Plant Growth Stimulation by Microbial Consortia
Abstract
:1. Introduction
2. Plant-Growth-Promoting Microorganisms
3. Bacterial Consortia
4. Bacteria–Bacteria Interactions
5. Plant Growth Stimulation by PGPB Consortia Under Non-Stress Conditions
6. Plant Growth Stimulation by PGPB Consortia Under Stress Conditions
6.1. Biotic Stress
6.2. Abiotic Stress
7. Plant Growth Stimulation by Fungal–Bacterial Consortia
7.1. Plant Growth Stimulation by AMF–PGPB Consortia
7.2. Plant Growth Stimulation by Trichoderma–PGPB Consortia
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, R.E.; Cornell, S.J.; Scharlemann, J.P.W.; Balmford, A. Farming and the fate of wild nature. Science 2005, 307, 550–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Is There a Need for a More Sustainable Agriculture? CRC Crit. Rev. Plant Sci. 2011, 30, 6–23. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Walvekar, V.A.; Sharma, S. Microbiome as Sensitive Markers for Risk Assessment of Pesticides. In Pesticides in Crop Production; Wiley: Hoboken, NJ, USA, 2020; pp. 89–108. [Google Scholar]
- Uhl, P.; Brühl, C.A. The Impact of Pesticides on Flower-Visiting Insects: A Review with Regard to European Risk Assessment. Environ. Toxicol. Chem. 2019, 38, 2355–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.M.D.; Cota, F.I.P.; Santoyo, G.; de los Santos Villalobos, S. Chlorothalonil tolerance of indole producing bacteria associated to wheat (Triticum turgidum L.) rhizosphere in the Yaqui Valley, Mexico. Ecotoxicology 2019, 28, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Stamati, P.N.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant Defense against Herbivorous Pests: Exploiting Resistance and Tolerance Traits for Sustainable Crop Protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Estrella, L.R.H.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-Generation Insect-Resistant Plants: RNAi-Mediated Crop Protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial services of arbuscular mycorrhizal fungi—From ecology to application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Glick, B.R.; Glick, B.R. Introduction to Plant Growth-Promoting Bacteria. In Beneficial Plant-Bacterial Interactions; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–28. [Google Scholar]
- Kashyap, P.L.; Solanki, M.K.; Kushwaha, P.; Kumar, S.; Srivastava, A.K. Biocontrol Potential of Salt-Tolerant Trichoderma and Hypocrea Isolates for the Management of Tomato Root Rot Under Saline Environment. J. Soil Sci. Plant Nutr. 2020, 20, 160–176. [Google Scholar] [CrossRef]
- Khatoon, Z.; Huang, S.; Rafique, M.; Fakhar, A.; Kamran, M.A.; Santoyo, G. Unlocking the potential of plant growth-promoting rhizobacteria on soil health and the sustainability of agricultural systems. J. Environ. Manag. 2020, 273, 111118. [Google Scholar] [CrossRef] [PubMed]
- Cedeño, L.R.M.; Mosqueda, M.d.C.O.; Lara, P.D.L.; Cota, F.I.P.; Villalobos, S.d.L.S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial Consortia: Promising Probiotics as Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Abhilash, P.C.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant Growth-Promoting Microorganisms for Environmental Sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef]
- Vázquez, I.T.; Cruz, R.S.; Domínguez, M.A.; Ruan, V.L.; Reyes, A.S.; Carbente, M.d.R.S.; Chacón, D.P.; García, R.A.B.; Mallol, J.L.F. Isolation and characterization of psychrophilic and psychrotolerant plant-growth promoting microorganisms from a high-altitude volcano crater in Mexico. Microbiol. Res. 2020, 232, 126394. [Google Scholar] [CrossRef]
- Khan, A.; Singh, J.; Upadhayay, V.K.; Singh, A.V.; Shah, S. Microbial Biofortification: A Green Technology Through Plant Growth Promoting Microorganisms. In Sustainable Green Technologies for Environmental Management; Springer: Singapore, 2019; pp. 255–269. ISBN 9789811327728. [Google Scholar]
- Mahmud, K.; Franklin, D.; Ney, L.; Cabrera, M.; Habteselassie, M.; Hancock, D.; Newcomer, Q.; Subedi, A.; Dahal, S. Improving inorganic nitrogen in soil and nutrient density of edamame bean in three consecutive summers by utilizing a locally sourced bio-inocula. Org. Agric. 2021, 1–11. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q.; Dahal, S. Impact of inoculation with local effective microorganisms on soil nitrogen cycling and legume productivity using composted broiler litter. Appl. Soil Ecol. 2020, 154, 103567. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Compant, S.; Ballhausen, M.B.; Ruppel, S.; Franken, P. The interaction between Rhizoglomus irregulare and hyphae attached phosphate solubilizing bacteria increases plant biomass of Solanum lycopersicum. Microbiol. Res. 2020, 240, 126556. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Merino, N.; Okamoto, A.; Gedalanga, P. Interkingdom microbial consortia mechanisms to guide biotechnological applications. Microb. Biotechnol. 2018, 11, 833–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Kreuzwieser, J.; Rennenberg, H. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 2001, 3, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Mercado-Blanco, J.; Bakker, P.A.H.M. Interactions between plants and beneficial Pseudomonas spp.: Exploiting bacterial traits for crop protection. Antonie Leeuwenhoek. 2007, 92, 367–389. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Pacheco, C.H.; Salmerón, J.H.; León, R.H. The role of abiotic factors modulating the plant-microbe-soil interactions: Toward sustainable agriculture. A review. Span. J. Agric. Res. 2017, 15, e03R01. [Google Scholar] [CrossRef] [Green Version]
- Carrión, V.J.; Jaramillo, J.P.; Cordovez, V.; Tracanna, V.; de Hollander, M.; Buck, D.R.; Mendes, L.W.; van Ijcken, W.F.J.; Exposito, R.G.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Bashan, Y. Inoculants of plant growth-promoting bacteria for use in agriculture. Biotechnol. Adv. 1998, 16, 729–770. [Google Scholar] [CrossRef]
