Girdling Effects on Fruit Maturity, Kernel Quality, and Nutritional Value of Walnuts (Juglans regia L.) alongside the Effects on Leaf Physiological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Leaf photosynthetic Variables
2.3. Maturity Stage
2.4. Weight of Nut and Color, Moisture, and Oil Percentage in Kernels
2.5. Extraction of Antioxidants
2.6. Total Phenolic Compounds (TP), Total Antioxidant Capacity (TAC) by FRAP and DDPH Methods
2.7. Extraction and Determination of Soluble Sugars (Sucrose, Glucose, Fructose)
2.8. Extraction of Oil for Quality Estimation and Fatty Acid Profile
2.9. Free Fatty Acid Content and Peroxide Value
2.10. Fatty Acid Analysis
2.11. Statistical Analysis
3. Results
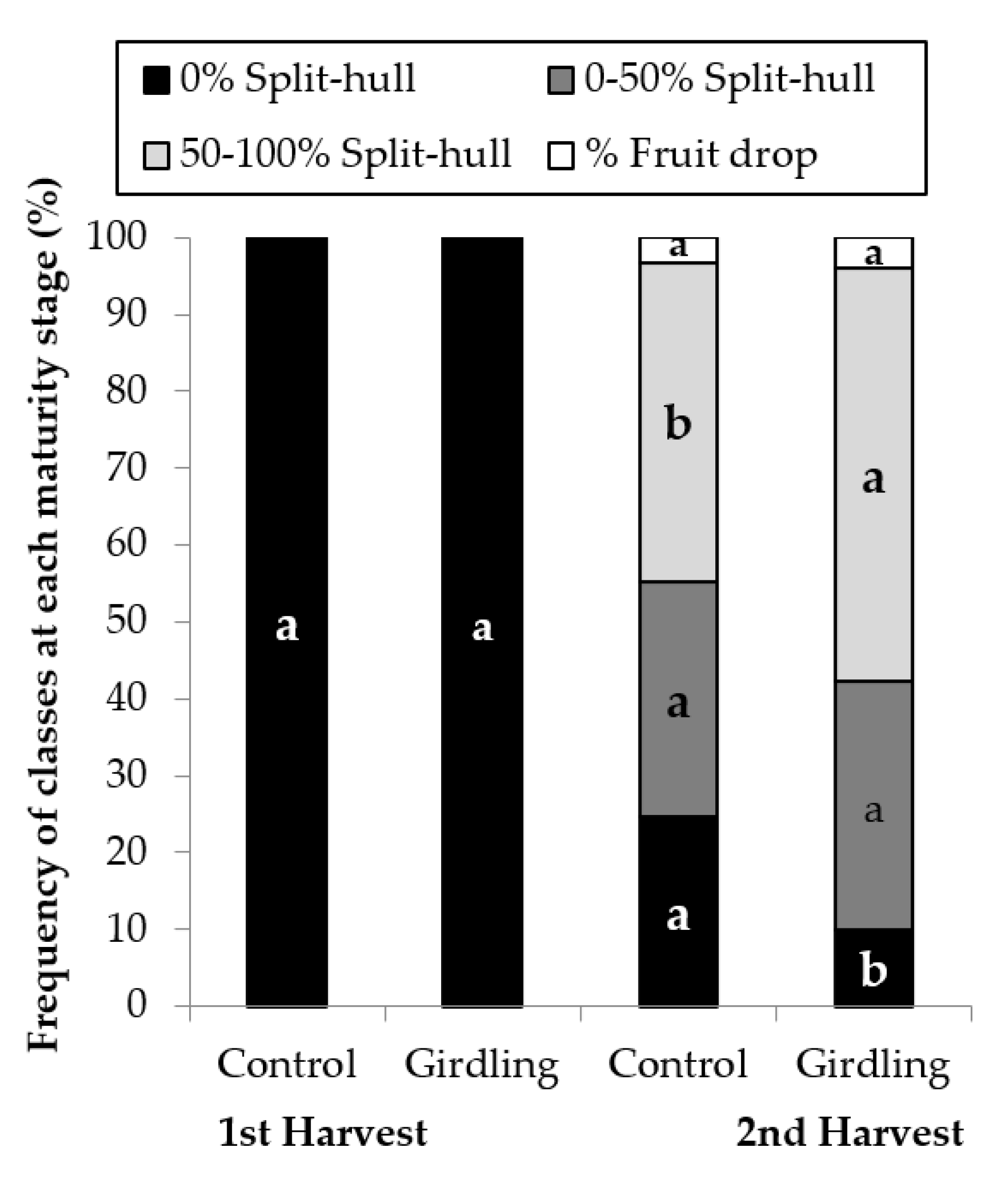
3.1. Girdling Effect on Maturity Stage
3.2. Nut and Kernel Characteristics
3.3. Oil Content in Kernel, Free Fatty Acids (FFA) and Peroxide Value (PV) in Oil, and Total Phenolics (TP) and Total Antioxidant Capacity (FRAP, DPPH) in Kernels
3.4. Oil Composition
3.5. Soluble Sugars (Sols)
3.6. Photosynthesis
4. Discussion
4.1. Fruit Maturation
4.2. Quality Characteristics Based on Consumer-Perception
4.3. Nutritional Value—Fatty Acids and Total Antioxidants
4.4. Leaf Photosynthesis and Soluble Sugars (Sols) in Leaves and Kernels
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 30 October 2020).
