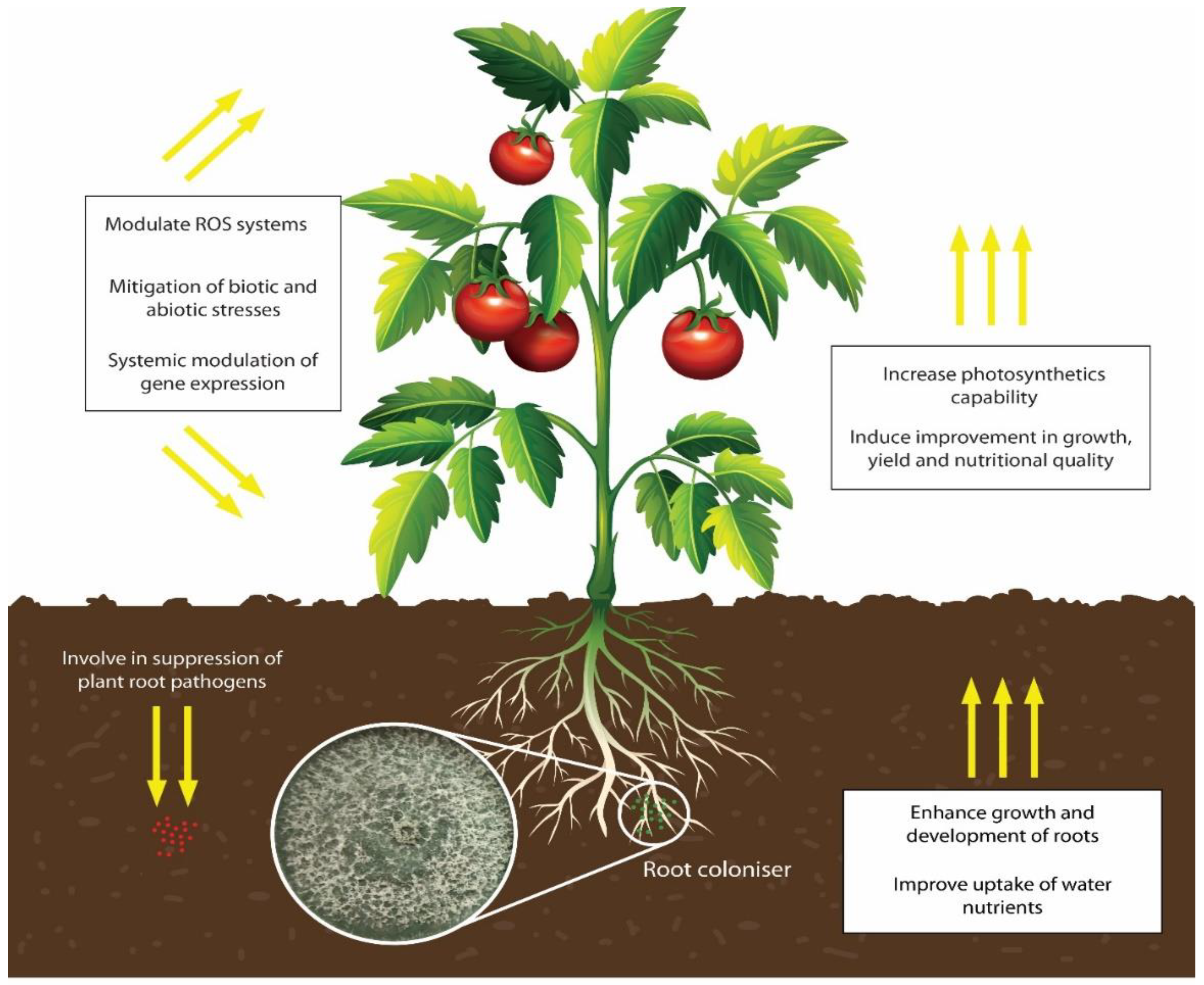
| Plants | Trichoderma Species | Genes | Observed Effects | References |
|---|---|---|---|---|
| Arabidopsis, cucumber | T. asperelloides | MDAR | Increased osmo-protection/oxidative stress. | [45] |
| Arabidopsis | T. atroviride, T. virens | AtERD14 | Mitigated cold stress effects. | [46] |
| Rapeseed | T. parareesei | NCED3, ACCO1, ERF1 and PYL4 | Improved tolerance to drought and salinity. | [47] |
| Wheat | T. longibrachiatum | SOD, POD, and CAT | Seedlings were protected from salinity. | [48] |
| Tomato | T. harzianum | TAS14 and P5CS | Improved tolerance to cold. | [49] |
| Potato | T. harzianum | Lox and GST1 | Induction of plant disease resistance. | [50] |
| Poplar | T. asperellum | PdPapARF1 | Promoted growth and defence responses. | [51] |
| Plants | Trichoderma Species | Phytopathogens | Observed Effects | References |
|---|---|---|---|---|
| Tomato | T. harzianum | Clavibacter michiganensis | Prevented the incidence of bacterial canker. | [73] |
| Tomato | T. harzianum and T. longibrachiatum | X. euvesicatoria, Alternaria solani | Reduced bacterial spots, triggering systemic acquired resistance (SAR) or induced systemic resistance (ISR). | [55] |
| Tomato | T. harzianum | Ralstonia spp. | Trichoderma spp. AA2 inhibited the growth and survival of Ralstonia spp. | [53] |
| Tomato | T. asperellum | R. solanacearum | Delayed wilt development, effectively decreased disease incidence, increased fruit yield, and improved plant growth promotion. | [54] |
| Tomato | T. asperellum | F. oxysporum, B. cinerea | Inhibited ROS production. | [70] |
| Arabidopsis thaliana | T. asperelloides | P. syringae | Lesser necrotic lesions surrounded by extensively spreading chlorosis. | [74] |
| Radish, lettuce, tomato | T. hamatum | X. campestris | Lowered bacterial population and disease severity (bacterial leaf spot). | [75] |
| Rice | T. harzianum | X. oryzae | Bacterial leaf blight severity was reduced while plant growth was improved. | [76] |
| Cucumber | T. asperellum | P. syringae pv. lachrymans | Transcript accumulation of biosynthetic defence related genes and accumulation of phenolic compounds (antimicrobial activity). | [56] |
| Citrus | T. harzianum | G. citricarpa | The involvement of protease affecting the germination of G. citricarpa conidia, able to deactivate the pathogen’s hydrolytic enzymes that are responsible for plant tissues necrosis. | [63] |
| Onion | T. koningii | S. cepivorum | Destroyed the hyphae, making it detached at septa, cell walls dissolved, and many hyphal apices burst. | [59] |
| Cotton | T. virens | R. solani | Induced terpenoid synthesis, toxic to the pathogen. | [65] |
| Cotton | T. virens and T. longibrachiatum | R. oryzae | Metabolized pathogen propagule germination stimulants that emanate from the germinating cotton seed. | [61] |