- Altieri, M.A.; Trujillo, J. The agroecology of corn production in Tlaxcala, Mexico. Hum. Ecol. 1987, 15, 189–220. [Google Scholar] [CrossRef]
- Finney, D.M.; Kaye, J.P. Functional diversity in cover crop polycultures increases multifunctionality of an agricultural system. J. Appl. Ecol. 2017, 54, 509–517. [Google Scholar] [CrossRef]
- Behera, B.; Das, T.K.; Raj, R.; Ghosh, S.; Raza, M.B.; Sen, S. Microbial Consortia for Sustaining Productivity of Non-legume Crops: Prospects and Challenges. Agric. Res. 2020, 1–14. [Google Scholar] [CrossRef]
- Bradáčová, K.; Kandeler, E.; Berger, N.; Ludewig, U.; Neumann, G. Microbial consortia inoculants stimulate early growth of maize depending on nitrogen and phosphorus supply. Plant Soil Environ. 2020, 66, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Goswami, M.; Deka, S. Plant growth-promoting rhizobacteria—Alleviators of abiotic stresses in soil: A review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Solis, D.R.; Guzmán, M.Á.V.; Sohlenkamp, C.; Santoyo, G. Antifungal and Plant Growth–Promoting Bacillus under Saline Stress Modify their Membrane Composition. J. Soil Sci. Plant Nutr. 2020, 20, 1549–1559. [Google Scholar] [CrossRef]
- Reed, M.L.E.; Glick, B.R. Applications of Plant Growth-Promoting Bacteria for Plant and Soil Systems. In Applications of Microbial Engineering; Gupta, V., Schmoll, M., Maki, M., Tuohy, M., Mazutti, M.A., Eds.; Taylor and Francis: Enfield, CT, USA, 2013; pp. 181–229. ISBN 9781466585782. [Google Scholar]
- Villalobos, S.d.l.S.; Cota, F.I.P.; Sepúlveda, A.H.; Aragón, B.V.; Mora, J.C.E. Colmena: Colección de microorganismos edáficos y endófitos nativos, para contribuir a la seguridad alimentaria nacional. Rev. Mex. Cienc. Agríc. 2018, 9, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Panwar, M.; Tewari, R.; Nayyar, H. Microbial Consortium of Plant Growth-Promoting Rhizobacteria Improves the Performance of Plants Growing in Stressed Soils: An Overview. In Phosphate Solubilizing Microorganisms: Principles and Application of Microphos Technology; Springer International Publishing: Cham, Switzerland, 2014; pp. 257–285. ISBN 9783319082165. [Google Scholar]
- Sarma, B.K.; Yadav, S.K.; Singh, S.; Singh, H.B. Microbial consortium-mediated plant defense against phytopathogens: Readdressing for enhancing efficacy. Soil Biol. Biochem. 2015, 87, 25–33. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Johnson, K.B.; Sugar, D.; Loper, J.E. Mechanistically compatible mixtures of bacterial antagonists improve biological control of fire blight of pear. Phytopathology 2011, 101, 113–123. [Google Scholar] [CrossRef] [Green Version]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Joshi, B.; Chaudhary, A.; Singh, H.; Kumar, P.A. Prospective evaluation of individual and consortia plant growth promoting rhizobacteria for drought stress amelioration in rice (Oryza sativa L.). Plant Soil 2020, 457, 225–240. [Google Scholar] [CrossRef]
- Nawaz, A.; Shahbaz, M.; Asadullah; Imran, A.; Marghoob, M.U.; Imtiaz, M.; Mubeen, F. Potential of Salt Tolerant PGPR in Growth and Yield Augmentation of Wheat (Triticum aestivum L.) Under Saline Conditions. Front. Microbiol. 2020, 11, 2019. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- Rodríguez, E.V.; Cota, F.P.; Longoria, E.C.; Cervantes, J.L.; de los Santos-Villalobos, S. Bacillus subtilis TE3: A promising biological control agent against Bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Biol. Control 2019, 132, 135–143. [Google Scholar] [CrossRef]
- Gosal, S.K.; Kaur, J. Microbial Inoculants: A Novel Approach for Better Plant Microbiome Interactions. In Probiotics in Agroecosystem; Springer: Singapore, 2017; pp. 269–289. ISBN 9789811040597. [Google Scholar]
- Bashan, Y.; Prabhu, S.R.; de-Bashan, L.E.; Kloepper, J.W. Disclosure of exact protocols of fermentation, identity of microorganisms within consortia, formation of advanced consortia with microbe-based products. Biol. Fertil. Soils 2020, 56, 443–445. [Google Scholar] [CrossRef]
- Verbruggen, E.; van der Heijden, M.G.A.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Bisht, S.; Sood, A.; Aeron, A.; Sharma, G.D.; Maheshwari, D.K. Consortium of Plant-Growth-Promoting Bacteria: Future Perspective in Agriculture. In Bacteria in Agrobiology: Plant Probiotics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9783642275159, pp. 185–200. ISBN 9783642275159. [Google Scholar]
- Morriën, E. Understanding soil food web dynamics, how close do we get? Soil Biol. Biochem. 2016, 102, 10–13. [Google Scholar] [CrossRef]
- Ney, L.; Franklin, D.; Mahmud, K.; Cabrera, M.; Hancock, D.; Habteselassie, M.; Newcomer, Q. Examining trophic-level nematode community structure and nitrogen mineralization to assess local effective microorganisms’ role in nitrogen availability of swine effluent to forage crops. Appl. Soil Ecol. 2018, 130, 209–218. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- van Deynze, A.; Zamora, P.; Delaux, P.-M.; Heitmann, C.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Ryu, J.; Kim, S.W. Microbial consortia including methanotrophs: Some benefits of living together. J. Microbiol. 2019, 57, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Roell, G.W.; Zha, J.; Carr, R.R.; Koffas, M.A.; Fong, S.S.; Tang, Y.J. Engineering microbial consortia by division of labor. Microb. Cell Fact. 2019, 18, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loccoz, Y.M.; Mavingui, P.; Combes, C.; Normand, P.; Steinberg, C. Microorganisms and Biotic Interactions. In Environmental Microbiology: Fundamentals and Applications; Springer: Dordrecht, The Netherlands, 2015; pp. 395–444. ISBN 9789401791182. [Google Scholar]