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) chemical composition and research in human health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Arranz, S.; Pérez-Jiménez, J.; Saura-Calixto, F. Antioxidant capacity of walnut (Juglans regia L.): Contribution of oil and defatted matter. Eur. Food Res. Technol. 2008, 227, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Storage of fresh walnuts (Juglans regia L.)—Low temperature and phenolic compounds. Postharvest Biol. Technol. 2012, 73, 80–88. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Oil composition in stored walnut cultivars—Quality and nutritional value. Eur. J. Lipid Sci. Technol. 2015, 117, 338–348. [Google Scholar] [CrossRef]
- Tapia, M.I.; Sánchez-Morgado, J.R.; García-Parra, J.; Ramírez, R.; Hernández, T.; González-Gómez, D. Comparative study of the nutritional and bioactive compounds content of four walnut (Juglans regia L.) cultivars. J. Food Compost. Anal. 2013, 31, 232–237. [Google Scholar] [CrossRef]
- Abdallah, I.B.; Tlili, N.; Martinez-Force, E.; Rubio, A.G.P.; Perez-Camino, M.C.; Albouchi, A.; Boukhchina, S. Content of carotenoids, tocopherols, sterols, triterpenic and aliphatic alcohols, and volatile compounds in six walnuts (Juglans regia L.) varieties. Food Chem. 2015, 173, 972–978. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Shahidi, F. Phytochemical Profiles and Potential Health Benefi ts of Heartnut (Juglans ailanthifolia var. cordiformis): A Comparison with the Common Walnut (Juglans regia L.). In Tree Nuts; CRC Press: Boca Raton, FL, USA, 2008; pp. 251–262. [Google Scholar]
- Ortiz, C.M.; Vicente, A.R.; Fields, R.P.; Grillo, F.; Labavitch, J.M.; Donis-Gonzalez, I.; Crisosto, C.H. Walnut (Juglans regia L.) kernel postharvest deterioration as affected by pellicle integrity, cultivar and oxygen concentration. Postharvest Biol. Technol. 2019, 156, 110948. [Google Scholar] [CrossRef]
- Donis-González, I.R.; Bergman, S.M.; Sideli, G.M.; Slaughter, D.C.; Crisosto, C.H. Color vision system to assess English walnut (Juglans Regia) kernel pellicle color. Postharvest Biol. Technol. 2020, 167, 111199. [Google Scholar] [CrossRef]
- Kader, A.A.; Thompson, J.E. Postharvest handling systems: Tree nuts. In Postharvest Technology of Horticultural Crops; Kader, A., Ed.; University of California, Division of Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 399–406. [Google Scholar]
- Goren, R.; Huberman, M.; Goldschmidt, E.E. Girdling: Physiological and Horticultural aspects. Hortic. Rev. 2004, 30, 1–36. [Google Scholar]
- Day, K.R.; DeJong, T.M. Girdling of early season ‘Mayfire’nectarine trees. J. Hortic. Sci. 1990, 65, 529–534. [Google Scholar] [CrossRef]
- Agusti, M.; Andreu, I.; Juan, M.; Almela, V.; Zacarias, L. Effects of ringing branches on fruit size and maturity of peach and nectarine cultivars. J. Hortic. Sci. Biotechnol. 1998, 73, 537–540. [Google Scholar] [CrossRef]
- Davie, S.J.; Stassen, P.J.C.; Van der Walt, M.; Snijder, B. Girdling avocado trees for improved production. SAAGA Yearb. 1995, 18, 51–53. [Google Scholar]
- Proietti, P. Changes in photosynthesis and fruit characteristics in olive in response to assimilate availability. Photosynthetica 2003, 41, 559–564. [Google Scholar] [CrossRef]
- Wargo, J.M.; Merwin, I.A.; Watkins, C.B. Nitrogen Fertilization, Midsummer Trunk Girdling, and AVG Treatments Affect Maturity and Quality of Jonagold Apples. HortScience 2004, 39, 493–500. [Google Scholar] [CrossRef] [Green Version]
- Rivas, F.; Gravina, A.; Agustí, M. Girdling effects on fruit set and quantum yield efficiency of PSII in two Citrus cultivars. Tree Physiol. 2007, 27, 527–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, B.; Chen, L.N.; Hao, J.B.; Zhang, Y.; Huang, J.C. Comparative transcription profiles reveal that carbohydrates and hormone signaling pathways mediate flower induction in Juglans sigillata after girdling. Ind. Crops Prod. 2020, 153, 112556. [Google Scholar] [CrossRef]
