Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Selection of Bilberry Accessions Based on AFLP Analysis
2.3. In Vitro Shoot Culture Initiation and Shoot Micropropagation
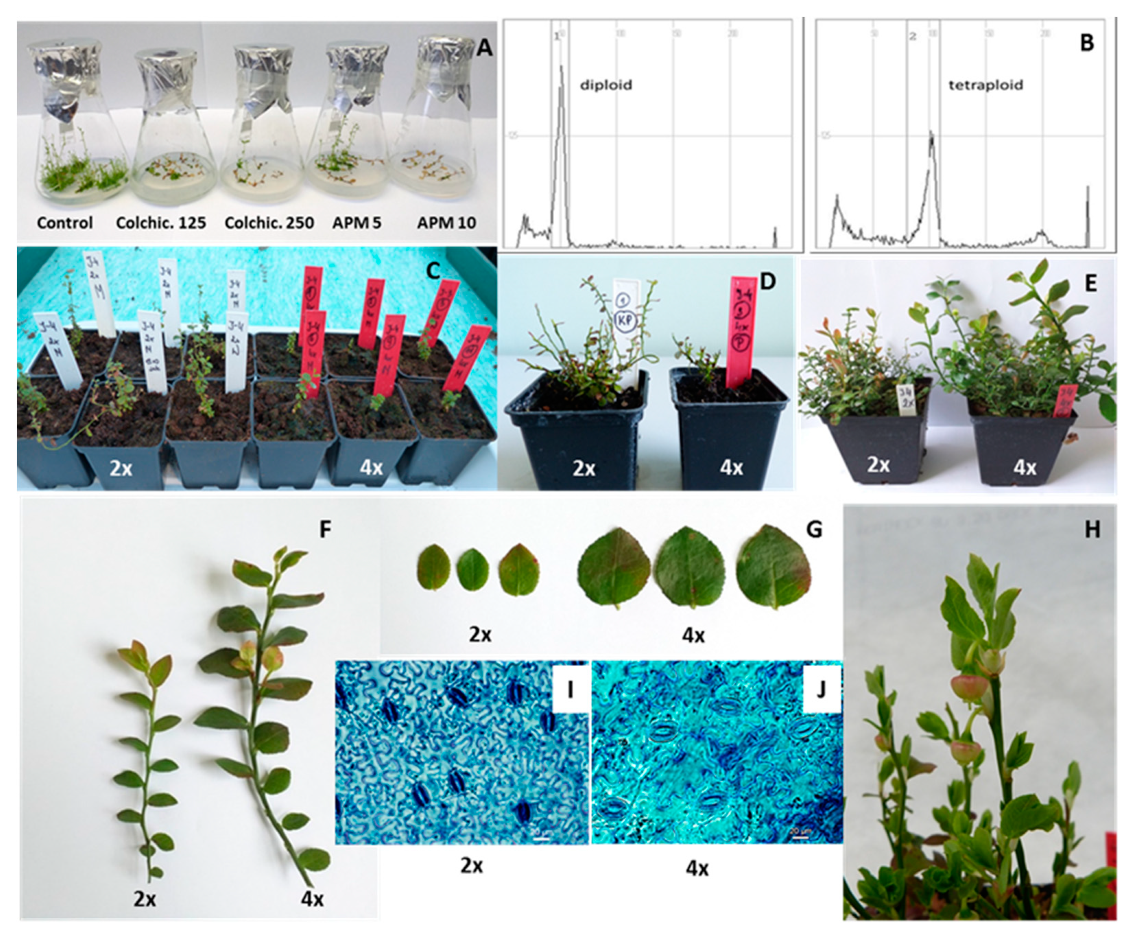
2.4. Optimisation of In Vitro Polyploidisation Method
2.5. Detection of Bilberry Tetraploids Based on Flow Cytometry
2.6. In Vitro Rooting Shoots of Selected Tetraploids and Their Diploid Counterparts, Acclimatisation to Ex Vitro Condition and Further Cultivation
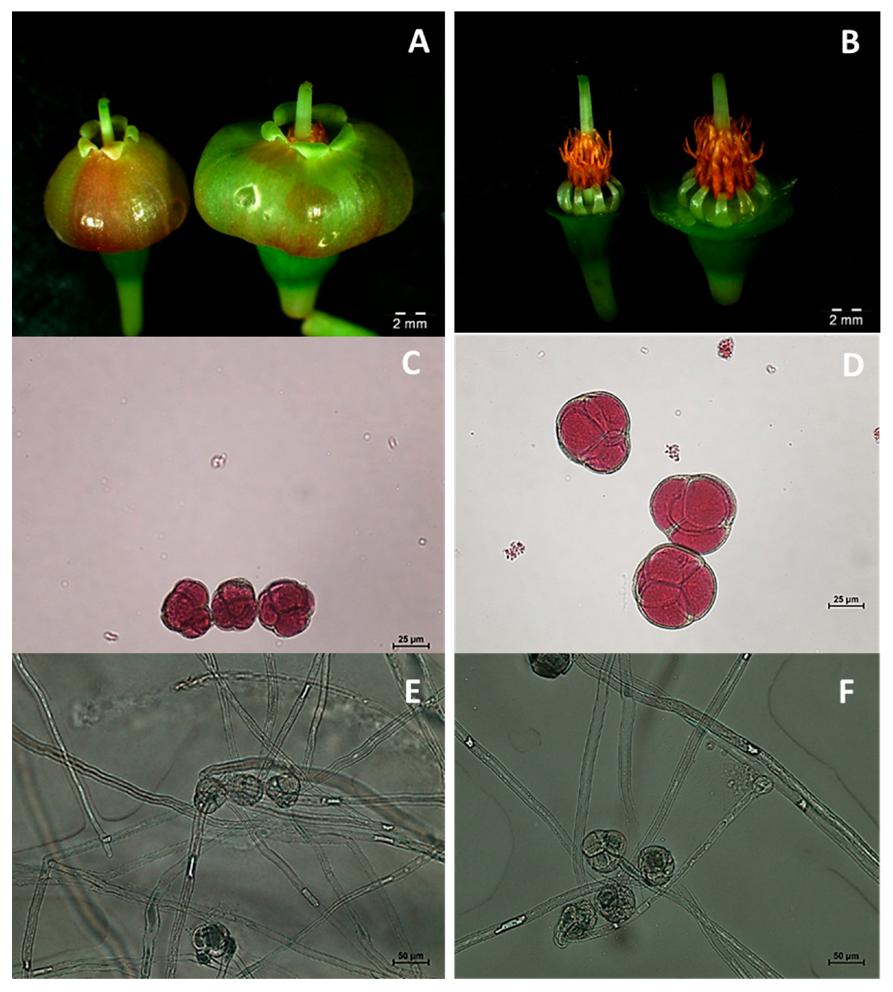
2.7. Phenotypic Evaluation
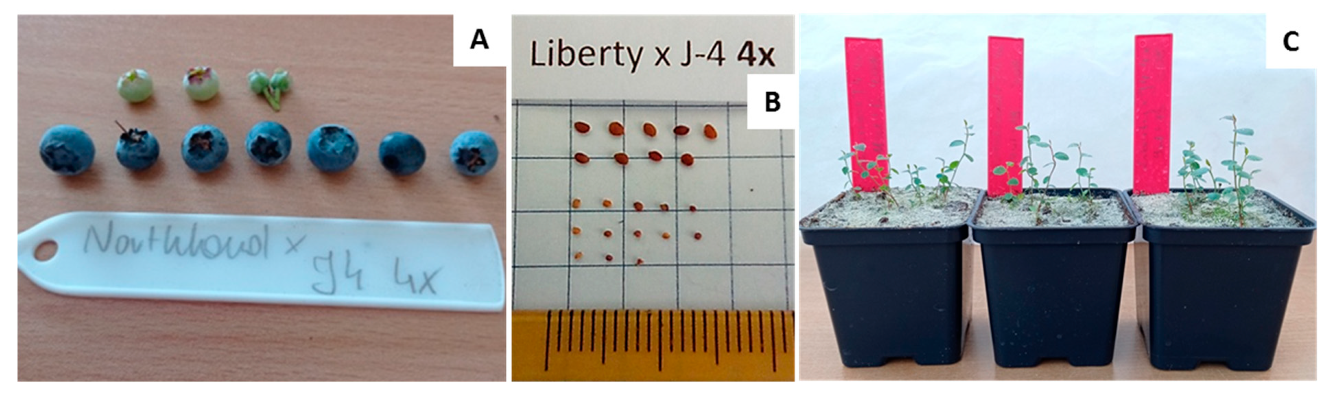
2.8. Hybridisation
2.9. Statistical Analysis
3. Results
3.1. Selection of Bilberry Accessions Based on AFLP Analysis
3.2. In Vitro Polyploidisation Efficiency
3.3. Phenotypic Evaluation
4. Discussion
4.1. Bilberry Genetic Variation
4.2. Bilberry Polyploidisation and Evaluation of Autotetraploids in Terms of Phenotype and Cross Ability
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Uleberg, E.; Rohloff, J.; Jaakola, L.; Trôst, K.; Junttila, O.; Häggman, H.; Martinussen, I. Effects of temperature and photoperiod on yield and chemical composition of northern and southern clones of bilberry (Vaccinium myrtillus L.). J. Agric. Food Chem. 2012, 60, 10406–10414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozd, J.; Anuszewska, E. Czarna jagoda-perspektywy nowych zastosowań w profilaktyce i wspomaganiu leczenia chorób cywilizacyjnych (Bilberry plant–Prospects of new applications in prevention and supportive treatment of civilisation diseases). Med. Rev. 2013, 2, 226–235. [Google Scholar]
- Peña-Sanhueza, D.; Inostroza-Blancheteau, C.; Ribera-Fonseca, A.; Reyes-Díaz, M. Anthocyanins in berries and their potential use in human health. In Superfood and Functional Food-The Development of Superfoods and Their Roles as Medicine; Shiomi, N., Waisundara, V., Eds.; InTech Open: Rijeka, Croatia, 2017; pp. 158–172. [Google Scholar]
- Pluta, S.; Żurawicz, E. The high-bush blueberry (Vaccinium corymbosum L.) breeding programme in Poland. Acta Hortic. 2014, 1017, 178–180. [Google Scholar] [CrossRef]
- Sady i Ogrody – Rynek Owoców i Warzyw - Polska Znaczącym Producentem Borówki w UE (Poland Is a Significant Producer of Blueberries in the EU). 2021. Available online: https://www.sadyogrody.pl/owoce/101/polska_znaczacym_producentem_borowki_w_ue,27958.html (accessed on 16 December 2021).