- Liu, F.; Bian, Z.; Jia, Z.; Zhao, Q.; Song, S. The GCR1 and GPA1 participate in promotion of Arabidopsis primary root elongation induced by N-Acyl-homoserine lactones, the bacterial quorum-sensing signals. Mol. Plant. Microbe Interact. 2012, 25, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, K.; Makaju, S.; Ibrahim, R.; Missaoui, A. Current Progress in Nitrogen Fixing Plants and Microbiome Research. Plants 2020, 9, 97. [Google Scholar] [CrossRef] [Green Version]
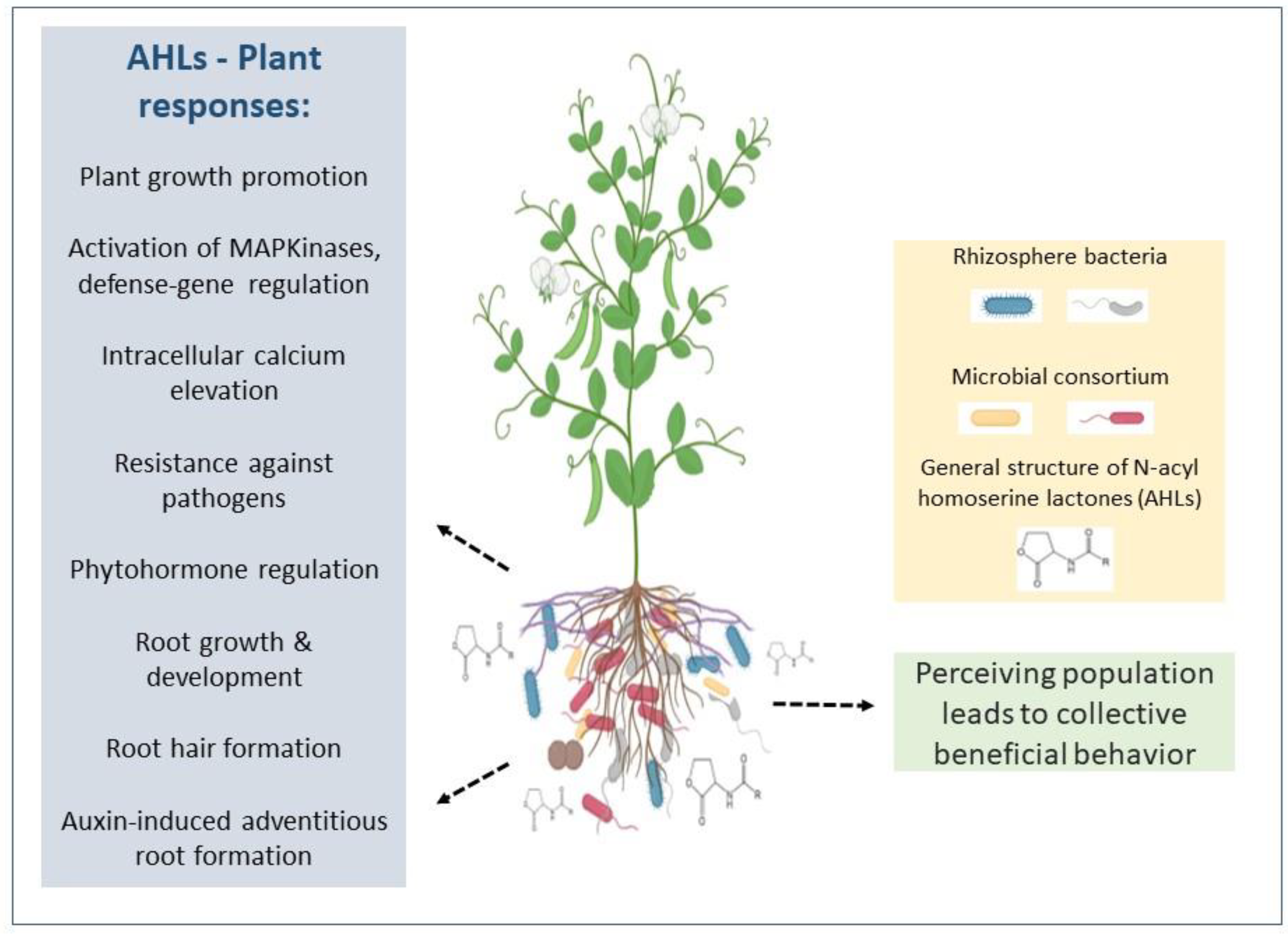
- Mukherjee, S.; Bassler, B.L. Bacterial quorum sensing in complex and dynamically changing environments. Nat. Rev. Microbiol. 2019, 17, 371–382. [Google Scholar] [CrossRef]
- Song, S.; Jia, Z.; Xu, J.; Zhang, Z.; Bian, Z. N-butyryl-homoserine lactone, a bacterial quorum-sensing signaling molecule, induces intracellular calcium elevation in Arabidopsis root cells. Biochem. Biophys. Res. Commun. 2011, 414, 355–360. [Google Scholar] [CrossRef]
- Montaño, F.P.; Guerrero, I.J.; Matamoros, R.C.S.; Baena, F.J.L.; Ollero, F.J.; Rodríguez-Carvajal, M.A.; Bellogín, R.A.; Espuny, M.R. Rice and bean AHL-mimic quorum-sensing signals specifically interfere with the capacity to form biofilms by plant-associated bacteria. Res. Microbiol. 2013, 164, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Vallejos, D.F.V.; van Noorden, G.E.; Yuan, M.; Mathesius, U. A Sinorhizobium meliloti-specific N-acyl homoserine lactone quorum-sensing signal increases nodule numbers in Medicago truncatula independent of autoregulation. Front. Plant. Sci. 2014, 5, 551. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.; Winzer, K.; Chan, W.C.; Cámara, M. Look who’s talking: Communication and quorum sensing in the bacterial world. Philos. Trans. R. Soc. B Biol. Sci. 2007, 362, 1119–1134. [Google Scholar] [CrossRef] [Green Version]
- Altaf, M.M.; Khan, M.S.A.; Abulreesh, H.H.; Ahmad, I. Quorum Sensing in Plant Growth-Promoting Rhizobacteria and Its Impact on Plant-Microbe Interaction. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; Volume 1, pp. 333–359. ISBN 9789811058134. [Google Scholar]
- Schikora, A.; Schenk, S.T.; Hartmann, A. Beneficial effects of bacteria-plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant. Mol. Biol. 2016, 90, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef] [PubMed]
- Fincheira, P.; Quiroz, A. Microbial volatiles as plant growth inducers. Microbiol. Res. 2018, 208, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Insam, H.; Seewald, M.S.A. Volatile organic compounds (VOCs) in soils. Biol. Fertil. Soils 2010, 46, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Bohm, K.S.; Sánchez, L.M.; Garbeva, P. Microbial volatiles: Small molecules with an important role in intra- and inter-kingdom interactions. Front. Microbiol. 2017, 8, 2484. [Google Scholar] [CrossRef]
- Delgado, M.F.V.; Rodríguez, E.D.V.; Chávez, L.A.C.; Alvarado, M.I.E.; Cota, F.I.P.; de los Santos-Villalobos, S. El género Bacillus como agente de control biológico y sus implicaciones en la bioseguridad agrícola. Rev. Mex. Fitopatol. Mex. J. Phytopathol. 2018, 36, 95–130. [Google Scholar] [CrossRef]
- Bitas, V.; Kim, H.S.; Bennett, J.W.; Kang, S. Sniffing on microbes: Diverse roles of microbial volatile organic compounds in plant health. Mol. Plant. Microbe Interact. 2013, 26, 835–843. [Google Scholar] [CrossRef] [Green Version]
- Díaz, I.F.C.; Molina, L.X.Z.; Cárdenas, C.I.C.; Anaya, E.R.; Ramírez, S.R.; de los Santos Villalobos, S. Consideraciones sobre el uso de biofertilizantes como alternativa agro-biotecnológica sostenible para la seguridad alimentaria en México. Rev. Mex. Cienc. Agríc. 2020, 11, 1423–1436. [Google Scholar] [CrossRef]
- Jha, C.K.; Saraf, M. Evaluation of Multispecies Plant-Growth-Promoting Consortia for the Growth Promotion of Jatropha curcas L. J. Plant. Growth Regul. 2012, 31, 588–598. [Google Scholar] [CrossRef]
- Jayashree, C.; Jagadeesh, K.S. Testing the Effect of the Microbial Consortium on Growth of Vegetable Seedlings in a Farmer’s Nursery. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1636–1639. [Google Scholar] [CrossRef] [Green Version]
- Molina-Romero, D.; Baez, A.; Quintero-Hernández, V.; Castañeda-Lucio, M.; Fuentes-Ramírez, L.E.; Bustillos-Cristales, M.D.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Munive, A.; Muñoz-Rojas, J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 2017, 12, e0187913. [Google Scholar] [CrossRef] [PubMed]
- Dary, M.; Pérez, M.A.C.; Palomares, A.J.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Malboobi, M.A.; Behbahani, M.; Madani, H.; Owlia, P.; Deljou, A.; Yakhchali, B.; Moradi, M.; Hassanabadi, H. Performance evaluation of potent phosphate solubilizing bacteria in potato rhizosphere. World J. Microbiol. Biotechnol. 2009, 25, 1479–1484. [Google Scholar] [CrossRef]
- Padilla, J.R.; Encinas, L.A.C.; Montoya, R.I.R.; de los Santos Villalobos, S. Promoción de crecimiento en trigo (Triticum turgidum L. subsp. durum) por la co-inoculación de cepas nativas de Bacillus aisladas del Valle del Yaqui, México. Nova Sci. 2020, 12, 1–27. [Google Scholar] [CrossRef]
- Montoya, R.I.R.; Encinas, L.A.C.; Cota, F.I.P.; de los Santos Villalobos, S. Mejorando rasgos biométricos de plántulas de trigo con la inoculación de un consorcio nativo de Bacillus. Rev. Mex. Cienc. Agrícolas 2020, 11, 229–235. [Google Scholar] [CrossRef] [Green Version]
- de Lajudie, P.M.; Andrews, M.; Ardley, J.; Eardly, B.; Bilak, E.J.; Kuzmanović, N.; Lassalle, F.; Lindström, K.; Mhamdi, R.; Martínez-Romero, E.; et al. Minimal standards for the description of new genera and species of rhizobia and agrobacteria. Int. J. Syst. Evol. Microbiol. 2019, 69, 1852–1863. [Google Scholar] [CrossRef]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The Sinorhizobium -Medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef] [Green Version]
- Gage, D.J. Infection and Invasion of Roots by Symbiotic, Nitrogen-Fixing Rhizobia during Nodulation of Temperate Legumes. Microbiol. Mol. Biol. Rev. 2004, 68, 280–300. [Google Scholar] [CrossRef] [Green Version]