- Moscatello, S.; Proietti, S.; Augusti, A.; Scartazza, A.; Walker, R.P.; Famiani, F.; Battistelli, A. Late summer photosynthesis and storage carbohydrates in walnut (Juglans regia L.): Feed-back and feed-forward effects. Plant Physiol. Biochem. 2017, 118, 618–626. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Zhang, Z.; Gao, Y.; Zhao, S.; He, F. Photosynthesis of two walnut cultivars as influenced by fruiting. Acta Hortic. 2010, 961, 297–302. [Google Scholar] [CrossRef]
- Zhang, C.F.; Pan, C.D.; Chen, H. The long-term response of photosynthesis in walnut (Juglans regia L.) leaf to a leaf-to-fruit ratio. Photosynthetica 2019, 57, 762–771. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, D.J.; Lliso, I.; Tadeo, F.R.; Talon, M. Regulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant. 2002, 116, 563–572. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Frak, E.; Le Roux, X.; Millard, P.; Dreyer, E.; Jaouen, G.; Saint-Joanis, B.; Wendler, R. Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant Cell Environ. 2001, 24, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Blanke, M.M.; Lenz, F. Fruit photosynthesis. Plant Cell Environ. 1989, 12, 31–46. [Google Scholar] [CrossRef]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘Antioxidant Power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Meyer, M.D.; Terry, L.A. Development of a rapid method for the sequential extraction and subsequent quantification of fatty acids and sugars from avocado mesocarp tissue. J. Agric. Food Chem. 2008, 56, 7439–7445. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Tsantili, E. Oil content and composition in relation to leaf photosynthesis, leaf sugars and fruit sugars in maturing Koroneiki olives—The mannitol effect on oil. J. Appl. Bot. Food Qual. 2016, 89. [Google Scholar] [CrossRef]
- AOAC. Oils and Fats. In Official Methods of Analysis, 16th ed.; Horwitz, W., Ed.; AOAC: Arlington, VA, USA, 1995; pp. 9–10, 17. [Google Scholar]
- Park, P.W.; Goins, R.E. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Li, Y.; Ma, S.; Wang, Y.; Xuan, X.; Hou, L.; Sun, Q.; Yang, K. The dynamics of fat, protein and sugar metabolism during walnut (Juglans regia L.) fruit development. Afr. J. Biotechnol. 2012, 11, 1267–1276. [Google Scholar]
- Pinney, K.; John, M. Fruit Growth and Development. Walnut Prod. Man. 1997, 3373, 139. [Google Scholar]
- Christopoulos, M.V.; Tsantili, E. Effects of temperature and packaging atmosphere on total antioxidants and colour of walnut (Juglans regia L.) kernels during storage. Sci. Hortic. 2011, 131, 49–57. [Google Scholar] [CrossRef]
- Ma, Y.; Li, P.; Watkins, C.B.; Ye, N.; Jing, N.; Ma, H.; Zhang, T. Chlorine dioxide and sodium diacetate treatments in controlled atmospheres retard mold incidence and maintain quality of fresh walnuts during cold storage. Postharvest Biol. Technol. 2020, 161, 111063. [Google Scholar] [CrossRef]
- Amin, F.; Masoodi, F.A.; Baba, W.N.; Khan, A.A.; Ganie, B.A. Effect of different ripening stages on walnut kernel quality: Antioxidant activities, lipid characterization and antibacterial properties. J. Food Sci. Technol. 2017, 54, 3791–3801. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.D.; White, P.J. Oxidative stability of walnut oils extracted with supercritical carbon dioxide. J. Am. Oil Chem. Soc. 2003, 80, 575–578. [Google Scholar] [CrossRef]
- Warmund, M.R.; Elmore, J.; Drake, M.; Yates, M.D. Descriptive analysis of kernels of selected black and Persian walnut cultivars. J. Sci. Food Agric. 2009, 89, 117–121. [Google Scholar] [CrossRef]
- United States Standards for Grades of Shelled Walnuts (Juglans regia L.); (82 FR39655); USDA: Washington, DC, USA, 2017.