- Nestby, R.; Percival, D.; Martinussen, I.; Opstad, N.; Rohloff, J. The European blueberry (Vaccinium myrtillus L.) and the potential for cultivation. Eur. J. Plant Sci. Biotechnol. 2011, 5, 5–16. [Google Scholar]
- Lyrene, P.; Vorsa, N.; Ballington, R. Polyploidy and sexual polyploidization in the genus Vaccinium. Euphytica 2003, 133, 27–36. [Google Scholar] [CrossRef]
- Lyrene, P.M. Phenotype and fertility of intersectional hybrids between tetraploid highbush blueberry and colchicine-treated Vaccinium stamineum. HortScience 2016, 51, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Norden, E.H.; Lyrene, P.M.; Chaparro, J.X. Ploidy, fertility, and phenotypes of F1 hybrids between tetraploid highbush blueberry cultivars and diploid Vaccinium elliottii. HortScience 2020, 55, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Cabezas, D.; de Bem Oliveira, I.; Acker, M.; Lyrene, P.; Munoz, P.R. Evaluating wild germplasm introgression into autotetraploid blueberry. Agronomy 2021, 11, 614. [Google Scholar] [CrossRef]
- Lafon-Placette, C.; Johannessen, I.M.; Hornslien, K.S.; Ali, M.F.; Bjerkan, K.N.; Bramsiepe, J.; Glöckle, B.M.; Rebernig, C.A.; Brysting, A.K.; Grini, P.E.; et al. Endosperm-based hybridization barriers explain the pattern of gene flow between Arabidopsis lyrata and Arabidopsis arenosa in Central Europe. Proc. Natl. Acad. Sci. USA 2017, 114, E1027–E1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chávez-Velásquez, D.J. Hybridization of various races of Vaccinium darrowi with cultivated highbush blueberry, V. arboreum, and V. fuscatum. Doctoral Dissertation, University of Florida, Gainesville, FL, USA, 2008. [Google Scholar]
- Haring, R.A.; Lyrene, P.M. Detection of colchicine induced tetraploids of Vaccinium arboreum with flow cytometry. Acta Hortic. 2009, 810, 133–138. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Gabryszewska, E.; Dyki, B.; Stępowska, A.; Kowalski, A.; Jasiński, A. Phenotypic and genome size changes (variation) in synthetic tetraploids of daylily (Hemerocallis) in relation to their diploid counterparts. Euphytica 2014, 203, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Podwyszyńska, M.; Sowik, I.; Machlańska, A.; Kruczyńska, D.; Dyki, B. In vitro tetraploid induction of Malus × domestica Borkh. using leaf or shoot explants. Sci. Hortic. 2017, 226, 379–388. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Trzewik, A.; Marasek-Ciolakowska, A. In vitro polyploidisation of tulips (Tulipa gesneriana L.)—Phenotype assessment of tetraploids. Sci. Hortic. 2018, 242, 155–163. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Pluta, S. In vitro tetraploid induction of the blackcurrant (Ribes nigrum L.) and preliminary phenotypic observations. Zemdirb. Agric. 2019, 106, 151–158. [Google Scholar] [CrossRef]