- Haag, A.F.; Arnold, M.F.F.; Myka, K.K.; Kerscher, B.; Dall’Angelo, S.; Zanda, M.; Mergaert, P.; Ferguson, G.P. Molecular insights into bacteroid development during Rhizobium-legume symbiosis. FEMS Microbiol. Rev. 2013, 37, 364–383. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Contador, C.A.; Fan, K.; Lam, H.M. Interaction and regulation of carbon, nitrogen, and phosphorus metabolisms in root nodules of legumes. Front. Plant. Sci. 2018, 871, 1860. [Google Scholar] [CrossRef] [Green Version]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.H.; Lin, Y.H.; Reid, D.E.; Gresshoff, P.M. Molecular analysis of legume nodule development and autoregulation. J. Integr. Plant. Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Tariq, M.; Hameed, S.; Yasmeen, T.; Zahid, M.; Zafar, M. Molecular characterization and identification of plant growth promoting endophytic bacteria isolated from the root nodules of pea (Pisum sativum L.). World J. Microbiol. Biotechnol. 2014, 30, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Muresu, R.; Polone, E.; Sulas, L.; Baldan, B.; Tondello, A.; Delogu, G.; Cappuccinelli, P.; Alberghini, S.; Benhizia, Y.; Benhizia, H.; et al. Coexistence of predominantly nonculturable rhizobia with diverse, endophytic bacterial taxa within nodules of wild legumes. FEMS Microbiol. Ecol. 2008, 63, 383–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidalgo, P.M.; Hirsch, A.M. The Nodule Microbiome: N 2-Fixing Rhizobia Do Not Live Alone. Phytobiomes 2017, 1, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Sturz, A.V.; Christie, B.R.; Matheson, B.G.; Nowak, J. Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol. Fertil. Soils 1997, 25, 13–19. [Google Scholar] [CrossRef]
- Leite, J.; Fischer, D.; Rouws, L.F.M.; Júnior, P.I.F.; Hofmann, A.; Kublik, S.; Schloter, M.; Xavier, G.R.; Radl, V. Cowpea nodules harbor non-rhizobial bacterial communities that are shaped by soil type rather than plant genotype. Front. Plant. Sci. 2017, 7, 2064. [Google Scholar] [CrossRef]
- Rajendran, G.; Patel, M.H.; Joshi, S.J. Isolation and characterization of nodule-associated Exiguobacterium sp. from the root nodules of fenugreek (Trigonella foenum-graecum) and their possible role in plant growth promotion. Int. J. Microbiol. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Korir, H.; Mungai, N.W.; Thuita, M.; Hamba, Y.; Masso, C. Co-inoculation Effect of Rhizobia and Plant Growth Promoting Rhizobacteria on Common Bean Growth in a Low Phosphorus Soil. Front. Plant. Sci. 2017, 8, 141. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.V.B.; Martinez, C.R.; Burity, H.A.; Chanway, C.P. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.). World J. Microbiol. Biotechnol. 2008, 24, 1187–1193. [Google Scholar] [CrossRef]
- Tavares, M.J.; Nascimento, F.X.; Glick, B.R.; Rossi, M.J. The expression of an exogenous ACC deaminase by the endophyte Serratia grimesii BXF1 promotes the early nodulation and growth of common bean. Lett. Appl. Microbiol. 2018, 66, 252–259. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Tavares, M.J.; Franck, J.; Ali, S.; Glick, B.R.; Rossi, M.J. ACC deaminase plays a major role in Pseudomonas fluorescens YsS6 ability to promote the nodulation of Alpha- and Betaproteobacteria rhizobial strains. Arch. Microbiol. 2019, 201, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Matse, D.T.; Huang, C.H.; Huang, Y.M.; Yen, M.Y. Effects of coinoculation of Rhizobium with plant growth promoting rhizobacteria on the nitrogen fixation and nutrient uptake of Trifolium repens in low phosphorus soil. J. Plant. Nutr. 2020, 43, 739–752. [Google Scholar] [CrossRef]
- Yadegari, M.; Rahmani, H.A.; Noormohammadi, G.; Ayneband, A. Plant growth promoting rhizobacteria increase growth, yield and nitrogen fixation in Phaseolus vulgaris. J. Plant. Nutr. 2010, 33, 1733–1743. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Bisht, J.K.; Kundu, S.; Gupta, H.S. Coinoculation of Bacillus thuringeinsis-KR1 with Rhizobium leguminosarum enhances plant growth and nodulation of pea (Pisum sativum L.) and lentil (Lens culinaris L.). World J. Microbiol. Biotechnol. 2009, 25, 753–761. [Google Scholar] [CrossRef]
- Elkoca, E.; Turan, M.; Donmez, M.F. Effects of single, dual and triple inoculations with Bacillus subtilis, Bacillus megaterium and Rhizobium leguminosarum bv. phaseoli on nodulation, nutrient uptake, yield and yield parameters of common bean (phaseolus vulgaris l. cv. ‘Elkoca-05’). J. Plant. Nutr. 2010, 33, 2104–2119. [Google Scholar] [CrossRef]
- Rajendran, G.; Sing, F.; Desai, A.J.; Archana, G. Enhanced growth and nodulation of pigeon pea by co-inoculation of Bacillus strains with Rhizobium spp. Bioresour. Technol. 2008, 99, 4544–4550. [Google Scholar] [CrossRef]
- Tilak, K.V.B.R.; Ranganayaki, N.; Manoharachari, C. Synergistic effects of plant-growth promoting rhizobacteria and Rhizobium on nodulation and nitrogen fixation by pigeonpea (Cajanus cajan). Eur. J. Soil Sci. 2006, 57, 67–71. [Google Scholar] [CrossRef]
- Petersen, D.J.; Srinivasan, M.; Chanway, C.P. Bacillus polymyxa stimulates increased Rhizobium etli populations and nodulation when co-resident in the rhizosphere of Phaseolus vulgaris. FEMS Microbiol. Lett. 1996, 142, 271–276. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Berg, G.; Lindström, K.; Räsänen, L.A. Co-inoculation of Pseudomonas spp. with Rhizobium improves growth and symbiotic performance of fodder galega (Galega orientalis Lam.). Eur. J. Soil Biol. 2010, 46, 269–272. [Google Scholar] [CrossRef]
- Zhang, F. Plant Growth Promoting Rhizobacteria and Soybean [Glycine max (L.) Merr.] Nodulation and Nitrogen Fixation at Suboptimal Root Zone Temperatures. Ann. Bot. 1996, 77, 453–460. [Google Scholar] [CrossRef]