| Cotton | T. virens | R. solani | Penetrated and destroyed some of the resting structures of the pathogen. | [60] |
| Sunflower | T. koningii, T. aureoviride, T. longibrachiatum | S. sclerotiorum | Head rot incidence was significantly reduced, delayed epidemic onset. | [77] |
| Wheat | T. harzianum, T. aureoviride, T. koningii | Pyrenophora triticirepentis | Pathogen mycelium on the leaf surface collapsed or disintegrated. | [78] |
| Rambutan | T. harzianum | Botryodiplodia theobromae, Colletotrichum gloeosporioides, Gliocephalotrichum microchlamydosporum | Reduced the occurrence of the three postharvest diseases, also retained the overall quality and colour of the fruits. | [79] |
| Chickpea | T. atroviride, T. koningii, T. harzianum, T. hamatum | F. oxysporum, Ascochyta rabiei | Suppressed fungal infections by mycoparasitism, antibiosis, and competition for space and/or nutrients. | [80] |
| Arabidopsis, Rapeseed | T. harzianum | B. cinerea | Induction of systemic defence, mediated by jasmonic acid. | [81] |
| Plants | Trichoderma Species | Mechanisms | References |
|---|---|---|---|
| Rice | T. harzianum | Promotion of root growth in water deficit conditions. | [95] |
| Maize | T. atroviride | Improved drought-induced damages such as fresh and dry weights of maize roots, lipid peroxidation, photosynthetic machinery and inducing antioxidant enzyme activity and hydrogen peroxide. | [96] |
| Maize | T. harzianum | High starch content. | [43] |
| Tomato | T. harzianum | Maintained a high level of growth regulators, modulated plant secondary metabolites. | [103] |
| Tomato | T. harzianum | Improved growth attributes together with reduced cold injuries. | [49] |
| Arabidopsis, Cucumber | T. asperelloides | Improved seed germination. | [45] |
| Indian mustard | T. harzianum | Restored photosynthetic pigment level. | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy 2021, 11, 2559. https://doi.org/10.3390/agronomy11122559
Abdullah NS, Doni F, Mispan MS, Saiman MZ, Yusuf YM, Oke MA, Suhaimi NSM. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy. 2021; 11(12):2559. https://doi.org/10.3390/agronomy11122559
Chicago/Turabian StyleAbdullah, Nur Syafikah, Febri Doni, Muhamad Shakirin Mispan, Mohd Zuwairi Saiman, Yusmin Mohd Yusuf, Mushafau Adebayo Oke, and Nurul Shamsinah Mohd Suhaimi. 2021. "Harnessing Trichoderma in Agriculture for Productivity and Sustainability" Agronomy 11, no. 12: 2559. https://doi.org/10.3390/agronomy11122559
APA StyleAbdullah, N. S., Doni, F., Mispan, M. S., Saiman, M. Z., Yusuf, Y. M., Oke, M. A., & Suhaimi, N. S. M. (2021). Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy, 11(12), 2559. https://doi.org/10.3390/agronomy11122559