- Mason, A.S. Polyploidy and Hybridization for Crop Improvement; CRS Press: Boca Raton, FL, USA, 2017; 490 p. [Google Scholar]
- Dhooghe, E.; Van Laere, K.; Eeckhaut, T.; Leus, L.; Van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell. Tiss. Organ. Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Nuortila, C.; Tuomi, J.; Aspi, J.; Laine, K. Early-acting inbreeding depression in a clonal dwarf shrub, Vaccinium myrtillus, in a northern boreal forest. Ann. Bot. Fenn. 2006, 43, 36–48. [Google Scholar]
- Prokaj, E.; Watanabe, H.; Suyama, Y.; Saigusa, M. Identification of rabbiteye blueberry cultivars (Vaccinium ashei Reade) and analysis of genetic relationships using amplified fragment length polymorphism (AFLP). Int. J. Hortic. Sci. 2004, 10, 27–30. [Google Scholar] [CrossRef]
- Ipek, A.; Barut, E.; Gulen, H.; Ipek, M. Genetic diversity among some blackberry cultivars and their relationship with boysenberry assessed by AFLP markers. Afr. J. Biotechnol. 2009, 8, 4830–4834. [Google Scholar]
- Debnath, S.C.; Siow, Y.L.; Petkau, J.; An, D.; Bykova, N.V. Molecular markers and antioxidant activity in berry crops: Genetic diversity analysis. Can. J. Plant Sci. 2012, 92, 1121–1133. [Google Scholar] [CrossRef]
- Debnath, S.C. Differentiation of Vaccinium cultivars and wild clones using RAPD markers. J. Plant Bioch. Biotech. 2005, 14, 173–177. [Google Scholar] [CrossRef]
- Albert, T.; Raspé, O.; Jacquemart, A.L. Diversity and spatial structure of clones in Vaccinium uliginosum populations. Can. J. Bot. 2005, 83, 211–218. [Google Scholar] [CrossRef]
- Debnath, S.C. An assessment of the genetic diversity within a collection of wild cranberry (Vaccinium macrocarpon Ait.) clones with RAPD-PCR. Genet. Resour. Crop Evol. 2007, 54, 509–517. [Google Scholar] [CrossRef]
- Debnath, S.C. Inter simple sequence repeat (ISSR) to assess genetic diversity within a collection of wild lingonberry (Vaccinium vitis-idaea L.) clones. Can. J. Plant Sci. 2007, 87, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Debnath, S.C. Development of ISSR markers for genetic diversity studies in Vaccinium angustifolium. Nordic J. Bot. 2009, 27, 141–148. [Google Scholar] [CrossRef]