- Masciarelli, O.; Llanes, A.; Luna, V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 2014, 169, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.I.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain s141 and bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Mishra, S.; Selvakumar, G.; Kundu, S.; Shankar Gupta, H. Enhanced soybean (Glycine max L.) plant growth and nodulation by Bradyrhizobium japonicum-SB1 in presence of Bacillus thuringiensis-KR. Acta Agric. Scand. Sect. B Soil Plant Sci. 2009, 59, 189–196. [Google Scholar] [CrossRef]
- Tsigie, A.; Tilak, K.V.B.R.; Saxena, A.K. Field response of legumes to inoculation with plant growth-promoting rhizobacteria. Biol. Fertil. Soils 2011, 47, 971–974. [Google Scholar] [CrossRef]
- Bai, Y.; Zhou, X.; Smith, D.L. Enhanced Soybean Plant Growth Resulting from Coinoculation of Bacillus Strains with Bradyrhizobium japonicum. Crop. Sci. 2003, 43, 1774–1781. [Google Scholar] [CrossRef]
- Soe, K.M.; Bhromsiri, A.; Karladee, D.; Yamakawa, T. Effects of endophytic actinomycetes and Bradyrhizobium japonicum strains on growth, nodulation, nitrogen fixation and seed weight of different soybean varieties. Soil Sci. Plant. Nutr. 2012, 58, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Soe, K.M.; Yamakawa, T. Evaluation of effective Myanmar Bradyrhizobium strains isolated from Myanmar soybean and effects of coinoculation with Streptomyces griseoflavus P4 for nitrogen fixation. Soil Sci. Plant. Nutr. 2013, 59, 361–370. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Moe, K.; Yamakawa, T. Effects of co-inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield of soybean in a field condition. Soil Sci. Plant. Nutr. 2018, 64, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Nimnoi, P.; Pongsilp, N.; Lumyong, S. Co-inoculation of soybean (Glycine max) with actinomycetes and Bradyrhizobium japonicum enhances plant growth, nitrogenase activity and plant nutrition. J. Plant. Nutr. 2014, 37, 432–446. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Hernandez, A.G.; Glick, B.R.; Rossi, M.J. The extreme plant-growth-promoting properties of Pantoea phytobeneficialis MSR2 revealed by functional and genomic analysis. Environ. Microbiol. 2020, 22, 1341–1355. [Google Scholar] [CrossRef]
- Pandya, M.; Rajput, M.; Rajkumar, S. Exploring plant growth promoting potential of non rhizobial root nodules endophytes of Vigna radiata. Microbiol. (Russ. Fed.) 2015, 84, 80–89. [Google Scholar] [CrossRef]
- Guiñazú, L.B.; Andrés, J.A.; Papa, M.F.D.; Pistorio, M.; Rosas, S.B. Response of alfalfa (Medicago sativa L.) to single and mixed inoculation with phosphate-solubilizing bacteria and Sinorhizobium meliloti. Biol. Fertil. Soils 2010, 46, 185–190. [Google Scholar] [CrossRef]
- Fox, S.L.; O’Hara, G.W.; Bräu, L. Enhanced nodulation and symbiotic effectiveness of Medicago truncatula when co-inoculated with Pseudomonas fluorescens WSM3457 and Ensifer (Sinorhizobium) medicae WSM. Plant Soil 2011, 348, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Verma, J.P.; Yadav, J.; Tiwari, K.N. Enhancement of Nodulation and Yield of Chickpea by Co-inoculation of Indigenous Mesorhizobium spp. and Plant Growth-Promoting Rhizobacteria in Eastern Uttar Pradesh. Commun. Soil Sci. Plant Anal. 2012, 43, 605–621. [Google Scholar] [CrossRef]
- Parmar, N.; Dadarwal, K.R. Stimulation of nitrogen fixation and induction of flavonoid-like compounds by rhizobacteria. J. Appl. Microbiol. 1999, 86, 36–44. [Google Scholar] [CrossRef]
- Malik, D.K.; Sindhu, S.S. Production of indole acetic acid by Pseudomonas sp.: Effect of coinoculation with Mesorhizobium sp. Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol. Mol. Biol. Plants 2011, 17, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Imran, A.; Mirza, M.S.; Shah, T.M.; Malik, K.A.; Hafeez, F.Y. Differential response of kabuli and desi chickpea genotypes toward inoculation with PGPR in different soils. Front. Microbiol. 2015, 6, 859. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, R.; Ramakrishnan, B.; Simranjit, K.; Ranjan, K.; Kanchan, A.; Hossain, F.; Nain, L. Cyanobacterial and rhizobial inoculation modulates the plant physiological attributes and nodule microbial communities of chickpea. Arch. Microbiol. 2017, 199, 1311–1323. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Zamin, F.R.; Sachdev, D.; Pour, N.K.; Engineer, A.; Pardesi, K.R.; Zinjarde, S.; Dhakephalkar, P.K.; Chopade, B.A. Characterization of plant-growth-promoting traits of Acinetobacter species isolated from rhizosphere of Pennisetum glaucum. J. Microbiol. Biotechnol. 2011, 21, 556–566. [Google Scholar] [CrossRef]
- Volpin, H.; Burdman, S.; Sowinski, S.C.; Kapulnik, Y.; Okon, Y. Inoculation with Azospirillum increased exudation of rhizobial nod- gene inducers by alfalfa roots. Mol. Plant. Microbe Interact. 1996, 9, 388–394. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant–bacterial interactions. Front. Plant. Sci. 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Guinel, F.C. Ethylene, a hormone at the center-stage of nodulation. Front. Plant. Sci. 2015, 6, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zgadzaj, R.; James, E.K.; Kelly, S.; Kawaharada, Y.; de Jonge, N.; Jensen, D.B.; Madsen, L.H.; Radutoiu, S. A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria. PLoS Genet. 2015, 11, e1005280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vimal, S.R.; Singh, J.S.; Arora, N.K.; Singh, S. Soil-Plant-Microbe Interactions in Stressed Agriculture Management: A Review. Pedosphere 2017, 27, 177–192. [Google Scholar] [CrossRef]
- de Vrieze, M.; Germanier, F.; Vuille, N.; Weisskopf, L. Combining Different Potato-Associated Pseudomonas Strains for Improved Biocontrol of Phytophthora infestans. Front. Microbiol. 2018, 9, 2573. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [Green Version]