- Gama, T.; Wallace, H.M.; Trueman, S.J.; Hosseini-Bai, S. Quality and shelf life of tree nuts: A review. Sci. Hortic. 2018, 242, 116–126. [Google Scholar] [CrossRef]
- Martínez, M.L.; Penci, M.C.; Ixtaina, V.; Ribotta, P.D.; Maestri, D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT-Food Sci. Technol. 2013, 51, 44–50. [Google Scholar] [CrossRef]
- Orm, R.B.; Citeau, M.; Comitis, A.; Savoire, R.; Harscoat-Schiavo, C.; Subra-Paternault, P.; Joffre, F. Walnut oil deacidification by liquid–liquid extraction with ethanol in a single-and multistage crossflow process. OCL 2020, 27, 35. [Google Scholar] [CrossRef]
- Lutsiak, V.; Lavrov, R.; Furman, I.; Smitiukh, A.; Mazur, H.; Zahorodnia, N. Economic Aspects and Prospects for the Development of the Market of Vegetable Oils in a Context of Formation of its Value Chain. Montenegrin J. Econ. 2020, 16, 155–168. [Google Scholar] [CrossRef]
- Mexis, S.F.; Badeka, A.V.; Riganakos, K.A.; Karakostas, K.X.; Kontominas, M.G. Effect of packaging and storage conditions on quality of shelled walnuts. Food Control 2009, 20, 743–751. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Pereira, J.A.; Seabra, R.M.; Oliveira, B.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003, 51, 7698–7702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, R.H.; Halim, L. Antioxidant and antiproliferative activities of common edible nut seeds. LWT-Food Sci. Technol. 2009, 42, 1–8. [Google Scholar] [CrossRef]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How can we make plants grow faster? A source–sink perspective on growth rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [CrossRef]
- Tombesi, A.; DeJong, T.M.; Ryugo, K. Net CO2 assimilation characteristics of walnut leaves under field and laboratory conditions. J. Am. Soc. Hortic. Sci. 1983, 108, 558–561. [Google Scholar]
- Ticho, R.J. Girdling, a means to increase avocado fruit production. Calif. Avocado Soc. Yearb. 1970, 54, 90–94. [Google Scholar]
- Bates, P.D.; Stymne, S.; Ohlrogge, J. Biochemical pathways in seed oil synthesis. Curr. Opin. Plant Biol. 2013, 16, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Fallahi, E.; Kiester, M.J.; Fallahi, B.; Mahdavi, S. Rootstock, Canopy Architecture, Bark Girdling, and Scoring Influence on Growth, Productivity, and Fruit Quality at Harvest in ‘Aztec Fuji’ Apple. HortScience 2018, 53, 1629–1633. [Google Scholar] [CrossRef] [Green Version]
- Boyd, L.M.; Barnett, A.M. Manipulation of whole-vine carbon allocation using girdling, pruning, and fruit thinning affects fruit numbers and quality in kiwifruit. HortScience 2011, 46, 590–595. [Google Scholar] [CrossRef] [Green Version]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Samiotaki, M.; Sarrou, E.; Karamanoli, K.; Molassiotis, A. Proteomic and metabolic analysis reveals novel sweet cherry fruit development regulatory points influenced by girdling. Plant Physiol. Biochem. 2020, 149, 233–244. [Google Scholar] [CrossRef] [PubMed]


| Harvest 1 | Treatment | Nut Weight (g) | Kernel (g) | Kernel to Nut % (w/w) | Kernel Moisture % (w/w) | L* | ho | C* |
|---|---|---|---|---|---|---|---|---|
| 1st | Control | - | 4.76 ± 0.25 d | - | 41.31 ± 1.89 a | 65.78 ± 0.74 a | 84.29 ± 0.39 ab | 29.98 ± 0.77 b |
| Girdling | - | 5.62 ± 0.27 c | - | 38.62 ± 1.61 ab | 64.73 ± 0.75 ab | 84.81 ± 0.93 a | 31.07 ± 0.86 ab | |