- Debnath, S.C. Structured diversity using EST-PCR and EST-SSR markers in a set of wild blueberry clones and cultivars. Biochem. Syst. Ecol. 2014, 54, 337–347. [Google Scholar] [CrossRef]
- Alam, Z.; Roncal, J.; Peña-Castillo, L. Genetic variation associated with healthy traits and environmental conditions in Vaccinium vitis-idaea. BMC Genom. 2018, 19, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cüce, M.; Sökmen, A. Micropropagation of Vaccinium myrtillus L. (bilberry) naturally growing in the Turkish flora. Turk. J. Biol. 2015, 39, 233–240. [Google Scholar] [CrossRef]
- Nedelcu, A.M.; Petrus-Vancea, A. In vitro propagation of Vaccinium myrtillus L. Nat. Resour. Sustain. Dev. 2016, 8, 122–129. [Google Scholar]
- Georgieva, M.; Badjakov, I.; Dincheva, I.; Yancheva, S.; Kondakova, V. In vitro propagation of wild Bulgarian small berry fruits (bilberry, lingonberry, raspberry and strawberry). Bul. J. Agric. Sci. 2016, 22, 46–51. [Google Scholar]
- Debnath, S.C.; Goyali, J.C. In vitro propagation and variation of antioxidant properties in micropropagated Vaccinium berry plants—A review. Molecules 2020, 25, 788. [Google Scholar] [CrossRef] [Green Version]
- Meiners, J.; Schwab, M.; Szankowski, I. Efficient in vitro regeneration systems for Vaccinium species. Plant Cell Tissue Organ Cult. 2007, 89, 169–176. [Google Scholar] [CrossRef]
- Litwińczuk, W. Micropropagation of Vaccinium sp. by in vitro axillary shoot proliferation. In Protocols for Micropropagation of Selected Economically-Important Horticultural Plants. Methods in Molecular Biology (Methods and Protocols); Lambardi, M., Ozudogru, E., Jain, S., Eds.; Humana Press: Totowa, NJ, USA, 2013; Volume 994, pp. 63–76. [Google Scholar]
- Anderson, W.C. Propagation of rhododendrons by tissue culture. I. Development of culture media for multiplication of shoots. Proc. Int. Plant Prop. Soc. 1976, 25, 129–135. [Google Scholar]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Lee, T.V.D.; Hornes, M.; Frijters, A.; Pot, J.; Peleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Money, T.; Reader, S.; Qu, L.J.; Dunford, R.P.; Moore, G. AFLP-based mRNA fingerprinting. Nucleic Acids Res. 1996, 24, 2616–2617. [Google Scholar] [CrossRef] [Green Version]