- Sharma, C.K.; Vishnoi, V.K.; Dubey, R.C.; Maheshwari, D.K. A twin rhizospheric bacterial consortium induces systemic resistance to a phytopathogen Macrophomina phaseolina in mung bean. Rhizosphere 2018, 5, 71–75. [Google Scholar] [CrossRef]
- Singh, A.; Jain, A.; Sarma, B.K.; Upadhyay, R.S.; Singh, H.B. Rhizosphere competent microbial consortium mediates rapid changes in phenolic profiles in chickpea during Sclerotium rolfsii infection. Microbiol. Res. 2014, 169, 353–360. [Google Scholar] [CrossRef]
- Marimuthu, S.; Ramamoorthy, V.; Samiyappan, R.; Subbian, P. Intercropping System with Combined Application of Azospirillum and Pseudomonas fluorescens Reduces Root Rot Incidence Caused by Rhizoctonia bataticola and Increases Seed Cotton Yield. J. Phytopathol. 2013, 161, 405–411. [Google Scholar] [CrossRef]
- Shahzad, S.; Khan, M.Y.; Zahir, Z.A.; Asghar, H.N.; Chaudhry, U.K. Comparative effectiveness of different carriers to improve the efficacy of bacterial consortium for enhancing wheat production under salt affected field conditions. Pak. J. Bot. 2017, 49, 1523–1530. [Google Scholar]
- Goswami, S.K.; Kashyap, P.L.; Awasthi, S. Deciphering rhizosphere microbiome for the development of novel bacterial consortium and its evaluation for salt stress management in solanaceous crops in India. Indian Phytopathol. 2019, 72, 479–488. [Google Scholar] [CrossRef]
- Xia, M.; Chakraborty, R.; Terry, N.; Singh, R.P.; Fu, D. Promotion of saltgrass growth in a saline petroleum hydrocarbons contaminated soil using a plant growth promoting bacterial consortium. Int. Biodeterior. Biodegrad. 2020, 146, 104808. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Cornejo, P.; Kannan, V.R. Role of plant growth–promoting rhizobacterial consortium in improving the Vigna radiata growth and alleviation of aluminum and drought stresses. Environ. Sci. Pollut. Res. 2019, 26, 27647–27659. [Google Scholar] [CrossRef]
- Wang, C.-J.; Yang, W.; Wang, C.; Gu, C.; Niu, D.-D.; Liu, H.-X.; Wang, Y.-P.; Guo, J.-H. Induction of Drought Tolerance in Cucumber Plants by a Consortium of Three Plant Growth-Promoting Rhizobacterium Strains. PLoS ONE 2012, 7, e52565. [Google Scholar] [CrossRef] [Green Version]
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.H.; Doty, S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant. Biol. 2016, 6, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Saikia, J.; Sarma, R.K.; Dhandia, R.; Yadav, A.; Bharali, R.; Gupta, V.K.; Saikia, R. Alleviation of drought stress in pulse crops with ACC deaminase producing rhizobacteria isolated from acidic soil of Northeast India. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef]
- Kumar, M.; Karthikeyan, N.; Prasanna, R. Priming of Plant Defense and Plant Growth in Disease-Challenged Crops Using Microbial Consortia. In Microbial-Mediated Induced Systemic Resistance in Plants; Springer: Singapore, 2016; pp. 39–56. ISBN 9789811003882. [Google Scholar]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Gamalero, E.; Glick, B.R. Ethylene and Abiotic Stress Tolerance in Plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer: New York, NY, USA, 2012; pp. 395–412. ISBN 9781461408154. [Google Scholar]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Etalo, D.W.; Jeon, J.S.; Raaijmakers, J.M. Modulation of plant chemistry by beneficial root microbiota. Nat. Prod. Rep. 2018, 35, 398–409. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial interactions within the plant holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.O.; Cornejo, H.A.C.; Rodríguez, L.M.; Bucio, J.L. The role of microbial signals in plant growth and development. Plant Signal. Behav. 2009, 4, 701–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barea, J.-M.; Pozo, M.J.; Azcón, R.; Aguilar, C.A. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corradi, N.; Bonfante, P. The Arbuscular Mycorrhizal Symbiosis: Origin and Evolution of a Beneficial Plant Infection. PLoS Pathog. 2012, 8, e1002600. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Allah, E.F.A.; Alqarawi, A.A.; Huqail, A.A.A.; Wirth, S.; Egamberdieva, D. The Interaction between Arbuscular Mycorrhizal Fungi and Endophytic Bacteria Enhances Plant Growth of Acacia gerrardii under Salt Stress. Front. Microbiol. 2016, 7, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusran, Y.; Roemheld, V.; Mueller, T. Effects of Pseudomonas sp. ”Proradix” and Bacillus amyloliquefaciens FZB42 on the Establishment of AMF Infection, Nutrient Acquisition and Growth of Tomato Affected by Fusarium oxysporum Schlecht f.sp. radicis-lycopersici Jarvis and Shoemaker. UC Davis Dep. Plant. Sci. 2009. [Google Scholar]
- Gao, X.; Lu, X.; Wu, M.; Zhang, H.; Pan, R.; Tian, J.; Li, S.; Liao, H. Co-inoculation with rhizobia and AMF inhibited soybean red crown rot: From field study to plant defense-related gene expression analysis. PLoS ONE 2012, 7, e33977. [Google Scholar] [CrossRef]
- Mosqueda, M.O.; del, C.; Granados, M.R.; del, C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef]
- Vivas, A.; Marulanda, A.; Lozano, J.M.R.; Barea, J.M.; Azcón, R. Influence of a Bacillus sp. on physiological activities of two arbuscular mycorrhizal fungi and on plant responses to PEG-induced drought stress. Mycorrhiza 2003, 13, 249–256. [Google Scholar] [CrossRef]
- Alam, M.; Khaliq, A.; Sattar, A.; Shukla, R.S.; Anwar, M.; Dharni, S. Synergistic effect of arbuscular mycorrhizal fungi and Bacillus subtilis on the biomass and essential oil yield of rose-scented geranium (Pelargonium graveolens). Arch. Agron. Soil Sci. 2011, 57, 889–898. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 4686. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef] [Green Version]