| 2nd | Control | 13.21 ± 0.41 a2 | 6.24 ± 0.22 b | 47.20 ± 0.35 b | 36.01 ± 1.20 bc | 63.89 ± 0.96 ab | 83.45 ± 0.36 b | 32.62 ± 0.77 a |
| Girdling | 13.95 ± 0.64 a | 7.20 ± 0.24 a | 51.70 ± 3.32 a | 34.37 ± 1.72 c | 62.68 ± 1.25 b | 83.03 ± 0.54 b | 32.39 ± 0.54 a | |
| Pg 3 | NS | *** | * | * | * | NS | NS | |
| Ph | - | *** | - | *** | ** | *** | *** | |
| Pg×h | - | NS | - | NS | NS | NS | NS |
| Harvest 1 | Treatment | Palmitic C16:0 | Linoleic C18:2 n-9,12 |
|---|---|---|---|
| 1st | Control | 10.41 ± 0.46 a2 | 49.52 ± 1.15 b |
| Girdling | 9.75 ± 0.64 a | 49.34 ± 1.19 b | |
| 2nd | Control | 8.08 ± 0.36 b | 50.57 ± 0.21 ab |
| Girdling | 8.02 ± 0.21 b | 52.32 ± 0.85 a | |
| Pg3 | NS | NS | |
| Ph | *** | ** | |
| Pg×h | NS | NS |
| Harvest 1 | Treatment | TFA TFA | SFA Cv:0 | ΤUFA | MUFA Cv:1 | PUFA Cv:n (n > 1) |
|---|---|---|---|---|---|---|
| 1st | Control | 98.05 ± 0.98 a2 | 13.91 ± 0.54 a | 84.14 ± 1.43 b | 20.11 ± 0.64 a | 64.03 ± 0.88 b |
| Girdling | 97.55 ± 0.86 a | 13.16 ± 0.82 a | 84.39 ± 1.60 b | 20.41 ± 0.37 a | 63.97 ± 1.28 b | |
| 2nd | Control | 97.97 ± 0.97 a | 11.46 ± 0.42 b | 86.50 ± 1.03 ab | 20.75 ± 0.40 a | 65.76 ± 0.64 ab |
| Girdling | 99.11 ± 1.30 a | 11.37 ± 0.32 b | 87.74 ± 1.35 a | 20.52 ± 1.92 a | 67.21 ± 0.44 a | |
| Pg3 | NS | NS | NS | NS | NS | |
| Ph | NS | *** | ** | NS | *** | |
| Pg×h | NS | NS | NS | NS | NS |
| Harvest 1 | Treatment | Pn (μmol m−2 s−1) | gs (mol m −2 s−1) | Ci (μmol mol air−1) | E Rate (mmol m −2 s−1) |
|---|---|---|---|---|---|
| 1st | Control | 17.59 ± 3.28 a2 | 239.63 ± 75.34 a | 149.38 ± 25.65 b | 3.93 ± 0.71 a |
| Girdling | 16.35 ± 2.37 a | 225.38 ± 62.43 a | 150.00 ± 35.27 b | 3.86 ± 0.68 a | |
| 2nd | Control | 5.75 ± 1.22 b | 132.00 ± 43.15 b | 219.25 ± 41.71 a | 2.67 ± 0.62 b |
| Girdling | 5.16 ± 0.91 b | 140.75 ± 52.58 b | 227.88 ± 52.22 a | 3.20 ± 0.89 b | |
| Pg3 | NS | NS | NS | NS | |
| Ph | *** | *** | *** | *** | |
| Pg×h | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christopoulos, M.V.; Kafkaletou, M.; Karantzi, A.D.; Tsantili, E. Girdling Effects on Fruit Maturity, Kernel Quality, and Nutritional Value of Walnuts (Juglans regia L.) alongside the Effects on Leaf Physiological Characteristics. Agronomy 2021, 11, 200. https://doi.org/10.3390/agronomy11020200
Christopoulos MV, Kafkaletou M, Karantzi AD, Tsantili E. Girdling Effects on Fruit Maturity, Kernel Quality, and Nutritional Value of Walnuts (Juglans regia L.) alongside the Effects on Leaf Physiological Characteristics. Agronomy. 2021; 11(2):200. https://doi.org/10.3390/agronomy11020200
Chicago/Turabian StyleChristopoulos, Miltiadis V., Mina Kafkaletou, Athanasia D. Karantzi, and Eleni Tsantili. 2021. "Girdling Effects on Fruit Maturity, Kernel Quality, and Nutritional Value of Walnuts (Juglans regia L.) alongside the Effects on Leaf Physiological Characteristics" Agronomy 11, no. 2: 200. https://doi.org/10.3390/agronomy11020200
APA StyleChristopoulos, M. V., Kafkaletou, M., Karantzi, A. D., & Tsantili, E. (2021). Girdling Effects on Fruit Maturity, Kernel Quality, and Nutritional Value of Walnuts (Juglans regia L.) alongside the Effects on Leaf Physiological Characteristics. Agronomy, 11(2), 200. https://doi.org/10.3390/agronomy11020200