- Bachem, C.W.; Van Der Hoeven, R.S.; De Bruijn, S.M.; Vreugdenhil, D.; Zabeau, M.; Visser, R.G. Visualization of differential gene expression using a novel method of RNA fingerprinting based on AFLP: Analysis of gene expression during potato tuber development. Plant J. 1996, 9, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Śliwinska, E. Zastosowanie cytometrii przepływowej do oznaczania zawartości DNA u roślin. (Estimation of DNA content in plants using flow cytometry). Postępy Biologii Komórki Suplement 2008, 24, 165–176. [Google Scholar]
- Dyki, B.; Habdas, H. The method of isolation of epidermis of tomato and cucumber leaves for microscopic investigation of pathogenic fungus development. Acta Agrobot. 1996, 49, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain. Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Guillaume, P.; Jacquemart, A.L. Early-inbreeding depression in Vaccinium myrtillus and V. vitis-idaea. Protoplasma 1999, 208, 107–114. [Google Scholar] [CrossRef]
- Hokanson, K.; Hancock, J. Earlyacting inbreeding depression in three species of Vaccinium (Ericaceae). Sex. Plant Reprod. 2000, 13, 145–150. [Google Scholar] [CrossRef]
- Raspé, O.; Guillaume, P.; Jacquemart, A.L. Inbreeding depression and biased paternity after mixed-pollination in Vaccinium myrtillus L. Int. J. Plant Sci. 2004, 165, 765–771. [Google Scholar] [CrossRef]
- Albert, T.; Raspé, O.; Jacquemart, A.L. Clonal structure in Vaccinium myrtillus L. revealed by RAPD and AFLP markers. Int. J. Plant Sci. 2003, 164, 649–655. [Google Scholar] [CrossRef]
- Zoratti, L.; Palmieri, L.; Jaakola, L.; Häggman, H. Genetic diversity and population structure of an important wild berry crop. AoB Plants 2015, 7, 117. [Google Scholar] [CrossRef] [Green Version]
- Albert, T.; Raspé, O.; Jacquemart, A.L. Clonal diversity and genetic structure in Vaccinium myrtillus populations from different habitats. Belg. J. Bot. 2004, 137, 155–162. [Google Scholar]
- Bjedov, I.; Obratov-Petković, D.; Mišić, D.; Šiler, B.; Aleksić, J.M. Genetic patterns in rangeedge populations of Vaccinium species from the central Balkans: Implications on conservation prospects and sustainable usage. Silva Fenn. 2015, 49, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Giordani, E.; Biricolti, S.; Ancillotti, C.; Petrucci, W.A.; Gori, M.; Calistri, E.; Orlandini, S.; Furlanetto, S.; Bubba, M.D. Genetic diversity and changes in phenolic contents and antiradical activity of Vaccinium myrtillus berries from its southernmost growing area in Italy. Genet. Resour. Crop Evol. 2018, 65, 1173–1186. [Google Scholar] [CrossRef]
- Gailīte, A.; Gaile, A.; Ruņģis, D.E. Genetic diversity and structure of wild Vaccinium populations V. myrtillus, V. vitis-idaea and V. uliginosum in the Baltic States. Silva Fenn. 2020, 54, 10396. [Google Scholar] [CrossRef]