- Zancarini, A.; Mougel, C.; Voisin, A.S.; Prudent, M.; Salon, C.; Jolain, N.M. Soil Nitrogen Availability and Plant Genotype Modify the Nutrition Strategies of M. truncatula and the Associated Rhizosphere Microbial Communities. PLoS ONE 2012, 7, e47096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vestberg, M.; Kukkonen, S.; Saari, K.; Parikka, P.; Huttunen, J.; Tainio, L.; Devos, N.; Weekers, F.; Kevers, C.; Thonart, P.; et al. Microbial inoculation for improving the growth and health of micropropagated strawberry. Appl. Soil Ecol. 2004, 27, 243–258. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seiboth, V.S.; Estrella, A.H.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Prudencio, O.G.R.; Castro, M.D.; Rivera, M.E.G.; López, M.d.C.G.; Moreno, S.J.; Flores, S.C. Trichoderma in the Rhizosphere: An Approach toward A Long and Successful Symbiosis with Plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Zeilinger, S., Singh, H.B., Druzhinina, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–38. ISBN 9780128194539. [Google Scholar]
- Marina, M.A.S.; Flores, M.A.S.; Rivera, E.E.U.; Longoria, E.C.; Estrella, A.H.; Flores, S.C. Colonization of Arabidopsis roots by Trichoderma atroviride promotes growth and enhances systemic disease resistance through jasmonic acid/ethylene and salicylic acid pathways. Eur. J. Plant Pathol. 2011, 131, 15–26. [Google Scholar] [CrossRef]
- Harman, G.E. Multifunctional fungal plant symbionts: New tools to enhance plant growth and productivity. New Phytol. 2011, 189, 647–649. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant. Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- de Rosa, A.; Napolitano, M.; Rouphael, Y.; Woo, S.L.; Gioia, L.; Fiorentino, N.; Lombardi, N.; Ventorino, V.; Romano, I.; Pepe, O.; et al. Trichoderma-Based Biostimulants Modulate Rhizosphere Microbial Populations and Improve N Uptake Efficiency, Yield, and Nutritional Quality of Leafy Vegetables. Front. Plant. Sci. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, P.G.; Troncoso, M.D.P.; Monfil, V.O.; Estrella, A.H. Trichoderma species: Versatile plant symbionts. Phytopathology 2019, 109, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youssef, S.A.; Tartoura, K.A.; Abdelraouf, G.A. Evaluation of Trichoderma harzianum and Serratia proteamaculans effect on disease suppression, stimulation of ROS-scavenging enzymes and improving tomato growth infected by Rhizoctonia solani. Biol. Control. 2016, 100, 79–86. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhuang, L.; Yu, Y.; Liu, J.; Zhang, L.; Gao, Z.; Wu, Y.; Gao, W.; Ding, G.; et al. A Rhizosphere-Derived Consortium of Bacillus subtilis and Trichoderma harzianum Suppresses Common Scab of Potato and Increases Yield. Comput. Struct. Biotechnol. J. 2019, 17, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, V.; Li, Y.; Sun, J.; Vallikkannu, M.; Chen, J. Vel1 regulates the growth of Trichoderma atroviride during co-cultivation with Bacillus amyloliquefaciens and is essential for wheat root rot control. Biol. Control. 2020, 151, 104374. [Google Scholar] [CrossRef]
- Junior, A.F.C.; Oliveira, A.G.; Santos, G.R.; Reis, H.B.; Chagas, L.F.B.; Miller, L.O. Combined inoculation of rhizobia and Trichoderma spp. on cowpea in the savanna, Gurupi-TO, Brazil. Rev. Bras. Ciênc. Agrár. Braz. J. Agric. Sci. 2015, 10, 27–33. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, J.D.G.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, H.; Behboudi, K.; Ahmadzadeh, M.; Javan-Nikkhah, M.; Zamioudis, C.; Pieterse, C.M.J.; Bakker, P.A.H.M. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps 14. Biol. Control. 2013, 65, 14–23. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, A.; Maji, D.; Awasthi, A.; Vajpayee, P.; Kalra, A. Evaluating the potential of combined inoculation of Trichoderma harzianum and Brevibacterium halotolerans for increased growth and oil yield in Mentha arvensis under greenhouse and field conditions. Ind. Crops Prod. 2019, 131, 173–181. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.Q.; Wang, M.; Sun, J.; Gao, J.X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium Stalk rot. Sci. Rep. 2017, 7, 1711. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Karlsson, M.; Rosberg, A.K.; Dorais, M.; Naznin, M.T.; Khalil, S.; Bergstrand, K.J. Light and microbial lifestyle: The impact of light quality on plant–microbe interactions in horticultural production systems—A review. Horticulturae 2019, 5, 41. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Schmid, M.; van Tuinen, D.; Berg, G. Plant-driven selection of microbes. Plant. Soil 2009, 321, 235–257. [Google Scholar] [CrossRef]
- León, R.H.; Solís, D.R.; Pérez, M.C.; Mosqueda, M.C.d.C.; Rodríguez, L.I.M.; la Cruz, H.R.d.; Cantero, E.V.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control. 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]


| Rhizobia | Co-Inoculant | Plant Host | Reference |
|---|---|---|---|
| R. tropici CIAT899 | Bacillus megaterium | Phaseolus vulgaris | [97] |
| R. tropici CIAT899 | Paenibacillus polymyxa (DSM 36) | Phaseolus vulgaris | [98] |
| R. tropici CIAT899 | Serratia grimesii BXF1 | Phaseolus vulgaris | [99] |
| R. tropici CIAT899 | Pseudomonas fluorescens YsS6 | Phaseolus vulgaris | [100] |
| Rhizobium spp. | Bacillus aryabhattai Sb Azotobacter vinelandii G31 | Trifolium repens | [101] |
| Rhizobium | Pseudomonas fluorescens P-93 Azospirillum lipoferum S-21 | Phaseolus vulgaris | [102] |
| Rhizobium leguminosarum bv. trifolii | Bacillus insolutis, Bacillus brevis Agrobacterium rhizogenes | Trifolium pratense | [94] |
| Rhizobium leguminosarum PR1 | Bacillus thuringiensis KR1 | Pisum sativum; Lens culinaris | [103] |
| Rhizobium | Bacillus subtilis OSU-142 Bacillus megaterium M-3 | Phaseolus vulgaris | [104] |
| Rhizobium spp. IC3123 | Bacillus megaterium NR4 and NR6 | Cajanus cajan | [105] |
| Rhizobium sp. AR-2–2 | Pseudomonas putida; P. fluorescens Bacillus cereus | Cajanus cajan | [106] |
| Rhizobium etli | Paenibacillus polymyxa | Phaseolus vulgaris | [107] |
| (Neo)Rhizobium galega bv. orientalis | Pseudomonas trivalis 3Re27 Pseudomonas extremorientalis TSAU20 | Galega orientalis | [108] |