- Zukauskiene, J.; Paulauskas, A.; Daubaras, R. Genetic structure of isolated Vaccinium oxycoccus populations in Lithuania. Proc. Latv. Acad. Sci. 2009, 63, 33–36. [Google Scholar]
- Rodriguez-Bonilla, L.; Williams, K.A.; Rodríguez Bonilla, F.; Matusinec, D.; Maule, A.; Coe, K.; Wiesman, E.; Diaz-Garcia, L.; Zalapa, J. The genetic diversity of cranberry crop wild relatives, Vaccinium macrocarpon Aiton and V. oxycoccos L., in the US, with special emphasis on National Forests. Plants 2020, 9, 1446. [Google Scholar] [CrossRef] [PubMed]
- Vega-Polo, P.; Cobo, M.M.; Argudo, A.; Gutierrez, B.; Rowntree, J.; Torres, M.D.L. Characterizing the genetic diversity of the Andean blueberry (Vaccinium floribundum Kunth.) across the Ecuadorian Highlands. PLoS ONE 2020, 15, e0243420. [Google Scholar] [CrossRef] [PubMed]
- Grzebelus, E.; Adamus, A. Effect of anti-mitotic agents on development and genome doubling of gynogenic onion (Allium cepa L.) embryos. Plant Sci. 2004, 167, 569–574. [Google Scholar] [CrossRef]
- Melchinger, A.E.; Molenaar, W.S.; Mirdita, V.; Schipprack, W. Colchicine alternatives for chromosome doubling in maize haploids for doubled-haploid production. Crop Sci. 2016, 56, 559–569. [Google Scholar] [CrossRef]
- Kondo, H.; Phlaetita, W.; Mii, M.; Kikuchi, S.; Deguchi, A.; Miyoshi, K. Efficient chromosome doubling of an interspecific hybrid Dendrobium Stardust ‘Fire Bird’ by treatment of amiprofos-methyl to protocorm-like body. Vitr. Cell. Dev. Biol. Plant 2020, 56, 738–749. [Google Scholar] [CrossRef]
- Haruka, K.; Ayumi, D.; Kazumitsu, M. Induction of adventitious shoots and tetraploids in Antirrhinum majus L. by treatment of antimitotic agents in vitro without plant growth regulators. Plant Biotechnol. 2021, 38, 145–152. [Google Scholar]
- Tsuda, H.; Kunitake, H.; Yamasaki, M.; Komatsu, H.; Yoshioka, K. Production of intersectional hybrids between colchicine-induced tetraploid shashanbo (Vaccinium bracteatum) and highbush blueberry ‘Spartan’. J. Am. Soc. Hortic. Sci. 2013, 138, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Perry, J.L.; Lyrene, P.M. In vitro induction of tetraploidy in Vaccinium darrowi, V. elliottii, and V. darrowi × V. elliottii with colchicine treatment. J. Am. Soc. Hortic. Sci. 1984, 109, 4–6. [Google Scholar]
- Dweikat, I.M.; Lyrene, P.M. Induced tetraploidy in a Vaccinium elliottii facilitates crossing with cultivated high-bush blueberry. J. Am. Soc. Hortic. Sci. 1991, 116, 1063–1066. [Google Scholar] [CrossRef] [Green Version]
- Rousi, A. Cytological observations on some species and hybrids of Vaccinium. Der Züchter 1966, 36, 352–359. [Google Scholar] [CrossRef]

| Antimitotic (mg L−1) | J-3 | J-4 | J-5 | J-8 | J-9 |
|---|---|---|---|---|---|
| Viable explants | |||||
| Control | 6.0 a | 6.0 a | 6.0 ab | 6.0 a | 6.0 a |
| Colchicine 125 | 5.8 a | 2.6 b | 5.0 ab | 3.4 b | 2.9 c |
| Colchicine 250 | 5.8 a | 0.8 b | 3.2 b | 4.8 ab | 3.6 bc |
| APM 5 | 5.0 a | 2.5 b | 4.2 ab | 2.8 b | 4.9 ab |
| APM 10 | 5.6 a | 1.4 b | 4.0 ab | 3.2 b | 2.3 c |
| p | 0.385 | 0.000 | 0.095 | 0.025 | 0. 000 |
| No. of shoots per explant | |||||
| Control | 6.7 a | 10.3 a | 9.7 a | 13.4 a | 34.3 a |
| Colchicine 125 | 3.1 b | 2.1 b | 2.9 bc | 5.0 b | 0.8 c |
| Colchicine 250 | 2.2 b | 0.3 b | 0.6 c | 3.4 b | 3.7 c |
| APM 5 | 6.9 a | 1.2 b | 4.0 b | 2.9 b | 12.0 b |
| APM 10 | 5.1 ab | 0.3 b | 2.7 bc | 2.5 b | 1.7 c |
| p | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 |
| Antimitotic Agents(mg L−1) | J-3 | J-4 | J-5 | J-8 | J-9 | Total Number of Tetraploids (Polyploidisation Efficiency, %) |
|---|---|---|---|---|---|---|
| Number of tetraploids (Polyploidisation efficiency, %) | ||||||
| Colchicine 125 | 1 (2.7%) | 3 (7%) | 3 (5.8%) | 1 (2.9%) | 6 (12.8%) | 14 (6.6%) |
| Colchicine 250 | 2 (3%) | 0 | 0 | 14 (22.2%) | 5 (10.4%) | 21 (10.4) |
| APM 5 | 2 (3.3%) | 3 (14.3%) | 2 (5.3%) | 8 (16.7%) | 1 (0.7%) | 16 (5.1%) |
| APM 10 | 11 (14.1%) | 2 (28.6%) | 2 (2.3%) | 6 (13.3%) | 2 (3.9%) | 23 (8.6%) |
| Total number of tetraploids, (%) | 16 (6.5%) | 8 (10.1%) | 7 (3.6%) | 29 (15.3%) | 14 (4.9% | 74 (7.4%) |
| Number of mixoploids (Polyploidisation efficiency, %) | ||||||
| Colchicine 125 | 5 (13.5%) | 10 (23.3%) | 7 (13.5%) | 8 (23.5%) | 16 (34.0%) | 46 (21.6%) |
| Colchicine 250 | 7 (10.4%) | 2 (25.0%) | 3 (20.0%) | 14 (22.2%) | 9 (18.8%) | 35 (17.4%) |
| APM 5 | 12 (19.0%) | 13 (61.9%) | 13 (34.2%) | 15 (31.3%) | 31 (21.8%) | 84 (26.0%) |
| APM 10 | 17 (21%) | 5 (71.0%) | 34 (38.6%) | 16 (35.6%) | 21 (41.2%) | 93 (34.6%) |
| Total number of mixoploid (%) | 41 (16.7%) | 30 (38.0%) | 57 (29.5) | 53 (27.9%)) | 77 (26.7%) | 258 (25.9%) |
| Total number of tested shoots | 245 | 79 | 193 | 190 | 288 | 995 |
| Trait | Diploid | Tetraploids | ||||
|---|---|---|---|---|---|---|
| 4x-1 | 4x-2 | 4x-5 | 4x-7 | p | ||
| Shoot length (cm) | 23.5 ab | 21.4 ab | 24.3 a | 22.1 ab | 20.2 b | 0.17 |