| Bradyrhizobium japonicum 532C | Serratia proteamaculans 1–102 Aeromonas hydrophila P73 Serratia liquefaciens 2–68 | Glycine max | [109] |
| B. japonicum E109 | Bacillus amyloliquefaciens LL2012 | Glycine max | [110] |
| B. diazoefficiens USDA110 | Bacillus velezensis S141 | Glycine max | [111] |
| B. japonicum SB1 | Bacillus thuringiensis KR1 | Glycine max | [112] |
| B. japonicum | B. subtilis | Glycine max | [113] |
| Bradyrhizobium japonicum | B. subtilis NEB4 and NEB5 B. thuringiensis NEB17 | Glycine max | [114] |
| B. japonicum spp. | Streptomyces griseoflavus P4 | Glycine max | [115,116] |
| B. elkanii BLY3–8 | Streptomyces griseoflavus P4 | Glycine max | [117] |
| B. japonicum | Nocardia alba Nonomuraea rubra Actinomadura glauciflava | Glycine max | [118] |
| Bradyrhizobium sp. BR1602 | Pantoea phytobeneficialis MSR2 | Calopogonium muconoides | [119] |
| Sinorhizobium meliloti | Exiguobacterium sp. M2N2c and B1N2b | Trigonella foenum-graecum | [96] |
| E. adhaerens | Bacillus anthracis Ml Paenibacillus taichungensis M10 Paenibacillus xylanilyticus M15 | Vigna radiata | [120] |
| Sinorhizobium meliloti B399 | Bacillus sp. M7c | Medicago sativa | [121] |
| Ensifer (Sinorhizobium) medicae WSM419 | Pseudomonas fluorescens WSM3457 | Medicago truncatula | [122] |
| Mesorhizobium sp. BHURC02 | Pseudomonas fluorescens BHUPSB06 | Cicer arietinum | [123] |
| Mesohizobium spp. Ca181 and Ca313 | Pseudomonas sp. CRP55b and CRS68 | Cicer arietinum | [124] |
| Mesohizobium spp. Ca181 | Pseudomonas spp. | Cicer arietinum | [125] |
| M. ciceri TAL-1148 | Ochrobactrum ciceri Ca-34 | Cicer arietinum | [126] |
| Mesorhizobium ciceri | Anabaena | Cicer arietinum | [127] |
| Cupriavidus taiwanensis | Pseudomonas fluorescens YsS6 | Mimosa pudica | [100] |
| Consortium | Crop | Stress | Effect | Reference |
|---|---|---|---|---|
| Pseudomonas spp. | Potato | Phytophthora infestans | Reduction of mycelial growth Decreased release of zoospores | [135] |
| Xanthomonas sp., Stenotrophomonas sp., and Microbacterium sp. | Arabidopsis thaliana | Hyaloperonospora arabidopsidis | induced systemic resistance (ISR) activation (reduced the number of pathogen spores) Plant growth promotion (shoot fresh weight) | [136] |
| Pseudomonas putida CRN-09 and Bacillus subtilis CRN-16 | Mung bean | Macrophomina phaseolina | ISR activation (peroxidase (PO), polyphenol oxidase (PPO), phenylalanine ammonia-lyase (PAL), β-1,3 glucanase and chitinase) Plant growth promotion (increased shoot and root length, shoot and root fresh weight) | [137] |
| Pseudomonas aeruginosa PHU094 and Mesorhizobium sp. RL091 | Chickpea | Sclerotium rolfsii | ISR activation (increased phenolic compounds) Plant growth promotion (increased shoot length and total biomass) | [138] |
| Pseudomonas fluorescens and Azospirillum | Cotton | Rhizoctonia bataticola | Decreased root rot incidence Increased seed cotton yield | [139] |
| Bacillus cereus Y5, Bacillus sp. Y14, and Bacillus subtilis Y16 | Wheat | Salinity | Increased photosynthetic rate, the content of carotenoids and crude protein Higher grain yield | [140] |
| Brevibacillus fluminis, Brevibacillus agri, and Bacillus paralicheniformis | Brinjal, potato, tomato, and chilli | Salinity | Increased fermination percentage Plant growth promotion (an increase in shoot length, root length, dry and fresh weight) | [141] |
| Pseudomonas sp. PFS1 and BSS3B2, Serratia proteamaculans S1BD1, Alcaligenes sp. PKS1, and Bacillus sp. PSS2 | saltgrass | Salinity, petroleum hydrocarbons | Plant growth promotion (increased plant biomass) | [142] |
| B. megaterium CAM12 and P. agglomerans CAH6 | Mung bean | Aluminum and drought | Reduced Al uptake in plants Plant growth promotion (increased plant biomass) Higher content of carotenoids, chlorophyll | [143] |
| Bacillus cereus AR156, Bacillus subtilis SM21, and Serratia sp. XY21 | Cucumber | Drought | Increased the leaf proline content Significantly enhanced the superoxide dismutase (SOD) activity | [144] |
| Rhodotorula graminis WP1, Burkholderia vietnamiensis WPB, Rhizobium tropici PTD1, Acinetobacter calcoaceticus WP19, Rahnella sp. WP5, Burkholderia sp. WP9, Enterobacter asburiae PDN3, Sphingomonas yanoikuyae WW5, Pseudomonas sp. WW6, and Curtobacterium sp. WW7 | poplar | Drought | Plant growth promotion (increased root dry weight, shoot dry weight, total dry weight, total nitrogen) Reduced damage by reactive oxygen species (ROS) | [145] |
| Ochrobactrum pseudogrignonense RJ12, Pseudomonas sp. RJ15, and Bacillus subtilis RJ46 | Black gram and Pea | Drought | Plant growth promotion (increased seed germination percentage, root length, shoot length, and dry weight) Elevated production of ROS scavenging enzymes and cellular osmolytes Higher leaf chlorophyll content | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoyo, G.; Guzmán-Guzmán, P.; Parra-Cota, F.I.; Santos-Villalobos, S.d.l.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Plant Growth Stimulation by Microbial Consortia. Agronomy 2021, 11, 219. https://doi.org/10.3390/agronomy11020219
Santoyo G, Guzmán-Guzmán P, Parra-Cota FI, Santos-Villalobos Sdl, Orozco-Mosqueda MdC, Glick BR. Plant Growth Stimulation by Microbial Consortia. Agronomy. 2021; 11(2):219. https://doi.org/10.3390/agronomy11020219
Chicago/Turabian StyleSantoyo, Gustavo, Paulina Guzmán-Guzmán, Fannie Isela Parra-Cota, Sergio de los Santos-Villalobos, Ma. del Carmen Orozco-Mosqueda, and Bernard R. Glick. 2021. "Plant Growth Stimulation by Microbial Consortia" Agronomy 11, no. 2: 219. https://doi.org/10.3390/agronomy11020219
APA StyleSantoyo, G., Guzmán-Guzmán, P., Parra-Cota, F. I., Santos-Villalobos, S. d. l., Orozco-Mosqueda, M. d. C., & Glick, B. R. (2021). Plant Growth Stimulation by Microbial Consortia. Agronomy, 11(2), 219. https://doi.org/10.3390/agronomy11020219