| Shoot thickness (mm) | 2.1 b | 2.4 ab | 2.6 a | 2.1 b | 2.6 a | 0.056 |
| Leaf area (cm2) | 1.6 c | 2.1 bc | 2.4 b | 2.5 b | 3.2 a | 0.000 |
| Chlorophyll content (CCI) | 11.4 d | 12.3 d | 15.4 ab | 13.5 bcd | 16.7 a | 0.001 |
| Stomata length (µm) | 31.6 c | 39.1 a | 39.0 a | 36.5 b | 40.6 a | 0.000 |
| Stomata density (field of view) | 27.5 a | 16.5 b | 16.1 b | 18.3 b | 16.6 b | 0.000 |
| Trait | Diploid | Tetraploid | p |
|---|---|---|---|
| First flower opening time | 20 April | 23 April | - |
| Flowering plants (%) | 66.7 | 20.0 | - |
| Number of flowers/flowering plant | 3.0 | 2.2 | - |
| Flower diameter (mm) | 4.8 b | 7.8 a | 0.008 |
| Pollen tetrad diameter (µm) | 41.9 b | 56.5 a | 0.000 |
| Pollen tetrad germination (%) | 94.7 | 89.3 | - |
| Parameter | ‘Bluecrop’ × J-4-4x | ‘Northland’ × J-4-4x | ‘Liberty’ × J-4-4x |
|---|---|---|---|
| Number of pollinated flowers | 61 | 70 | 15 |
| Total number of fruits formed (green and matured) | 56 | 11 | 7 |
| % of fruits formed ) in relation to the number of pollinated flowers | 91.8 | 15.7 | 46.7 |
| Number of matured fruits | 37 | 7 | 7 |
| Mature fruit mass (g) | 0.64 | 0.38 | 0.60 |
| Number of well-formed (plump) seeds | 11 | 14 | 9 |
| Number of seedlings obtained | 9 | 7 | 8 |
| % of seedlings in relation to the number of pollinated flowers | 14.8 | 10.0 | 53.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podwyszynska, M.; Mynett, K.; Markiewicz, M.; Pluta, S.; Marasek-Ciolakowska, A. Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.). Agronomy 2021, 11, 2584. https://doi.org/10.3390/agronomy11122584
Podwyszynska M, Mynett K, Markiewicz M, Pluta S, Marasek-Ciolakowska A. Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.). Agronomy. 2021; 11(12):2584. https://doi.org/10.3390/agronomy11122584
Chicago/Turabian StylePodwyszynska, Malgorzata, Katarzyna Mynett, Monika Markiewicz, Stanisław Pluta, and Agnieszka Marasek-Ciolakowska. 2021. "Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.)" Agronomy 11, no. 12: 2584. https://doi.org/10.3390/agronomy11122584
APA StylePodwyszynska, M., Mynett, K., Markiewicz, M., Pluta, S., & Marasek-Ciolakowska, A. (2021). Chromosome Doubling in Genetically Diverse Bilberry (Vaccinium myrtillus L.) Accessions and Evaluation of Tetraploids in Terms of Phenotype and Ability to Cross with Highbush Blueberry (V. corymbosum L.). Agronomy, 11(12), 2584. https://doi.org/10.3390/agronomy11122584







